Abstract
Androgen deprivation therapy (ADT) has been associated with adverse effects on cognition. However, we currently lack understanding of the neurobiology and prognostic markers of these effects. Given that ADT acts via the hypothalamus–pituitary–gonadal axis, we assessed whether baseline hypothalamic resting state functional connectivity (rsFC) could predict changes in working memory and quality of life in prostate cancer patients following androgen deprivation. In a prospective observational study, 28 men with non-metastatic prostate cancer receiving ADT and 38 patients not receiving ADT (controls), matched in age, years of education and Montreal Cognitive Assessment score, participated in brain imaging at baseline, and N-back task and quality-of-life (QoL) assessments at baseline and at 6 months follow-up. Imaging data were processed with published routines and evaluated at a corrected threshold. ADT and control groups did not differ in N-back performance or QoL across time points. In ADT, the changes in 0-back correct response rate (follow-up—baseline) were correlated with baseline hypothalamus-precentral gyrus rsFC; the changes in 1-back correct response rate and reaction time were each correlated with hypothalamus-middle frontal gyrus and superior parietal lobule rsFC. The changes in physical well-being subscore of QoL were correlated with baseline hypothalamus-anterior cingulate and cuneus rsFC. The hypothalamus rsFCs predicted N-back and QoL change with an area under the receiver operating characteristic curve of 0.93 and 0.73, respectively. Baseline hypothalamus-frontoparietal and salience network rsFC’s predict inter-subject variations in the changes in working-memory and QoL following 6 months of ADT. Whether and how hypothalamic rsFCs may predict the cognitive and QoL effects with longer-term ADT remain to be investigated.
Similar content being viewed by others
Introduction
Androgen deprivation therapy (ADT) is the standard primary treatment for men with prostate cancer1. Despite the potential survival benefits, ADT is accompanied with physiological side effects that may compromise patient’s quality of life (QoL)1,2. Further, testosterone affects cognition possibly via modulation of neuronal signaling and connectivity, and plays a role in limiting the production of β-amyloid peptide and preventing N-methyl-d-aspartate excitotoxicity1,3. ADT results in reduction in the level of bioavailable testosterone and may negatively affect cognition4. However, according to a recent review, testosterone levels alone did not appear to predict cognitive decline or development of Alzheimer’s disease, nor does testosterone replacement therapy significantly affect cognition5 in elderly men. Similarly, in prostate cancer patients, the neurobiology of ADT-associated cognitive changes are not fully understood, and the predictors of such changes have yet been investigated systematically3,6.
Resting-state functional connectivity (rsFC) characterizes intrinsic functional organization of the brain and has successfully predicted changes in cognitive functions during healthy aging and in neurological illnesses7,8,9,10. Studies have noted the contribution of sex steroids to modulation of brain functional connectivity11. In support, prostate cancer patients with and without ADT demonstrate different patterns of regional connectivities. For instance, a recent study showed enhanced rsFC in regions with high androgen receptor expression in prostate cancer patients undergoing ADT, as compared to those who did not receive ADT12. Although a direct correlation between connectivity metrics and cognition was not reported, cognitive performance was worse in ADT patients and the changes in rsFC may reflect impaired brain functionality12. Importantly, in mild cognitive impairment, baseline rsFC predicted later development of dementia and Alzheimer disease with 80–90% accuracy13,14. RsFC also predicted treatment responses in patients with neuropsychiatric disorders15,16,17,18, suggesting its utility as a prognostic marker of clinical outcomes.
In the present study, we assessed whether baseline hypothalamic rsFC could predict changes in working memory and quality of life in prostate cancer patients at 6 months following androgen deprivation. ADT acts via the hypothalamus–pituitary–gonadal (HPG) axis and achieves its therapeutic effects19 by lowering the levels of androgen, which plays a critical role in driving proliferation of prostate cancer possibly via inducing cell cycle growth and blocking apoptosis20. Hypothalamus secretes gonadotropin-releasing hormone (GnRH) in a pulsatile manner, which stimulates the release of luteinizing hormone (LH)21. ADT with GnRH agonists initially stimulates this pathway, resulting in enhanced release of LH and androgens in men22,23,24. With continued administration, GnRH receptors on the pituitary become desensitized, and the levels of gonadotropin and androgen decline, thereby inhibiting tumor growth23,25. Thus, ADT with GnRH agonists achieves its therapeutic effects by acting directly on the hypothalamus.
In accord, animal studies have extensively documented the effects of androgen deprivation on hypothalamic structures and functions26,27,28. Importantly, hypothalamic circuits regulate object memory formation29 and play an instrumental role in motivation, arousal, and affect processing30,31. A meta-analysis reported HPG dysregulation, as evidenced in high night-time cortisol level as well as diurnal drop and awakening response, as a potential predictor of cognitive impairment in older individuals32. Another meta-analysis reported that variants of HPG axis-related genes, including those for corticotropin releasing hormone (CRH), CRH-binding protein, CRH-receptor, glucocorticoid and mineralocorticoid receptors, may differentially affect cognitive performance33. Global dysfunction of the HPG axis has often been described in the pathophysiology of Alzheimer’s disease34,35. Further, hypothalamic neuronal activities conduce to learning and memory independently of motivation, arousal, and anxiety36,37,38. A recent study associated N-back working memory performance and HPG axis response in healthy young adults39. Another study noted reduced hypothalamus-middle frontal rsFC in link with diminished attention in sleep-deprived men40. Further, we previously reported altered hypothalamus-precentral gyrus rsFC in association with 0-back correct response rate in prostate cancer patients on ADT41.
A review of longitudinal studies in humans and animals showed working memory, visuospatial memory, and executive functioning to be the cognitive domains most affected by androgen deprivation1. However, a meta-analysis noted effects of ADT on visuomotor performance but not working memory42. Another study reported worse visuospatial function and visual memory but overall intact cognitive performance in prostate cancer patients on ADT relative to controls in a longitudinal setting43. On the other hand, despite the less-than-consistent findings, studies have reported substantial inter-subject variation in cognitive performance, as also noted in women with breast cancer who received chemotherapy44,45,46,47. In particular, no studies to our knowledge have aimed to identify the neural predictors of individual variation in the impact of ADT on cognition.
Here, we evaluated hypothalamus rsFC as a predictor of ADT-associated changes in working memory and quality of life. We hypothesized that hypothalamus rsFC would predict individual variation in accuracy and reaction time in an N-back task and quality of life in prostate cancer patients receiving ADT.
Materials and methods
Participants and clinical profiles
Patient recruitment criteria and procedures followed our earlier studies2,41,48. Patients 55–75 years of age and with biopsy-proven prostate adenocarcinoma without distant metastases were recruited from the Medical Oncology and Urology Clinics at the West Haven VA Connecticut Healthcare System. Following current National Comprehensive Cancer Network and American Urological Society practice guidelines, treatments were not affected by patients’ decision of participation in the study. All patients prescribed ADT as adjuvant treatment or due to biochemical recurrence were contacted for participation. ADT consisted of medical castration with an LH-RH agonist (Goserelin or Leuprolide) subcutaneously for 6 months, after a lead-in period with Bicalutamide 50 mg daily. Patients with non-metastatic prostate cancer who had never been treated with ADT participated as controls (CON). For both ADT and CON, exclusion criteria were: Eastern Cooperative Oncology Group Performance Status > 1; active second malignancy; significant cardiovascular, liver, renal, or neurological disease; use of any investigational drugs or contraindications, including claustrophobia, for magnetic resonance imaging (MRI); current substance (except nicotine) use disorders (use of illicit substances were verified by a urine test); history of Axis I psychiatric illness; history of traumatic brain injury or concussions causing loss of consciousness. All participants underwent a health questionnaire interview to ensure eligibility for MRI. Participants who had a prostatectomy were at least 3 months from their surgery and had fully recovered from anesthesia before study entry. Participants who were to receive radiation to the prostate underwent baseline assessment and MR scan before starting any treatment and had to be fully recovered from any acute side effects of radiation at the time of their follow-up assessments. In addition to measuring serum testosterone and prostate-specific antigen as part of their routine bloodwork at every assessment, all participants underwent determination of other hormonal (e.g., cortisol and thyroid hormone) levels that could potentially affect cognitive function.
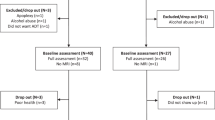
Among 100 candidates with non-metastatic prostate cancer, 78 who had never been treated with ADT were enrolled in the study. Thirty-six patients were scheduled for ADT (ADT group) and 42 patients served as controls (CON group). Thirty ADT and 40 CON completed both baseline (clinical assessment and MRI) and follow-up (clinical) assessments. However, 2 ADT and 2 CON were excluded due to excessive head movements during MR scans. Thus, the data from 28 ADT and 38 CON were included in the analyses (Supplementary Fig. S1).
Study procedures and assessment of working memory and quality of life
All participants underwent clinical, including quality of life (QoL), and cognitive (N-back task) assessment at baseline and at 6-month follow-up. At baseline, participants were also assessed for global cognition using Montreal Cognitive Assessment (MoCA) and underwent MR imaging.
N-back task is a widely used paradigm to assess working memory, a form of short-term memory that provides temporary storage and manipulation of information necessary for complex cognitive tasks49 (Supplementary Fig. S2). Briefly, 0-, 1-, and 2-back trials were included in the N-back task, each imposing increasingly higher demand on working memory. We used the correct response rate (CR) and reaction time (RT) as outcome measures of N-back performance.
As a general measure of QoL, participants completed the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire at baseline and at 6-month follow-up50,51. The cumulative score of FACT-P subscale scores: physical well-being (PWB), social well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and prostate cancer subscale (PCS) formed the total QoL scores.
Imaging protocol and data processing
Details of imaging data acquisition, processing, and analyses are described in the Supplement and follow our earlier study41. Briefly, subjects were scanned at baseline with a 3-Tesla Siemens Prisma system using a protocol as in our previous studies41. We pre-processed the imaging data and computed whole brain resting state functional connectivity (rsFC) of the hypothalamus for individual participants, following published routines in Statistical Parametric Mapping (SPM12)31,52.
Nuisance signals unlikely to reflect neural activity were removed using linear regression with the six head motion parameters from realignment, signals of the whole brain, ventricular system, white matter, and their first-order derivatives as covariates31,53. Images were checked for micro-head motions, followed by “scrubbing” to remove time points affected by head motions54 or DVARS(t) > 7555. We applied a temporal band-pass filter (0.009 Hz < f < 0.08 Hz) to the time course to obtain low-frequency fluctuations and computed the correlation maps to estimate rsFC of the hypothalamus56,57. We employed the hypothalamus mask from the WFU Pick-Atlas58 as the seed, as in an earlier study52. The correlation coefficients between the averaged time course of the hypothalamus seed and time courses of all other brain voxels were computed for each participant. Next, the correlation maps were converted into z-score maps by Fisher’s Z transform: z = 0.5loge [1 + r/1 − r] (r = correlation coefficient) to obtain normally distributed maps41.
Statistical analyses of clinical, behavioral, and imaging data
All statistical analyses of clinical and behavioral data were conducted with Stata (Stata Corp LLC, Texas, USA). We used linear mixed model via restricted maximum likelihood (REML) with random intercept across subjects to assess changes during follow-up from baseline in longitudinal variables, with group (ADT vs. CON) as a between-subject and time-point (follow-up vs. baseline) as a within-subject variable (fixed effects in model). In post-hoc analyses, between and within group differences were assessed using two- and paired-sample t-test, respectively. The results that met p < 0.05 (two-tailed) were considered statistically significant.
We employed SPM12 in group statistics of rsFC data. To examine hypothalamus rsFCs that could predict changes (follow-up vs. baseline) in N-back and QoL scores, we assessed the correlations between baseline hypothalamus rsFC and changes in N-back and QoL scores in ADT, using multiple regression module of SPM with baseline age, education, and MoCA scores as covariates. Clusters with hypothalamus rsFCs that met cluster p < 0.05, family-wise error (FWE)-corrected with a cluster-forming voxel p < 0.001, uncorrected were reported in MNI coordinates. We extracted cluster parameter (β) estimates for graphical presentation and further statistical analyses.
In an additional set of analyses, we targeted subprocesses of memory by contrasting the metrics of N-back performance across block conditions. For both CR and RT, we extracted these parameters: 2-back minus 0-back; 1-back minus 0-back; and 2-back minus 1-back, each representing maintenance load (2- vs. 0-item), replacement (1-step vs. no replacement), and shifting (2- shift vs. 1-step shift) subprocess of memory59. Replacement and shifting are components of updating. We repeated multiple regression, as described above, with these derived scores and presented the results in the Supplement.
Prediction of change for worse vs. no-worse in N-back performance and QoL in ADT
We assessed how well hypothalamus rsFCs at baseline could predict inter-subject variation in the changes (follow-up—baseline) in N-back performance metrics and QoL scores using logistic regression. Changes in N-back and QoL scores were coded as 0 (worse) and 1 (no-worse). A worse change was assessed as for a difference > 0.5 SD (decrease in CR and QoL, and increase in RT) during follow-up as compared to baseline60; otherwise, no-worse. As there were multiple N-back performance metrics, we performed latent class analysis (LCA) of all six N-back metrics, i.e., 0-, 1-, 2-back CR and 0-, 1-, 2-back RT, each coded in 0 and 1, to obtain representative classes of overall worse/no-worse performance. LCA returned two classes of overall changes in N-back performance, with 0/1 to indicate worse/no-worse performance during follow-up vs. baseline (score distribution shown in Supplementary Fig. S3). We applied logistic regression using LCA (for N-back) and QoL classes as the response variable, hypothalmaus rsFCs as predictor, and baseline age, education, and MoCA scores as covariates. Model prediction accuracy was represented by the area under the curve (AUC) in receiver operating characteristic analysis.
Ethics approval and consent to participate
The study was approved by the Human Investigation Committee at both the West Haven VA and Yale University School of Medicine (Ref. No.: HIC#2000020501) and was conducted in accordance with Declaration of Helsinki. All participants provided a written informed consent prior to the study.
Results
Baseline clinical profiles of the participants
At baseline, ADT and CON patients were comparable in age, years of education, MoCA score, testosterone (t64 = 1.0, p = 0.309), and cortisol (t64 = 0.3, p = 0.798) levels (Table 1). In addition, we observed a significant treatment × time interaction in testosterone level, as expected of the effects of ADT, but not in the cortisol level (Table 1).
N-back performance and quality of life scores
In mixed model analyses, treatment (ADT/CON) × time (baseline/follow-up) interaction was not significant for any of the N-back performance metrics (Supplementary Table S1). Overall, mean correct response rate was higher and mean RT was lower in CON compared to ADT at both baseline and follow-up (Fig. 1, Supplementary Table S2). Addditionally, we observed a significant effect of treatment on 0-back and 1-back correct response rate, and a significant effect of time on 2-back correct response rate (Supplementary Table S1). Among the N-back metrics, baseline 0-back correct response rate (t64 = 2.4, p = 0.02) and follow-up 1-back correct response rate (t64 = 2.6, p = 0.01) were significantly higher in CON than in ADT. For other N-back metrics, baseline and follow-up measures were comparable between groups (p’s > 0.05).
Distribution of (A) N-back correct response rates (%), (B) N-back response time (ms), (C) quality of life (QoL) score and sub-scores—physical well-being (PWB), subjective well-being (SWB), (D) emotional well-being (EWB), functional well-being (FWB)—and prostate cancer specific (PCS) score at baseline and follow-up in ADT (red) and CON (green). Data shown in group mean ± SD. CR correct response, RT reaction time. *p < 0.05, #p = 0.078 in paired t-test.
Among memory subprocesses, correct response rate during replacement was better in CON compared to ADT during follow-up. Other baseline and follow-up comparisons were comparable between the two groups (Supplementary Table S3).
Treatment × time interaction was not significant for QoL scores and sub-scores (Supplementary Table S1). However, the mean scores were higher in CON than in ADT both during baseline and follow-up (Fig. 1, Supplementary Table S2). Significant main effect of treatment was noted for QoL, PWB and PCS scores; and significant main effect of time for PWB scores. The follow-up scores of QoL (t64 = 2.4, p = 0.019), PWB (t64 = 2.7, p = 0.008), and PCS (t64 = 2.7, p = 0.009) were lower in ADT than in CON. The other scores were not significantly different.
Baseline hypothalamic rsFC predicting changes in N-back and QoL scores in ADT
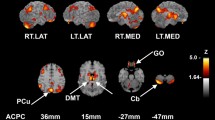
The hypothalamus seed is shown in Fig. 2A. We evaluated how baseline hypothalamus rsFC associated with changes (follow-up vs. baseline) in N-back and QoL scores for the whole-brain with baseline age, education and MoCA score as covariates at cluster p < 0.05 FWE-corrected with a cluster-forming voxel p < 0.001, uncorrected. In the ADT group, hypothalamus-bilateral precentral gyrus (PCG) rsFC was negatively correlated with change in 0-back correct response rate, hypothalamus-right middle frontal gyrus (MFG) rsFC was negatively correlated with change in 1-back correct response rate, and hypothalamus-left superior parietal lobule (SPL) rsFC was negatively correlated with change in 1-back RT (Fig. 2B).
Hypothalamus (A: seed) rsFC correlates of change (follow-up versus baseline) in N-back scores (B) and QoL sub-score PWB (C) in ADT. The clusters were obtained using whole-brain regression with baseline age, education, MoCA as covariates, at cluster p < 0.05 FWE-corrected and cluster-forming voxel p < 0.001, uncorrected. Color bar shows voxel T-values. Warm/cool colors: positive/negative correlations. Hit: correct response rate (%), RT: reaction time (ms), PWB: physical well-being.
Additionally, for memory subprocesses, the change in replacement RT, shift correct response rate and shift RT correlated negatively with hypothalamus-left SPL rsFC, positively with hypothalamus-left cerebellum rsFC, and negatively with hypothalamus-right cerebellum rsFC, respectively (Supplementary Fig. S4A). Regressions with the other measures did not reveal significant clusters.
Among the QoL scores, change in PWB was correlated positively with hypothalamus-left anterior cingulate cortex (ACC) rsFC and negatively with hypothalamus-bilateral cuneus rsFC (Fig. 2C). At the same threshold no clusters showed significant hypothalamus rsFCs in correlation with the changes in other QoL scores.
We also assessed whether baseline testosterone and cortisol levels could predict N-back and QoL changes in ADT. The results showed that baseline testosterone levels were not correlated with any of the N-back (all p’s > 0.102), and QoL (all p’s > 0.299) measures or hypothalamus rsFCs (all p’s > 0.164). Likewise, baseline cortisol levels were not correlated with any of the N-back (all p’s > 0.080), and QoL (p’s > 0.228) measures or hypothalamus rsFCs (all p’s > 0.170) across subjects.
We computed the β estimtaes of hypothalamus rsFC of the regions identified in ADT for all participants (ADT and CON). Figure 3A–C show the correlations for ADT and CON separately and slope tests confirmed the differences in slope in the regressions of N-back metrics vs. rsFCs between groups. None of the regressions were significant in CON. Supplementary Figure S4B–D show the correlations with memory subprocesses (N-back derived metrics) separately in ADT and CON. Likewise, we computed the β estimtaes of hypothalamus rsFC with ACC and bilateral cuneus for all participants. Figure 3D–F show the correlations for ADT and CON separately and slope tests confirmed the differences in slope in the regressions of PWB score vs. rsFCs between groups.
Pearson’s partial correlation between baseline hypothalamus (HT)—(A) bilateral precentral gyrus (PCG) rsFC and change (follow-up—baseline) in 0-back correct response rate (hit %); (B) right MFG rsFC and change in 1-back hit %; (C) left superior parietal lobule (SPL) rsFC and change in 1-back reaction time (RT); (D) left anterior cingulate cortex (ACC) rsFC and change in PWB; (E/F) right/left cuneus rsFC and change in PWB. Note that, because of the inclusion of covariates in the regression, the residuals are plotted here. The covariates included baseline age, years of education, and MoCA score. The insets show the t- and p-values of slope tests of ADT vs. CON in the regressions.
Accuracy of baseline hypothalamic rsFC in predicting worse vs. no-worse grouping in N-back and QoL scores in ADT
Additionally, with LCA classes of N-back performance as the response variable (see “Methods”), the logistic regression model with the hypothalamus rsFCs (as noted in previous section) as predictors and baseline age, education, and MoCA scores as covariates was significant (p = 0.010) with an AUC = 0.93 (Fig. 4A). In contrast, the regression model with the covariates alone was not significant (p = 0.119) and showed an AUC = 0.73.
Receiver operating characteristic (ROC) analysis showing the accuracy (area under the curve, AUC) of baseline hypothalamus (HT) rsFC and covariates (solid line and circles) and covariates alone (dashed lines and open circles) in predicting overall changes (follow-up vs. baseline) in (A) N-back metrics (as revealed in latent class analysis) and (B) QoL score (total QoL score). Covariates (COV) included baseline age, education, and MoCA score.
For overall QoL, the logistic regression models with hypothalamus-ACC and cuneus rsFC predictiors along with covariates, as well as the model with covariates alone were not significant (p’s > 0.151), each with an AUC = 0.79 and 0.75, respectively (Fig. 4B).
Discussion
ADT is widely used for the treatment of prostate cancer. ADT offers survival benefits but the associated adverse effects, especially cognitive dysfunction, are not fully understood; and individuals demonstrated significant variation in ADT-elicited cognitive impairment6. Despite extensive research on the neural processes underlying the cognitive impairment, there has been no studies aiming specifically to identify neural predictors of individual variation in the impairment. In the present study, we showed that baseline hypothalamus rsFCs may predict individual variation in changes in working memory and QoL following six months of ADT.
ADT for six months did not appear to affect N-back performance and QoL differently from patients who did not receive ADT. ADT for a longer duration may cause greater cognitive changes1, although studies have reported no effects on working memory after 1261 and 3643 months of treatment. Similarly, a meta-analysis noted no adverse effects of ADT on working memory42. Thus, the present findings are consistent with this literature, including our earlier studies2,41. However, despite a lack of effects on behavioral measures, we showed earlier significant differences in brain activity and functional connectivity. Notably, our previous studies implicated middle frontal cortical activation2 and hypothalamus-middle frontal cortical functional connectivity41 in ADT N-back performance.
Despite the absence of an overall effect, individuals demonstrated variations in cognitive performance at follow-up vs. baseline. Of the 6 N-back scores, 17 among 28 ADT patients demonstrated impairment in ≥ 2 scores, while a smaller number (n = 6) performed better at follow-up vs. baseline in all 6 scores. Such inter-subject variation in cognitive performance has been noted in women with breast cancer on chemotherapy44,45. In a longitudinal assessment, a sub-population (~ 60%) of breast cancer patients experienced subtle chemotherapy-linked cognitive decline62 along with altered frontal-cerebellar rsFC in comparison to those who did not undergo chemotherapy or healthy controls47. Decrement in frontal cortical gray matter density mediated inter-subject variation in chemotherapy dosage-related impairment in verbal fluency46. Here, we demonstrated that hypothalamus connectivity may predict such variations in working memory and QoL in individuals undergoing ADT, as discussed in more detail below.
Studies have also documented the effects of ADT on patients’ quality of life63,64,65. We assessed different domains of QoL using FACT-P questionnaire and did not observe significant changes in QoL, except possibly PWB, which was marginally reduced (p = 0.078) during follow-up as compared to baseline in ADT. These findings appear to accord with an earlier study which noted worsening physical but comparable emotional and social well-being in prostate cancer patients with 24 weeks of ADT, relative to controls64.
Hypothalamus regulates the activity of the HPA axis66. Although best known for its role in supporting survival-related responses, the hypothalamus connects structurally with many brain regions, including the amygdala, cingulate and other frontal cortical regions67, suggesting that it may partake in cognitive and affective processes beyond those to meet immediate physiological needs. We assessed hypothalamus rsFCs in predicting inter-subject variations in N-back performance and QoL score/sub-scores post-ADT and observed both positive and negative associations. Higher baseline hypothalamus-frontal cortical rsFC predicted worse N-back score. Although we could not explain the precise mechanism of this association, hypothalamus-frontal cortical rsFC is implicated in cognitive and attention control both in health68 and illnesses9,31,69,70. In our earlier studies too, we noted significant ADT-associated alterations in frontal cortical activation and connectivity2,41. We also observed that lower baseline hypothalamus-SPL rsFC predicted longer reaction time and thus, worse attention, in the N-back task, consistent with SPL being part of dorsal attention network71. Hypothalamus-ACC and cuneus rsFC each predicted better and worse physical well-being. A hub of the salience network, the ACC connects with both limbic and prefrontal cortex72. With the hypothalamus, it regulates autonomic72 and visceromotor functions73, and hypothalamus-ACC connectivity has been implicated in reward74 and pain75 processing. The functional significance of hypothalamus-cuneus connectivity is not clear, but may involve visual processing of emotionally relevant stimuli76,77. Notably, the associations were not significant in CON, possibly indicating the specificity of hypothalamic rsFCs for ADT-associated changes.
Prediction analyses revealed reasonable accuracy of hypothalamus rsFCs in determining the N-back performance and QoL. The best model constituted hypothalamus-frontoparietal rsFC in predicting N-back performance and hypothalamus-cingulate/occipital rsFC in predicting QoL. Thus, prognostic functional connectivity markers may prove of clinical utility as the acquisition and analysis of functional brain imaging data is easy to standardize, less demanding to the patients, and less prone to subjective errors.
Limitations and conclusions
A number of limitations need to be considered. First, the study comprised a small sample and the results should be replicated in a larger population. On the other hand, we wish to emphasize that the imaging results on hypothalamic rsFCs were obtained at a corrected threshold and would likely be robust. Second, studies are also needed to employ a more comprehensive battery of neuropsychological tests to fully investigate potential cognitive dysfunction in prostate cancer patients receiving ADT. It is possible that patients may compensate functionally in certain but not in other cognitive domains. Thus, a detailed assessment would reveal the cognitive side effects of ADT. Third, although we targeted the hypothalamus on the basis of the biological effects of androgen deprivation, other brain regions, e.g., frontal cortex, should be thoroughly investigated in future studies. Fourth, as patients may undergo ADT for a longer duration, the current findings should be considered as specific to patients with only 6 months of androgen deprivation. Finally, as we did not observe significant changes in N-back/QoL scores at follow-up compared to baseline in ADT with respect to HC, we cannot entirely rule out the effect of time in driving the changes at follow-up. Nonetheless, in predicting the changes we showed the hypothalamus rsFC associations to be significant in ADT but not in CON, suggesting that these findings may be specific to androgen deprivation.
To conclude, androgen deprivation for 6 months did not lead to significant changes in working memory or quality of life. However, individuals varied in the changes in working memory and quality of life likely as a manifestation of the effects of ADT and indirect effects in functional compensation. Baseline hypothalamus-frontoparietal and occipital/limbic rsFCs predict inter-subject variations in working-memory and QoL in prostate cancer patients at six months after ADT. Studies with longer-term ADT and other cognitive/behavioral markers are needed to fully evaluate the effects of androgen deprivation on cognition.
Data availability
The datasets generated and/or analyzed in the paper are part of an on-going study and thus not publicly available. However, the data would be available from the corresponding author on reasonable request.
Abbreviations
- ADT:
-
Androgen deprivation therapy
- ACC:
-
Anterior cingulate cortex
- AUC:
-
Area under the curve
- CON:
-
Controls
- EWB:
-
Emotional well-being
- FACT-P:
-
Functional Assessment of Cancer Therapy-Prostate
- FWB:
-
Functional well-being
- HPG:
-
Hypothalamus–pituitary–gonadal
- LCA:
-
Latent class analysis
- MFG:
-
Middle frontal gyrus
- MoCA:
-
Montreal cognitive assessment
- MRI:
-
Magnetic resonance imaging
- PCS:
-
Prostate cancer subscale
- PCG:
-
Precentral gyrus
- PWB:
-
Physical well-being
- QoL:
-
Quality of life
- rsFC:
-
Resting-state functional connectivity
- SPL:
-
Superior parietal lobule
- SWB:
-
Social well-being
References
Nelson, C. J., Lee, J. S., Gamboa, M. C. & Roth, A. J. Cognitive effects of hormone therapy in men with prostate cancer: A review. Cancer 113, 1097–1106 (2008).
Chao, H. H. et al. Effects of androgen deprivation on brain function in prostate cancer patients—A prospective observational cohort analysis. BMC Cancer 12, 371 (2012).
Shim, M., Bang, W. J., Oh, C. Y., Lee, Y. S. & Cho, J. S. Androgen deprivation therapy and risk of cognitive dysfunction in men with prostate cancer: Is there a possible link?. Prostate Int. https://doi.org/10.1016/j.prnil.2021.02.002 (2021).
Jenkins, V. A., Bloomfield, D. J., Shilling, V. M. & Edginton, T. L. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 96, 48–53 (2005).
Kaufman, J.-M. & Lapauw, B. Role of testosterone in cognition and mobility of aging men. Andrology 8, 1567–1579 (2020).
Kluger, J., Roy, A. & Chao, H. H. Androgen deprivation therapy and cognitive function in prostate cancer. Curr. Oncol. Rep. 22, 24 (2020).
Hausman, H. K. et al. The role of resting-state network functional connectivity in cognitive aging. Front. Aging Neurosci. 12, 177 (2020).
Lin, Q. et al. Resting-state functional connectivity predicts cognitive impairment related to Alzheimer’s disease. Front. Aging Neurosci. 10, 94 (2018).
Hawellek, D. J., Hipp, J. F., Lewis, C. M., Corbetta, M. & Engel, A. K. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc. Natl. Acad. Sci. 108, 19066–19071 (2011).
Wang, S. et al. Using fractional amplitude of low-frequency fluctuations and functional connectivity in patients with post-stroke cognitive impairment for a simulated stimulation program. Front. Aging Neurosci. 13, 724267 (2021).
Peper, J. S., van den Heuvel, M. P., Mandl, R. C. W., Hulshoff Pol, H. E. & van Honk, J. Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrinology 36, 1101–1113 (2011).
Plata-Bello, J. et al. Changes in resting-state measures of prostate cancer patients exposed to androgen deprivation therapy. Sci. Rep. 11, 23350 (2021).
Gullett, J. M. et al. Baseline neuroimaging predicts decline to dementia from amnestic mild cognitive impairment. Front. Aging Neurosci. 13, 828 (2021).
Hojjati, S. H., Ebrahimzadeh, A., Khazaee, A. & Babajani-Feremi, A. Predicting conversion from MCI to AD using resting-state fMRI, graph theoretical approach and SVM. J. Neurosci. Methods 282, 69–80 (2017).
Sarpal, D. K. et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am. J. Psychiatry 173, 69–77 (2015).
Wu, G.-R., Wang, X. & Baeken, C. Baseline functional connectivity may predict placebo responses to accelerated rTMS treatment in major depression. Hum. Brain Mapp. 41, 632–639 (2020).
Wilcox, C. E. et al. Functional network connectivity predicts treatment outcome during treatment of nicotine use disorder. Psychiatry Res. Neuroimaging 265, 45–53 (2017).
Mehta, U. M. et al. Resting-state functional connectivity predictors of treatment response in schizophrenia—A systematic review and meta-analysis. Schizophr. Res. 237, 153–165 (2021).
Kluth, L. A. et al. The hypothalamic–pituitary–gonadal axis and prostate cancer: implications for androgen deprivation therapy. World J. Urol. 32, 669–676 (2014).
Ashikari, D. et al. Androgen induces G3BP2 and SUMO-mediated p53 nuclear export in prostate cancer. Oncogene 36, 6272–6281 (2017).
Chapin, R. E. Reproductive System, Male, 3rd ed (ed. Wexler, P. B. T.-E.) 82–92 (Academic Press, 2014). https://doi.org/10.1016/B978-0-12-386454-3.00059-2.
Chrisp, P. & Goa, K. L. A review of its pharmacodynamic and pharmacokinetic properties, and clinical use in sex hormone-related conditions. Drugs 41, 254–288 (1991).
Eckstein, N. & Haas, B. Clinical pharmacology and regulatory consequences of GnRH analogues in prostate cancer. Eur. J. Clin. Pharmacol. 70, 791–798 (2014).
Swayzer, D. V & Gerriets, V. Leuprolide. (2022).
Harris, W. P., Mostaghel, E. A., Nelson, P. S. & Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 6, 76–85 (2009).
Spratt, D. P. & Herbison, A. E. Regulation of preoptic area gonadotrophin-releasing hormone (GnRH) mRNA expression by gonadal steroids in the long-term gonadectomized male rat. Brain Res. Mol. Brain Res. 47, 125–133 (1997).
Lindzey, J. et al. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-α knockout mice. Endocrinology 139, 4092–4101 (1998).
Han, X. et al. Surgical castration but not immuncastration is associated with reduced hypothalamic GnIH and GHRH/GH/IGF-I axis function in male rats. Theriogenology 86, 657–665 (2016).
Kosse, C. & Burdakov, D. Natural hypothalamic circuit dynamics underlying object memorization. Nat. Commun. 10, 2505 (2019).
Sternson, S. M. Hypothalamic survival circuits: Blueprints for purposive behaviors. Neuron 77, 810–824 (2013).
Zhang, S., Wang, W., Zhornitsky, S. & Li, C.-S.R. Resting state functional connectivity of the lateral and medial hypothalamus in cocaine dependence: An exploratory study. Front. Psychiatry 9, 344 (2018).
Gardner, M. et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: Individual participant meta-analysis of five cohorts. Sci. Rep. 9, 4555 (2019).
Ferrer, A. et al. Hypothalamic–pituitary–adrenal axis-related genes and cognition in major mood disorders and schizophrenia: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 101, 109929 (2020).
Vercruysse, P., Vieau, D., Blum, D., Petersén, Å. & Dupuis, L. Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front. Mol. Neurosci. 11, 2 (2018).
Dovrolis, N., Nikou, M., Gkrouzoudi, A., Dimitriadis, N. & Maroulakou, I. Unlocking the memory component of Alzheimer’s Disease: Biological processes and pathways across brain regions. Biomolecules 12, 263 (2022).
Burdakov, D. & Peleg-Raibstein, D. The hypothalamus as a primary coordinator of memory updating. Physiol. Behav. 223, 112988 (2020).
Varas, M., Pérez, M., Ramírez, O. & de Barioglio, S. R. Melanin concentrating hormone increase hippocampal synaptic transmission in the rat. Peptides 23, 151–155 (2002).
Varas, M. M., Pérez, M. F., Ramírez, O. A. & de Barioglio, S. R. Increased susceptibility to LTP generation and changes in NMDA-NR1 and -NR2B subunits mRNA expression in rat hippocampus after MCH administration. Peptides 24, 1403–1411 (2003).
Lin, L., Wu, J., Yuan, Y., Sun, X. & Zhang, L. Working memory predicts hypothalamus–pituitary–adrenal axis response to psychosocial stress in males. Front. Psychiatry 11, 142 (2020).
Qi, J. et al. Altered hypothalamic functional connectivity following total sleep deprivation in young adult males. Front. Neurosci. 15, 688247 (2021).
Chaudhary, S. et al. The effects of androgen deprivation on working memory and quality of life in prostate cancer patients: The roles of hypothalamic connectivity. Cancer Med. https://doi.org/10.1002/cam4.4704 (2022).
McGinty, H. L. et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support. Care Cancer Off J. Multinatl. Assoc. Support. Care Cancer 22, 2271–2280 (2014).
Alibhai, S. M. H. et al. Effects of long-term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer 123, 237–244 (2017).
Ahles, T. A. et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 20, 485–493 (2002).
Ahles, T. A. et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 28, 4434–4440 (2010).
Li, X. et al. Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Sci. Rep. 8, 13801 (2018).
Park, H. Y. et al. Increased resting-state cerebellar-cortical connectivity in breast cancer survivors with cognitive complaints after chemotherapy. Sci. Rep. 11, 12105 (2021).
Chao, H. H. et al. Effects of androgen deprivation on cerebral morphometry in prostate cancer patients—an exploratory study. PLoS ONE 8, e72032 (2013).
Baddeley, A. Working memory. Science 255, 556–559 (1992).
Esper, P. et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 50, 920–928 (1997).
Esper, P., Hampton, J. N., Smith, D. C. & Pienta, K. J. Quality-of-life evaluation in patients receiving treatment for advanced prostate cancer. Oncol. Nurs. Forum 26, 107–112 (1999).
Le, T. M. et al. The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus. Int. J. Obes. 44, 1097–1107 (2020).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 102, 9673–9678 (2005).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Li, J. et al. Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage 196, 126–141 (2019).
Fair, D. A. et al. A method for using blocked and event-related fMRI data to study ‘resting state’ functional connectivity. Neuroimage 35, 396–405 (2007).
Cordes, D. et al. Frequencies contributing to functional connectivity in the cerebral cortex in ‘resting-state’ data. AJNR. Am. J. Neuroradiol. 22, 1326–1333 (2001).
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
Chen, Y.-N., Mitra, S. & Schlaghecken, F. Sub-processes of working memory in the N-back task: An investigation using ERPs. Clin. Neurophysiol. 119, 1546–1559 (2008).
Wolinsky, F. D. et al. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. J. Gerontol. Ser. A 61, 1324–1329 (2006).
Alibhai, S. M. H. et al. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J. Clin. Oncol. 28, 5030–5037 (2010).
Wefel, J. S., Lenzi, R., Theriault, R. L., Davis, R. N. & Meyers, C. A. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma. Cancer 100, 2292–2299 (2004).
Fowler, F. J. Jr., McNaughton Collins, M., Walker Corkery, E., Elliott, D. B. & Barry, M. J. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer 95, 287–295 (2002).
Gagliano-Jucá, T. et al. Effects of androgen deprivation therapy on pain perception, quality of life, and depression in men with prostate cancer. J. Pain Symptom Manag. 55, 307-317.e1 (2018).
Sharma, A. et al. A prospective longitudinal study to evaluate bone health, implication of FRAX tool and impact on quality of life (FACT-P) in advanced prostate cancer patients. Am. J. Clin. Exp. Urol. 9, 211–220 (2021).
Averbeck, B. B. & Murray, E. A. Hypothalamic interactions with large-scale neural circuits underlying reinforcement learning and motivated behavior. Trends Neurosci. 43, 681–694 (2020).
Risold, P. Y., Thompson, R. H. & Swanson, L. W. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res. Brain Res. Rev. 24, 197–254 (1997).
Kullmann, S. & Veit, R. Chapter 7—Resting-state functional connectivity of the human hypothalamus. In The Human Hypothalamus: Anterior Region (eds. Swaab, D. F. et al.) vol. 179 113–124 (Elsevier, 2021).
Kaiser, R. H. et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology 41, 1822–1830 (2016).
Boehm, I. et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front. Behav. Neurosci. 8, 346 (2014).
Petersen, S. E. & Posner, M. I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 35, 73–89 (2012).
Stevens, F. L. et al. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 23, 121–125 (2011).
Johansen-Berg, H. et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex 18, 1374–1383 (2008).
Frank, G. K. W., Shott, M. E., Riederer, J. & Pryor, T. L. Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Transl. Psychiatry 6, e932–e932 (2016).
Kong, J. et al. Altered functional connectivity between hypothalamus and limbic system in fibromyalgia. Mol. Brain 14, 17 (2021).
Roger, C. et al. The role of the human hypothalamus in food intake networks: An MRI perspective. Front. Nutr. 8, 1191 (2022).
Lukoshe, A. et al. Altered functional resting-state hypothalamic connectivity and abnormal pituitary morphology in children with Prader–Willi syndrome. J. Neurodev. Disord. 9, 12 (2017).
Acknowledgements
We are grateful for all patients’ participation in the study and for the help of the Urology clinic at the CT West Haven VA in assistance with the recruitment. We are indebted to our colleague and friend Dr. Ruth McCorkle for her assistance in study design in the early phase of this work.
Funding
The current study is supported by NIH grant CA218501 and VA Merit Award CX 001301. The NIH and VA otherwise not responsible for the design of the study or data analyses and interpretation or in the decision to publish the current results.
Author information
Authors and Affiliations
Contributions
S.C.: data curation, formal analysis, writing of the original draft, and editing. A.R.: project administration, data acquisition. C.S.: project administration, data acquisition. S.Z.: data acquisition, manuscript editing. T.A.: conceptualization, methodology, and manuscript editing. C.-S.R.L.: conceptualization, methodology and data analyses, manuscript editing, and supervision. H.H.C.: conceptualization, investigation, methodology, manuscript editing, and supervision. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaudhary, S., Roy, A., Summers, C. et al. Hypothalamic connectivities predict individual differences in ADT-elicited changes in working memory and quality of life in prostate cancer patients. Sci Rep 12, 9567 (2022). https://doi.org/10.1038/s41598-022-13361-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13361-4
- Springer Nature Limited