Abstract
Human milk oligosaccharides (HMOs) support the development of a healthy gut microbiome and the growth of infants. We aimed to determine the association of different HMOs with severe acute malnutrition (SAM) among Bangladeshi young infants. This study was nested within a single-blind, randomized, pilot clinical trial (NCT0366657). A total of 45 breastmilk samples from mothers of < 6 months old infants who had SAM (n = 26) or were non-malnourished (n = 19) and were analyzed for constituent HMOs. Of the infants with SAM, 14 (53.85%) had secretor mothers, and 11 (57.89%) of the non-malnourished infants had secretor mothers. A one-unit increase in the relative abundance of sialylated HMOs was associated with higher odds of SAM in age and sex adjusted model (aOR = 2.00, 90% CI 1.30, 3.06), in age, sex, and secretor status adjusted model (aOR = 1.96, 90% CI 1.29, 2.98), and also in age and sex adjusted model among non-secretor mothers (aOR = 2.86, 90% CI 1.07, 7.62). In adjusted models, there was no evidence of a statistically significant association between SAM and fucosylated or undecorated HMOs. Our study demonstrates that a higher relative abundance of sialylated HMOs in mothers’ breastmilk may have a negative impact on young infants’ nutritional status.
Similar content being viewed by others
Introduction
Globally 144 million children under the age of five years are stunted, 47 million are wasted and 38 million are overweight1. The risk of mortality associated with acute malnutrition is the highest among infants aged under six months2. Pre-term birth, sub-optimal breastfeeding, acute infection or congenital anomaly disrupting appetite, and being born small for gestational age or as a twin have been reported as risk factors for infants becoming malnourished3,4. The updated WHO guidelines focus on possible initiation and/or re-establishment of exclusive breastfeeding as a management strategy for infant malnutrition5. Consequently, an emerging body of evidence suggests that commensal microbial communities, residing primarily in the lower GI tract and collectively known as the “gut microbiome” play an important role in the growth during early life6. The GI tract of well-nourished breastfed infants is known to be colonized by lactic acid-producing bacteria (LAB), including Bifidobacterium sp. and Lactobacillus sp, which confer beneficial effects on the host physiology7. Insufficient breast milk intake has been reported among infants suffering from severe acute malnutrition (SAM)8,9. Concurrent studies conducted at icddr,b reported chronic depletion of the aforementioned beneficial members of the gut microbiota among children with SAM, which is not replenished upon nutritional rehabilitation following current treatment protocols including low-cost, culturally appropriate, diet for nutritional rehabilitation (halwa and khichuri) for SAM10.
Human milk contains a number of bioactive components, among which human milk oligosaccharides (HMOs) have been reported to exhibit pivotal roles in the overall health and nutritional status of infants11. Human milk contains human milk oligosaccharide (HMO) more than 20 g/L in colostrum and mature milk contains approximately 12–13 g/L12. Also the composition of HMOs vary with maternal nutritional status and dietary intake13. The building blocks of these milk oligosaccharides are the five monosaccharides, namely: D-glucose (Glc3), D-galactose (Gal), N-acetylglucosamine (GlcNAc), L-Fucose (Fuc), and sialic acid (Sia; N-acetyl neuraminic acid [Neu5Ac])11. Usually, HMOs range from 3 to 8 sugars in size, mostly containing fucose as a constituent although highly complex oligosaccharides containing up to 32 sugars have been reported14.
HMOs are not digestible by infants and arrive intact to the large intestine, where they exert prebiotic roles by supporting the development of selected beneficial groups of the gut microbiota, including Bifidobacterium sp and some Lactobacillus sp. by providing metabolic products for their existence, growth, and ultimate colonization in the gut of infant15,16. In addition to helping in healthy gut microbiome, HMOs deliver a number of assistances to infants, including brain development17, acting as decoys for harmful organism18, and averting disease and infection19. Certain HMOs have also been found to reduce frequencies of moderate to severe diarrhea among infants, in particular diarrhea caused by Campylobacter and calicivirus20.
Human milk oligosaccharides differ between secretor and non-secretor mothers21, whereby fucosylation in the human milk oligosaccharides are a result of gene products that regulate Lewis and secretor blood group types21. Concurrent shifts in gut microbiota depending on secretor status have been previously reported22,23,24. Subsequently, a Malawian study reported that mothers with undernourished infants had an overall decreased content of milk oligosaccharides25. It was also observed that particular human milk oligosaccharides support age-appropriate growth of infants26.
A recent study reported that milk oligosaccharides influences the nutritional status of the offspring27. However, there has been no report on the variation of HMO content and its effect on the nutritional outcome of young infants, particularly among those of under six months of age. In light of this knowledge gap, we conducted an observational study at the Dhaka Hospital of icddr,b, where we aimed to determine possible relationships between HMO content of breast milk with the nutritional status, anthropometric outcomes, and abundance of Bifidobacterium infantis in the gut of these young infants.
Materials and methods
Study site and participants
This study was conducted at the Dhaka Hospital of icddr,b. We recruited 49 mothers of infants who were already enrolled in a randomized controlled pilot trial (RCT) entitled “Pilot of a prebiotic and probiotic trial in young infants with severe acute malnutrition (NCT0366657)”. These infants were between 2 and 6 months of age, of either sex, admitted to the Dhaka Hospital of icddr,b due to gastrointestinal or respiratory infection and were categorized into two groups based on their nutritional status: 1) SAM: infants with weight for length Z score of less than − 3 (WLZ < − 3) or with the presence of bilateral pedal edema, irrespective of anthropometric measurements and non-malnourished: infants with weight for length Z score of greater than − 1 (WLZ > − 1). The final analysis included 45 infants; 4 samples (3 SAM and 1 non-malnourished infant) were excluded because the secretor status of the women could not be determined.
Collection of breast milk samples
Breast milk samples from mothers were collected under aseptic conditions. Mothers were asked to stop feeding the infant 30 min before sample collection. Iodine-soaked gauze was used to clean the breast. Samples were collected in a sterile bottle using a breast pump. The samples were aliquoted in pre-labeled cryovials and flash-frozen in liquid nitrogen immediately after collection. The samples were transferred to − 80 °C freezers within 24 h of collection and were stored until further analyses.
HMO extraction and quantitation
The technique of extraction of milk oligosaccharide and quantification has been described in detail in the cited literature28,29. In brief, 25 µL aliquots of each breastmilk sample were diluted and defatted via centrifugation. Proteins were precipitated with ethanol and the resulting glycans were reduced with 1M NaBH4 for 90 min in a dry incubator set at 65 °C and then purified on solid-phase extraction graphitized carbon cartridges (GCC). The resulting eluents were combined, followed by reconstitution by solvent evaporation and dilution for subsequent analyses. HPLC QqTOF Mass Spectrometry was used for quantitation of the individual HMO constituents, using previously optimized protocols29. The specific structures were identified and assigned by matching retention time and exact mass which was matched with the data available in previously developed HMO libraries30,31 and the glycans were categorized in the manner as followed in previous publications28,29,30,31,32.
Evaluation of secretor status from breast milk
Secretor status was determined by phenotyping method, as described elsewhere following earlier published methods33. Briefly, oligosaccharides with known α(1,2)-Fuc linkages were identified by matching exact mass and retention times with a previously developed in-house library30,31. The signal responses of the most abundant α(1,2)-Fuc containing structures, namely 2’-fucosyllactose (2’FL), lactodifucotetraose (LDFT), difucosyllacto-N-hexaose a(DFLNHa) and trifucosyllacto-N-hexaose (TFLNH), were summed and normalized to the total HMO abundance present in a given sample. Secretor status was assigned based on a previously established and validated threshold33. The sum of the relative abundances was obtained and when it exceeded this threshold the mother was deemed to be a secretor; conversely, if it fell below the threshold they were deemed to be non-secretors.
Identifying human milk oligosaccharides
Fucosylated and sialylated HMOs
To identify the HMOs, we followed reports from previously published literature34. The compositions of HMOs were given as hexose, N-acetylhexoseamine (HexNAc), fucose, N-acetylneuraminic acid (sialic acid). The compound name of HMOs was expressed with a four-digit number. For example, the compound identifier 4110 indicated that the compound has 4 hexoses, 1 HexNAc, 1 fucose, and 0 sialic acid molecule. Thus, compounds with a nonzero number in the last position are sialylated HMO; those with a nonzero number in the third position are fucosylated HMO.
Undecorated HMOs
Other than fucosylated or sialylated HMOs are called undecorated HMOs.
Evaluation of the B. infantis levels in the fecal samples of the infants at enrolment
Stool samples were collected at baseline, prior to the start of supplementation. Stool samples collected were aliquoted in pre-labeled cryovials and flash-frozen in liquid nitrogen within 20 min after defecation, as practiced in other studies35. Aliquots were preserved in − 80 °C freezers until analysis. The laboratory assays were carried out at the Laboratory of Evolve bioSystems, Inc California. The levels of B.infantis, the predominant member of the gut flora among young infants was measured from the collected stool samples by qPCR, using protocols described elsewhere36.
Statistical analysis
We presented the characteristics of mothers and infants using percentage and median (interquartile range (IQR)), as appropriate. To assess the association of the relative abundance of different HMOs with severe acute malnutrition, simple and multivariable binary logistic regression models were used separately for fucosylated, sialylated, and undecorated HMOs. As the sample size is relatively small, we used firth logistic regression instead of conventional logistic regression. Firth logistic regression provides more reliable estimates in instances of small sample size, complete separation, and rare events using penalized maximum likelihood estimation37. We expressed the strength of association as odds ratio (OR) and adjusted odds ratio (aOR) with a 90% confidence interval (90% CI) for each unit increase in the relative abundance of a certain HMO.
Since this is an exploratory study, to better understand the relationship between the relative abundance of the HMOs and SAM, we fitted infant age- and infant sex-adjusted models, and infant age-, infant sex-, and mother’s secretor status-adjusted models. We also fitted infant age- and infant sex-adjusted models separately for secretor and non-secretor mothers. To examine the discriminative performance of the logit models for the detection of SAM, the area under the receiver operating characteristic (AUROC) curves was estimated38.
All statistical tests were two-sided. Since the study is pilot in nature, the statistical significance was evaluated at p < 0.10, and 90% CIs was reported39. Data analysis was done in Stata v15.1 (Stata Corp, College Station, TX, USA). Z scores for nutritional status were calculated using WHO Anthro software.
Ethical approval
The randomized controlled trial was registered at ClinicalTrials.gov (NCT03666572) on 12/09/2018 (https://clinicaltrials.gov/ct2/show/NCT03666572) and the research protocol was approved by the Institutional Review Board (IRB) of International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) on 16 February, 2018 (approval number: PR- 17112). All clinical aspects of the study were supervised by the investigators and study physicians. All activities were conducted in accordance with the Helsinki Declaration of 1975, revised in 1983. Names of the participants were de-identified prior to analysis.
Patient consent for publication
Details that disclose the identity of the participants or their information were kept confidential. All procedures were approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was taken prior to sample collection and intervention.
Results
We analyzed a total of 45 breast milk samples, of which 26 (26/45, 57.7%) were from the mothers of SAM infants and 19 were from mothers of non-malnourished infants (19/45, 42.2%). Among the mothers of SAM infants, 14 were secretors (14/26, 53.85%) and 12 were non-secretors (12/26,46.15%). Among the mothers of non-malnourished infants, 11 were secretors (11/19, 57.89%) and 8 were non-secretors (8/19, 42.11%).
In comparison to the mothers of non-malnourished infants, the mothers of the SAM infants were younger in age (23.42 years vs. 25.89 years), had lower educational qualification (38.46% vs. 21.05%, education level less than 5 years), had babies delivered with lower birth weight (2.76 kg vs. 3.04 kg) and lower proportion of term infant (65.38% vs. 89.74%). Higher proportion of cesarean section (LUCS) was predominant among the SAM infants in comparison to the non-malnourished infants (50% vs. 21.05%). Moreover, SAM infants presented with a short duration of diarrheal illness, when compared to the non-malnourished infants (4.46 days vs. 7.26 days). Antibiotic use before hospitalization was significantly higher in non-malnourished infants (34.78% vs. 94.44%, p < 0.01). Fecal B. infantis levels baseline were lower in non-malnourished infants compared to the SAM infants (6.00 vs. 4.88 Log10 CFU/µg of DNA), but this difference was not statistically significant (Table 1).
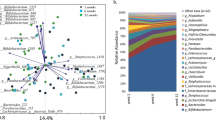
Figure 1 shows the distribution of individual oligosaccharides in breastmilk of mothers of SAM and non-malnourished infants. In the analysis, we identified 73 HMOs out of which 26 HMOs had a chemical name and others only had a compound identifier.
Distribution of different HMOs in mothers of different secretor status and having infants with different nutritional status. [6′SL, 6′-Sialyllactose; 3′SL, 3′-Sialyllactose; 3′FL, 3′-Fucosyllactose; 2′FL, 2′-Fucosyllactose; LDFT, lactodifucosyllactose; LNT&LnNT, lacto-N-tetraose& lacto-N-neotetraose; LNFP II, Lacto-N-fucosylpentose-II; LNFP III, Lacto-N-fucosylpentose-III; LNFP I, Lacto-N-fucosylpentose-I; LNDFH I,lacto-N-difucosylhexose-I; LNDFH II, lacto-N-difucosylhexose-II; LNH, lacto-N-hexaose; LNnH, lacto-N-neohexaose; MFLNH I, monofucosyllacto-N-hexaose I; MFLNH III, monofucosyllacto-N-hexaose III; IFLNH III, isomer 3 fucosyl-paralacto-Nhexaose; IFLNH I, isomer 1 fucosyl-paralacto-N-hexaose; P-LNH, para-lacto-N-hexaose; SLNH, Monosialyllacto-N-hexaose; a+S-LNnH II, No literature name + sialyllacto-N-neohexaose II; MFpLNH IV, Monofucosyl-paralacto-N-hexaose; DFLNHb, Difucosyllacto-Nhexaose b; DFLNHa, Difucosyllacto-Nhexaose a; DFS-LNH, Difucosylmonosialyllacto-N-neohexaose; DFS-LNHnH, Difucosylmonosialyllacto-N-neohexaose; TFLNH, Trifucosyllacto-N-hexaose.]
In Table 2 shows the association of malnutrition status of the young infants with different types of HMOs. Sialylated HMOs were associated with higher odds of severe acute malnutrition status in age and sex adjusted model (AOR = 2.00, 90% CI 1.30, 3.06), in age, sex, secretor status adjusted model (AOR = 1.96, 90% CI 1.29, 2.98) and also among non-secretor mothers when age and sex adjusted model was used (AOR = 2.86, 90% CI 1.07, 7.62). All these different statistical models show statistically significant association with sialylated HMO and severe acute malnutrition among the young infants. Fucosylated HMOs were less likely associated with severe acute malnutrition but there was no significant association between these. Undecorated HMOs showed association with higher odds severe acute malnutrition among the young infants in all the models but associations were not statistically significant. In Supplementary Table, we included the conventional logistic regression.
Different adjusted models showed modest to strong ability to distinguish association with different HMOS and severe acute malnutrition (AUROC range: 0.74–0.86) (Supplementary Figs. 1,2,3).
Discussion
The study was conducted among 45 infant-mother dyads. The data presented here were based on the HMO analysis of the breast milk of mothers who had either non-malnourished or SAM infants. In the study, we observed that the association of sialylated HMOs with severe acute malnutrition among young infants. Conversely, fucosylated HMOs and undecorated HMOs had no significant association with severely malnourished infants. To our knowledge, ours is the first study to assess the relation of different HMOs with malnutrition status among Bangladeshi infant-mother dyads without any supplementation of HMOs.
Breast milk contains complex oligosaccharides that escape digestion by the intestinal enzymes and transit through the gastrointestinal tract of the infant40. The variety of HMOs appears to be endless; to date over 200 different structures of HMOs have been identified in breast milk. HMO profile is affected by host secretor status, encoded by fucosyltransferase 2 (FUT2) gene, based on the secretor/Lewis blood group21,41. Secretor status phenotyping depends upon the expression of the histo blood group antigens (HBGAs): fucosyltransferase FUT2 (secretor gene), and FUT3 (Lewis gene) in secretions such as saliva and breast milk42. These antigens may act as innate host factors that differentially influence the susceptibility of individuals and their offspring43. The analytical procedure that was used in this study for the secretor status phenotyping of the mothers involves the measurement of a certain level of HBGAs in the breast milk samples, using a set cut-off value33. FUT2 and FUT3 genes that are rendered non-functional due to distinct polymorphisms are thus unable to express the corresponding HBGAs in the secretions44. Henceforth, the secretor status of such individuals with non-functional FUT2 and FUT3 genes cannot be determined by analytical methods, as used in our study. This is a probable explanation of their “Undetermined” secretor status.
The oligosaccharide abundance in breast milk is also affected by maternal genetics, particularly by the FUT2 gene, which encodes an enzyme accountable for the addition of a fucose residue at the α1-2 position on a backbone of abundant glycans containing galactose45. The absence or trace amount of α1-2 fucosylated oligosaccharides is responsible for the decreased amount of HMOs in non-secretor mothers’ breast milk33.
In our study, we have observed an association of higher odds of severe acute malnutrition among the young infants with sialylated HMOs and this association was statistically significant. On the contrary, fucosylated HMOs were less likely to be associated with severely malnourished infants. Recent studies demonstrated that human milk oligosaccharides (HMOs) are linked to growth in early infancy. In a study conducted among 37 mother-infant dyads, it was observed that an increase in lacto-N-fucopentaose (LNFP) I was linked with lower infant weight at 1 and at 6 months and with lower lean and fat mass at 6 months. Further, At 6 months, LNFP II and disialyl-lacto-N-tetraose (DSLNT) were related to higher fat mass46. The amount of fucosylation in breast milk changes over the course of lactation47, which may affect the protection deliberated to an infant. HMOs containing α1,2-fucosyl linkages have been revealed to help the growth of members of the Bifidobacterium genus due to prebiotic action48.
Different HMOs have been shown to exert an impact on the weight-for-age z score (WAZ) of infants, particularly 3’-SL, as observed in the Gambian study49. In this study, the relative sialylation of HMOs was not a predictor for WAZ of the infants49. However, in our study, we observed that severely malnourished infants consumed breast milk which had significantly higher sialylated HMOs than non-malnourished infants. Sialylated HMOs was also found to have impact on linear growth in animal model study50. The report from a Malawian cohort showed that among only the secretor mothers, the total abundance of HMOs was positively associated with increments in length-for-age z-score (LAZ) from 6 to 12 months of age51. Analysis of HMOs of two Malawian birth cohort also reported that severely stunted infants had significantly low sialylated HMO25. Observation of this study was also used in animal model study which indicated relationship between sialylated HMO and growth promotion25. On enrolment our study infants were with severe acute malnutrition, so we could not follow them to get association of linear growth with sialylated HMOS. In our model, there was significant association with sialylated HMOs with severe acute malnutrition both in age, sex-adjusted model and age, sex, secretor status adjusted model. Comparatively lower sample size in this study may have concealed the significant effect of fucosylated HMOs on the nutritional status of the infants enrolled in our study. Recent review article reported that the results of different studies on HMO were also contradictory due to mixed interpretations52. With our exploratory study we have found association of sialylated HMOs with severe acute malnutrition but we could not find causal association from this cross-sectional design.
The infant's intestinal microbiome predominantly consists of two subspecies of Bifidobacterium longum: subsp. infantis (B. infantis) and subsp. longum (B. longum). Competitive growth of B. infantis in the neonatal intestine has been connected to the use of human milk oligosaccharides (HMO)53. Previous studies have shown that different fucosylated HMOs have an impact on the incidence of diarrhea49. Our study infants exhibited diarrhea on admission, so we were unable to establish a specific role of any of the constituent HMOs on diarrheal incidences among these young infants with different nutritional statuses. We have observed that malnourished infants presented with shorter duration of diarrhea compared to non-malnourished infants. Malnourished infants present with increased disease severity due to their impaired immune system which could have an impact on early presentation of such infants54.
Fucosylated HMOs and N-glycans on milk proteins are beneficial for the growth of healthy gut microbiota, acting as prebiotics25,48. Changes in gut microbiota depending on secretor status have been reported earlier22,23,24. The impact of maternal secretor status in microbiota is well documented and affects different bacterial groups during lactation55. Different studies reported that HMOs in breast milk are key factors which promote the development of members of the Bifidobacterium genus in the gut56,57. Bifidobacterium longum subsp. infantis (B. infantis) is unique among other gut microbes as this members of this particular subspecies of B.longum areequipped with the appropriate machinery to consume the full range of human milk oligosaccharides (HMOs)58. A large proportion of breast milk comprises fucosylated HMOs which can also be utilized by select B. breve strains and B. bifidum through extracellular fucosidase activity59,60. The gut microbiota is often exposed to a variety of antimicrobial agents, like antibiotics, which are used to combat bacterial infection in humans61. This antibiotic therapy may cause depletion of the beneficial members of the gut microbiota, a phenomenon known as “dysbiosis’62. Results from our study suggest that different types of HMOs consumed by infants have an impact on the nutritional status of the infants. However, the use of antibiotic may have masked the effect of HMOs on gut microbiota especially of the Bifidobacterium species which showed that even with exclusive breastfeeding, the levels of Bifidobacterium infantis was comparatively lower in non-malnourished infants. This association explains context-specific correlation, which also has causational support. Different studies also reported that infants without exposure to antibiotics presented with a higher percentage of Bifidobacteriaceae63,64. A number of metagenomics studies have illustrated associations between the altered gut microbiota and child malnutrition. Early depletion of Bifidobacterium longum appears to be the first step in gut microbiota alteration in severe acute malnutrition65,66 since any change in the relative abundance of Bifidobacterium in early infancy marks a change in the infant gut microbiome, potentially indicating dysbiosis.
The smaller sample size of this study makes it underpowered for certain study findings. Nevertheless, differences were detected in the relative abundance of particular HMO constituents in the breast milk of mothers of infants of different nutritional statuses, and as a result, the concentration of each constituent HMO was not measured. Moreover, the SAM infants were only partially breastfed and this variable might have had influenced the overall study findings and we could not assess the total amount of HMOs. A prospective large data set is required to have clear understanding on relationship of HMOs with ponderal as well as linear growth of infants.
This study provides primary observational data that the nutritional status of infants is affected by breast milk HMO composition of mothers and that additional supplementation of certain deficient HMO along with B. longum subsp infantis could be an intervention approach for the management of malnutrition in early infancy.
Data availability
This data set contains some personal information of the study patients (such as name, admission date, month, area of residence). Our IRB has required that the personal information of the participants is not disclosed. Thus, the policy of our centre (icddr,b) is that we should not make the availability of whole data set in the manuscript, the supplemental files, or a public repository. However, data related to this manuscript are available upon request and for researchers who meet the criteria for access to confidential data may contact with Armana Ahmed (armana@icddrb.org) to the Research Administration of icddr,b (http://www.icddrb.org/).
References
Organization, W. H. UNICEF/WHO/The World Bank Group joint child malnutrition estimates: Levels and trends in child malnutrition: Key findings of the 2020 edition. (2020).
Grijalva-Eternod, C. S. et al. Admission profile and discharge outcomes for infants aged less than 6 months admitted to inpatient therapeutic care in 10 countries. A secondary data analysis. Matern. Child Nutr. 13, e12345 (2017).
Bhutta, Z. A. et al. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost?. Lancet 382, 452–477 (2013).
Thurstan, R. et al. Filling historical data gaps to foster solutions in marine conservation. Ocean Coast. Manag. 115, 31–40 (2015).
Prudhon, C., Prinzo, Z. W., Briend, A., Daelmans, B. M. & Mason, J. B. Proceedings of the WHO, UNICEF, and SCN informal consultation on community-based management of severe malnutrition in children. Food Nutr. Bull. 27, S99–S104 (2006).
Moore, R. E. & Townsend, S. D. Temporal development of the infant gut microbiome. Open Biol. 9, 190128 (2019).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. 108, 4578–4585 (2011).
Mishra, K., Kumar, P., Basu, S., Rai, K. & Aneja, S. Risk factors for severe acute malnutrition in children below 5 y of age in India: a case-control study. Indian J. Pediatrics 81, 762–765 (2014).
Organization, W. H. Guideline: Updates on the management of severe acute malnutrition in infants and children. (World Health Organization, 2013).
Subramanian, S. et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014).
Bode, L. Human milk oligosaccharides: Prebiotics and beyond. Nutr. Rev. 67, S183–S191 (2009).
Urashima, T. et al. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv. Nutr. 3, 473S-482S (2012).
Biddulph, C. et al. Human milk oligosaccharide profiles and associations with maternal nutritional factors: A scoping review. Nutrients 13, 965 (2021).
Stahl, B. et al. Oligosaccharides from human milk as revealed by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Biochem. 223, 218–226 (1994).
Coppa, G. V. et al. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum. J. Pediatr. Gastroenterol. Nutr. 53, 80–87 (2011).
Thongaram, T., Hoeflinger, J. L., Chow, J. & Miller, M. J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 100, 7825–7833 (2017).
Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 29, 177–222 (2009).
Newburg, D. S., Ruiz-Palacios, G. M. & Morrow, A. L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 25, 37–58 (2005).
Chaturvedi, P. et al. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 11, 365–372 (2001).
Morrow, A. L. et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 145, 297–303 (2004).
Thurl, S., Henker, J., Siegel, M., Tovar, K. & Sawatzki, G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 14, 795–799 (1997).
Wacklin, P. et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS ONE 6, e20113 (2011).
Wacklin, P. et al. Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS ONE 9, e94863 (2014).
Kumar, H. et al. Secretor status is strongly associated with microbial alterations observed during pregnancy. PLoS ONE 10, e0134623 (2015).
Charbonneau, M. R. et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164, 859–871 (2016).
Puccio, G. et al. Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J. Pediatr. Gastroenterol. Nutr. 64, 624 (2017).
Lagström, H. et al. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am. J. Clin. Nutr. 111, 769–778 (2020).
Totten, S. M. et al. Rapid-throughput glycomics applied to human milk oligosaccharide profiling for large human studies. Anal. Bioanal. Chem. 406, 7925–7935 (2014).
Davis, J. C. et al. Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 7, 40466 (2017).
Wu, S., Tao, N., German, J. B., Grimm, R. & Lebrilla, C. B. Development of an annotated library of neutral human milk oligosaccharides. J. Proteome Res. 9, 4138–4151 (2010).
Wu, S., Grimm, R., German, J. B. & Lebrilla, C. B. Annotation and structural analysis of sialylated human milk oligosaccharides. J. Proteome Res. 10, 856–868 (2011).
Porfirio, S. et al. New strategies for profiling and characterization of human milk oligosaccharides. Glycobiology 30, 774 (2020).
Totten, S. M. et al. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J. Proteome Res. 11, 6124–6133 (2012).
Totten, S. M. et al. Rapid-throughput glycomics applied to human milk oligosaccharide profiling for large human studies. 406, 7925-7935 (2014).
Mahfuz, M. et al. Bangladesh Environmental Enteric Dysfunction (BEED) study: Protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. 7, e017768 (2017).
Frese, S. A. et al. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. MSphere 2 (2017).
Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38 (1993).
Hasan, S., Khan, M. A. & Ahmed, T. Institute of medicine recommendations on the rate of gestational weight gain and perinatal outcomes in rural Bangladesh. Int. J. Environ. Res. Public Health 18, 6519 (2021).
Huebner, M., Zhang, Z., Therneau, T., McGrath, P. & Pianosi, P. Modeling trajectories of perceived leg exertion during maximal cycle ergometer exercise in children and adolescents. BMC Med. Res. Methodol. 14, 1–9 (2014).
De Leoz, M. L. A. et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J. Proteome Res. 11, 4662–4672 (2012).
Kunz, C., Rudloff, S., Baier, W., Klein, N. & Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20, 699–722 (2000).
Nordgren, J. et al. Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin. Infect. Dis. 59, 1567–1573 (2014).
Ramani, S. & Giri, S. Influence of histo blood group antigen expression on susceptibility to enteric viruses and vaccines. Curr. Opin. Infect. Dis. 32, 445–452 (2019).
Koda, Y., Soejima, M. & Kimura, H. The polymorphisms of fucosyltransferases. Leg. Med. 3, 2–14 (2001).
Kunz, C. et al. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. J. Pediatr. Gastroenterol. Nutr. 64, 789–798 (2017).
Alderete, T. L. et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 102, 1381–1388 (2015).
Davidson, B., Meinzen-Derr, J. K., Wagner, C. L., Newburg, D. S. & Morrow, A. L. in Protecting Infants through Human Milk 427–430 (Springer, 2004).
Yu, Z.-T. et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 23, 169–177 (2013).
Davis, J. C. et al. Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. 7, 1–16 (2017).
Cowardin, C. A. et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc. Natl. Acad. Sci. 116, 11988–11996 (2019).
Jorgensen, J. M. et al. Associations of human milk oligosaccharides and bioactive proteins with infant morbidity and inflammation among Malawian mother–infant dyads. Current Developments in Nutrition (2021).
Sudarma, V., Hegar, B., Hidayat, A. & Agustina, R. Human milk oligosaccharides as a missing piece in combating nutritional issues during exclusive breastfeeding. Pediatric Gastroenterol. Hepatol. Nutr. 24, 501 (2021).
Garrido, D. et al. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci. Rep. 6, 35045 (2016).
Tickell, K. D. et al. Impact of childhood nutritional status on pathogen prevalence and severity of acute diarrhea. Am. J. Trop. Med. Hyg. 97, 1337 (2017).
Cabrera-Rubio, R. et al. Association of maternal secretor status and human milk oligosaccharides with milk microbiota: An observational pilot study. J. Pediatr. Gastroenterol. Nutr. 68, 256–263 (2019).
Asakuma, S. et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 286, 34583–34592 (2011).
Ashida, H. et al. Two distinct α-L-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 19, 1010–1017 (2009).
Underwood, M. A., German, J. B., Lebrilla, C. B. & Mills, D. A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 77, 229–235 (2015).
Ruiz-Moyano, S. et al. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 79, 6040–6049 (2013).
Sakanaka, M. et al. Varied pathways of infant gut-associated Bifidobacterium to assimilate human milk oligosaccharides: Prevalence of the gene set and its correlation with bifidobacteria-rich microbiota formation. Nutrients 12, 71 (2020).
Marshall, B. M. & Levy, S. B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24, 718 (2011).
Francino, M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front. Microbiol. 6, 1543 (2016).
Arboleya, S. et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 166, 538–544 (2015).
Fouhy, F. et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 56, 5811 (2012).
Kumar, M. et al. Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: lessons from genome-scale metabolic modeling. Metab. Eng. 49, 128–142 (2018).
Million, M., Diallo, A. & Raoult, D. Gut microbiota and malnutrition. Microb. Pathogenesis 106, 127–138 (2017).
Acknowledgements
The authors sincerely thank the study participants and their families for adherence to the rigorous study guidelines. This research protocol/activity/study was funded by Bill and Melinda Gates Foundation, grant number OPP1179599. icddr,b acknowledges with gratitude the commitment of BMGF to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support.
Funding
This research study was funded by Bill and Melinda Gates Foundation, USA (Grant no: OPP1179599). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The funders had no role in the study design, implementation, data collection, data analysis and manuscript drafting.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.N., P.P., M.M., S.M.T.H., M.R.I., T.A.; Methodology, S.N., P.P., M.M., S.M.T.H., M.R.I., M.M.I., S.A.S., D.J.K., R.L.F., A.V., C.B.L., T.A.; Software, S.N., P.P.; Validation, S.N., P.P., S.M.T.H., M.M., T.A.; Formal Analysis, S.N., S.M.T.H., P.P.; Investigation, S.N., P.P., M.R.I., A.V., C.B.L., D.J.K., R.L.F.; Data Curation, S.N., P.P.; Writing—Original Draft Preparation, S.N., P.P., S.M.T.H., M.M., M.R.I., M.M.I., S.A.S., D.J.K., R.L.F., A.V., C.B.L., T.A.; Writing—Review & Editing,. M.M., M.R.I., S.M.T.H., M.M.I., S.A.S., D.J.K., R.L.F., A.V., C.B.L., T.A.; Visualization, S.A.S., D.J.K., C.B.L., T.A.; Supervision, S.A.S., T.A.; Project Administration. S.N., P.P., M.M., M.R.I., M.M.I., R.L.F., A.V.; Funding Acquisition, T.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nuzhat, S., Palit, P., Mahfuz, M. et al. Association of human milk oligosaccharides and nutritional status of young infants among Bangladeshi mother–infant dyads. Sci Rep 12, 9456 (2022). https://doi.org/10.1038/s41598-022-13296-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13296-w
- Springer Nature Limited
This article is cited by
-
The intersection of undernutrition, microbiome, and child development in the first years of life
Nature Communications (2023)
-
Associations between human milk oligosaccharides and infant growth in a Bangladeshi mother–infant cohort
Pediatric Research (2023)