Abstract
Many insects show plasticity in the area of the brain called the mushroom bodies (MB) with foraging and social experience. MBs are paired neuropils associated with learning and memory. MB volume is typically greater in mature foragers relative to young and/or inexperienced individuals. Long-term studies show that extended experience may further increase MB volume, but long-term studies have only been performed on non-reproductive social insect workers. Here we use the subsocial bee Ceratina calcarata to test the effect of extended foraging experience on MB volume among reproductive females. Ceratina calcarata females forage to provision their immature offspring in the spring, and then again to provision their adult daughters in the late summer. We measured the volume of the MB calyces and peduncle, antennal lobes (AL), optic lobes (OL), central complex (CX), and whole brains of three groups of bees: newly emerged females, reproductive females in spring (foundresses), and post-reproductive mothers feeding their adult daughters in late summer. Post-reproductive late summer mothers had smaller MB calyces and ALs than foundresses. Moreover, among late mothers (but not other bees), wing wear, which is a measure of foraging experience, negatively correlated with both MB and OL volume. This is contrary to previously studied non-reproductive social insect workers in which foraging experience correlates postiviely with MB volume, and suggests that post-reproductive bees may reduce neural investment near the end of their lives.
Similar content being viewed by others
Introduction
Adult brain plasticity in response to accumulated experience or environmental change is widespread among animals. In insects, plasticity in response to adult experience is especially prominent in the mushroom body (MB)1,2. The mushroom bodies are paired neuropils that support higher-order cognitive processes such as sensory integration, learning and memory2,3. Previous studies have shown expansion in the MBs of adult insects resulting from foraging experience, social interactions, and age, termed ‘experience dependant plasticity’1.
MBs may increase in volume in response to foraging experience, especially central-place foraging, which requires spatial memory and navigation4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20. Typically, this increase in MB volume is demonstrated by comparing young or inexperienced individuals with experienced foragers7,15 or by comparing an experimentally restricted group with one allowed to forage11,19,20.
Another type of adult experience that can lead to increased MB volume is social experience. Drosophila housed in groups had larger MBs than those reared alone21. In the ant Camponotus floridanus, socially isolated individuals had smaller MB calyces than ants in a social nest22. The solitary bee Nomia melanderi showed increased growth in the lip region of the MB calyx when experimentally paired with another bee23. In some species of bees and paper wasps social dominance of reproductive queens over subordinate workers, rather than social interactions themselves, result in increased MB calyx volume of social dominants relative to subordinates12,15,24,25,26. However, this may be a result of reduced MB calyx volume in workers, rather than, or in addition to, increased volume in queens15,27, or a result of different developmental pathways rather than adult plasticity28. Rehan et al.15 used the facultatively social and solitary bee Ceratina australensis to show that foraging experience (in both social and solitary reproductives) and social dominance (in the social ones) each contributed to increased MB calyx volume.
Lastly, MB volume may increase with age in early adulthood before the onset of foraging. Workers of honeybees increase MB neuropil volume immediately following adult emergence, but before foraging (this is termed ‘experience-expectant’ plasticity)5,18,20,29. Other species with similar ‘experience-expectant’ increases in MB volume in early adulthood include bumblebees30, paper wasps25, and some ants7,16,22. These are all species that live in social groups where young workers do not need to begin foraging immediately. Data are mixed on whether experience-expectant plasticity occurs in the facultatively social and solitary sweat bee Megalopta genalis27,31, but experience-expectant plasticity was not found in the only two species of solitary bees in which it was investigated19,23.
Studies that follow insects into 'old age' (well beyond the onset of foraging) have been conducted on honeybee and ant workers, where individuals accumulate experience, but do not reproduce. These studies show incremental increases in MB volume in ants7,10,17,32, and no effect of extended foraging experience on MB volume in honeybees11,33. No study has examined the effect of extended foraging through and beyond the reproductive period in a solitary or small-colony insect.
Here we use the small carpenter bee Ceratina calcarata to test for an effect of extended age, experience, and social interactions on brain development. Ceratina calcarata mothers engage in two distinct rounds of foraging. First, in spring they initiate a nest and provision their offspring with pollen and nectar, similarly to most other bees (this is the reproductive foundress stage). When their daughters emerge in late summer, the mothers undertake a second round of foraging to further provision their now adult daughters; this is atypical among bees (this is the late mother stage)34,35. The adult daughters will enter diapause during the fall and winter, and emerge the following spring to initiate their own nests. Mothers forage at the same rate as their daughters, and the second bout of foraging from the late mothers increases overwintering success of the daughters. The late mothers die soon after they cease foraging35.
The life history of C. calcarata results in three groups of bees of increasing age and foraging experience: newly emerged daughters, reproductive foundresses, and late mothers. Based on previous studies of adult brain plasticity, reproductive foundresses should have greater MB calyx volume than newly emerged daughters because they have foraging experience and are older. Likewise, late mothers should have greater MB calyx volume than reproductive foundresses because they have even more foraging experience, are older still, and have social interactions with their adult daughters. The maternal care of late mothers is different than the social dominance of C. australensis or other social bees and wasps cited above because the mother is supporting her daughter, who will be a reproductive female the following spring, rather than supressing reproduction or establishing dominance15.
In order to examine brain development with increasing age and experience in C. calcarata, we measured the MB calyces and MB peduncle, as well as the optic lobes (OL), antennal lobes (AL), and central complex (CX) of the brain. We measured the MB calyx because this was the area of the brain affected by foraging and social dominance in another species of Ceratina bee, C. australensis15. We measured the OL and AL because these areas may respond to increased visual and chemical stimulation, respectively, associated with foraging and social experience (e.g.36,37,38). And we measured the CX because it is also involved in learning and sensory integration39. In addition to the brain areas, we measured ovary size to investigate the effect of reproductive status on brain area volume, and also wing wear, which correlates with flight activity, as another measure of foraging experience15. We predicted that the MB calyx, OL, and AL would all increase with age and experience. An alternative prediction is that if females reduce the expense of maintaining neural tissue after reproduction, late mothers would have reduced MB calyx, OL, and AL volume relative to reproductive foundresses.
Methods
Collections
Females were collected from nests of staghorn sumac (Rhus typhina) in and around Durham, NH in May–August 2018. Nest entrances were covered with masking tape and the base of the stem cut with pruning shears to remove dead stems from the trees. Nests were stored at 4 °C until dissection the same day in the lab. Adult females were placed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at collection and stored them at 4 °C until dissection. Females establish nests in late spring (May) and forage to provision nests (June) followed by guarding developing brood (July) and interacting with adult offspring (August)34. Foundresses were collected in May of 2018. Late summer mothers and recently emerged late summer daughters were collected in August of 2018.
Brain volume measurements
We dissected head capsules in PBS from seven females in each group (21 total) to remove the brain which was immediately placed in glutaraldehyde (2%) for 48 h, bleached in a formamide solution, and dehydrated in a series of ethanol washes of increasing concentration following40. Prior to imaging, brains were mounted in methyl salicylate. Brains were imaged using an Olympus Fluoview FV1000 confocal microscope using autofluorescence at 10X magnification and a step size of 10 μm (Fig. 1a). We calculated volumes of the whole brain (excluding the subesophegeal ganglion) and different neuropils through tracing and serial reconstruction using the software program Reconstruct41 (Fig. 1b). We measured the MB calyces, MB peduncle, AL, and OL (medulla + lobula) of one side of the brain and multiplied the resulting volumes by two to calculate total volume. Likewise, we measured the right hemisphere and multiplied by two to calculate whole brain volume. We measured the right side of the brain (viewed frontally) unless that side was damaged. We measured the CX, including the ellipsoid body, the superior arch, and the fan shaped body, but not the paired noduli or protocerebral bridge. Brain and neuropil volumes were standardized to average body size (head width was our measure of body size) by calculating a correction factor that was applied to each bee: mean body size of all bees in the study divided by the individual’s body size. This correction factor was then multiplied to brain and neuropil volume for each bee.
(a) Confocal micrograph showing mushroom body calyces (MB) and peduncle (ped), the central complex (CX), antennal lobes (AL), and optic lobes; scale bar = 500 μm. (b) Reconstructed volume of a representative brain, with AL in green, CX in royal blue, MB peduncle in red, MB calyces in light blue, and OL in pink.
Physical measurements
We used head width as a measure of body size. Wing wear was scored on a scale of 0–5 following15,42 in which zero represents bees with unworn forewing margins, and 5 represents bees with none of the origninal forewing margin remaining and wear extending to the wing veins. Wings were examined under a dissecting microscope at 10 × magnification for scoring. Ovary development was scored on a scale of 1–5 following43 in which one represents ovaries with no visible developing oocytes and five represents ovaries with nearly fully developed eggs. Ovaries were exposed by removing the abdominal tergites and gut at 10 × magnification under a dissecting light microscope. Scores for left and right forewings and left and right ovaries, respectively, were averaged for each individual bee.
Stastical analyses
Wing wear scores and ovary ratings were analyzed with non-parametric statistics (Kruskal–Wallis and Spearman’s rank correlations) because they are ordinal variables. Head width and volumes of brain areas were compared between groups using ANOVA followed by Tukey’s post-hoc comparisons. Antennal lobe volume and central complex volume were both log-transformed to fit the assumption of normality.
Results
Physical measures
There were no differences in body size, measured as head width, between groups (F2,18 = 1.929, p = 0.174; Fig. 2a). Late mothers had more worn wings than reproductive foundresses and newly emerged daughters; newly emerged daughters showed no wing wear at all (Kruskal–Wallis test = 16.633, df = 2, p < 0.001; Bonferroni corrected post-hoc p values = 0.020 and p < 0.001, respectively, Fig. 2b). Foundresses and newly emerged daughters did not differ in degree of wing wear (p = 0.604; Fig. 2b).
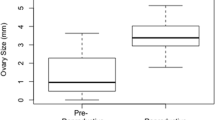
Boxplots showing head width, a measure of body size (a), wing wear (b) and ovary development (c) among newly emerged daughters, foundresses, and late mothers. N = 7 for each group. Note that no newly emerged daugthers exhibited wing wear, and only one exhibited ovarian development. Groups that do not share a letter are significantly different from each other based on ANOVA (a) or Kruskil-Wallis test followed by Bonferroni-corrected pairwise comparisons (b,c).
Ovary size increased from the newly emerged daughter to reproductive foundress stage (Kruskal–Wallis test = 6.629, df = 2, p = 0.036; Fig. 2c). Foundresses had significantly larger ovaries than newly emerged daughters, but not late mothers (Bonferroni corrected post-hoc p values = 0.037 and p = 1.00, respectively). Newly emerged daughters and late mothers did not differ (p = 1.00; Fig. 2c). Only one of the newly emerged daugthers showed any ovary development.
Neurobiological measures
Our predictions for enlargement of the MB calyx, OL, and AL were not met. Late mothers had smaller MB calyces than foundresses (F2,18 = 3.691, p = 0.045; Tukey’s post-hoc p = 0.041; Fig. 3a). There were no other significant pairwise differences in MB calyx volume (Fig. 3a). Both late mothers and newly emerged daughters had smaller antennal lobes than foundresses (F2,18 = 5.543, p = 0.013; Fig. 3b; Tukey’s post-hoc p = 0.040 and 0.018, respectively). There were no significant differences in optic lobe volume among groups (F2,18 = 0.994, p = 0.389; Fig. 3c). There were no significant differences in whole brain size (F2,18 = 2.296, p = 0.124), nor in the size of the central complex (F2,18 = 1.596, p = 0.230; Fig. 3d) or the MB peduncle (F2,18 = 2.313, p = 0.128; Fig. 3e) among groups.
Size-corrected volumes of the mushroom body calyces (a), Antennal lobes (b), optic lobes (c), central complex (d), and MB peduncle (f) for each group (N = 7 for each group). Groups that do not share a letter are significantly different from each other based on an ANOVA followed by Tukey’s HSD pairwise comparisons.
Interactions between physical and neurobiological measures
Wing wear, a correlate of foraging experience, negatively correlated with MB calyx volume (rho = − 0.43, p = 0.049; Fig. 4a). This relationship is driven entirely by the late mothers group (rho = − 0.83, N = 7, p = 0.021). There is no correlation between wing wear and MB calyx volume among the foundresses (rho = 0.23, N = 7, p = 0.625); newly emerged daughters showed no wing wear so were not analyzed separately. Antennal lobe volume did not correlate with wing wear, whether all bees were analyzed together (rho = − 0.06, p = 0.801), or separately by group (late mothers rho = − 0.23, N = 7, p = 0.613; foundress rho = 0.61, N = 7, p = 0.150; Fig. 4b). Optic lobe volume did not correlate with wing wear when all bees were analyzed together (rho = − 0.41, p = 0.069). However, wing wear strongly and negatively correlated with OL volume among the late mothers (rho =− 0.92, N = 7, p = 0.003), but not the foundresses (rho = 0.44, N = 7, p = 0.330; Fig. 4c). Whole brain volume did not significantly correlate with wing wear for all bees (rho =− 0.14, p = 0.552) or the foundress and late mothers analyzed separately (foundresses: rho = 0.23, N = 7, p = 0.625; late mothers: rho =− 0.60, N = 7, p = 0.159). There was no correlation between CX and wing wear (rho = 0.08, p = 0.744), nor MB peduncle and wing wear (rho = − 0.17, p = 0.475).
Degree of wing wear plotted against the volume of the MB calyx (a), AL (b), and OL (c). In all panels, late mothers are represented by green filled triangles, foundresses by blue filled squares, and newly emerged daughters by open red circles. Regression lines are shown for statistically significant within-group relationships (Spearman’s rank correlation) only, and represent linear regressions (N = 7 for each group).
Ovary development did not correlate with MB calyx volume for all bees in the study analyzed together (rho = 0.17, p = 0.173), or separately by group (late mothers rho = 0.17, N = 7, p = 0.168; foundress rho = 0.26, N = 7, p = 0.582; only one newly emerged daughter showed any ovarian development, so we did not calculate correlations for this group, Fig. 5a). Likewise, AL volume did not correlate with ovary development for all bees in the study (rho = 0.29, p = 0.202) or individual groups (late mothers rho = 0.43, N = 7, p = 0.335; foundress rho = − 0.53, N = 7, p = 0.224; Fig. 5b). OL volume did not correlate with ovary development across all bees (rho = − 0.08, p = 0.740), or late mothers analyzed alone (rho = 0.58, N = 7, p = 0.172) but there was a strong correlation within the foundress group (rho = 0.82, N = 7, p = 0.024; Fig. 5c). There was no correlation between CX and ovary size (rho = 0.28, p = 0.225) nor MB peduncle and ovary size (rho = − 0.07, p = 0.763). Ovary development did not correlate with wing wear (rho = 0.24, p = 0.304), although a non-significant negative trend was seen in late mothers (rho = − 0.67, N = 7, p = 0.099; Fig. 6).
Ovary development plotted against the volume of the MB calyx (a), AL (b), and OL (c). In all panels, late mothers are represented by green filled triangles, foundresses by blue filled squares, and newly emerged daughters by open red circles. Regression lines are shown for statistically significant within-group relationships (Spearman’s rank correlation) only, and represent linear regressions (N = 7 for each group).
Discussion
Here we show that neural investment in the MB calyces and AL, but not the OL, declines in advanced age in the subsocial bee Ceratina calcarata. This is contrary to what we predicted based on previous studies on the effect of experience on the insect brain. However, most previous studies of experience on insect brains have not followed individuals well past the onset of foraging, and those that did focused on the non-reproductive workers of eusocial ants or bees. Below, we first describe our ovary size and wing wear data in terms of reproductive development and foraging behavior. We then interpret our brain volume data in terms of the bees’ life history. We next discuss how our data comparing newly emerged daughters and reproductive foundresses are consistent with previous studies of the influence of experience on brain development. We then explain how our data showing a decline in MB calyx and AL volume in the late mothers relative to foundresses are not consistent with previous studies. Lastly, we contrast the influence of social experience in C. calcarata with C. australensis and outline directions for future study.
Ovary size and wing wear
Females in this species emerge as adults in late summer (August) before entering diapause, after which they initiate a new nest and forage to provision offspring the following spring, continuing into the summer34. Our ovarian development measurements show that late summer, recently emerged daughters have little ovarian development, as expected. However, foundresses, which are actively provisioning nests, have enlarged ovaries. The large variability in foundress ovarian development of this group may arise from some bees being collected before fully enlarging their ovaries, while others were collected upon reproductive maturity44. For example, a study of the sweat bee Megalopta genalis showed small ovaries in newly emerged bees and large ovaries in established reproductives, but wide variation in the ovaries of foundresses45. The lack of wing wear in some C. calcarata foundress females in this study supports the interpretation that some foundresses have only just begun foraging, as wing wear generally correlates with flight activity34,35. By late summer, females have ceased reproduction and provisioning, and as a result ovarian development regresses, as reflected in the lower mean ovary rating of late summer mothers, and the negative, but non-significant, correlation between wing wear and ovary development.
Life history and brain development
Our brain measurements suggest that after reproduction and initial foraging have ceased, C. calcarata females reduce, or cease to maintain, neural investment in the MB calyces and AL. The size-corrected volume of both the MB calyces and AL are smaller in late mothers than in foundresses; there were no significant differences in OL volume. Examination of the correlations between wing wear, which is a correlate of lifetime foraging activity, and neural investment further support the interpretation of reduced neural investment as females become post-reproductive. Among the late mothers, those with the most worn wings also had the smallest MB calyces and OLs. Interestingly, all three brain areas we studied (MB calyces, AL, OL) showed a negative correlation with wing wear in the late mothers (although the AL correlation was not significant), while all three brain areas showed a positive correlation with wing wear in foundresses (but none of these correlations were significant). This might indicate that experience may lead to increased neural investment in foundresses, who are enlarging their ovaries, initiating nests, and provisioning offspring. However, in late-summer mothers, experience, as indicated by wing wear, may instead be associated with post-reproductive senescence and neural decline. An alternative hypothesis is that foundresses, but not late mothers, require higher investment in MB calyces and ALs for initial larval provisioning that then regresses in late mothers. Further investigation, such as experiments in which the experience of foundresses and late-summer mothers is controlled [e.g.5,11,15,19,30] and observations are extended throughout the reproductive season [e.g.7,32] is required to test this hypotheses.
The typical pattern seen in adult insects is for MB and sensory neuropil volume to increase with age and foraging experience4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,32. Studies that disentangle the two factors show that neuropil growth often occurs independent of experience at the beginning of adult life, after which accumulated sensory experience leads to further volume expansion5,11,15,19,30,32. In our study, AL volume shows a pattern of increased investment in foundresses relative to newly emerged daughters, which suggests a combined effect of age and experience, but MB calyx and OL volumes do not differ between these two groups. However, the positive, albeit non-significant, wing-wear correlations among foundresses might indicate that neuropil may generally increase with experience as females begin reproducing. Further studies examining newly emerged daughter brains immediately after adult emergence and experimentally manipulating the foraging experience of foundresses (e.g.19) are required to determine to what extent brain development between the newly emerged daughter and foundress stages is age vs. experience dependent. While our study was not designed to explicitly test for experience-expectant plasticity, this result is consistent with the two other studies of solitary bees which showed no experience-expectant plasticity19,23.
Post-reproductive reduction of MB calyx and AL volume
The most dramatic finding of our study, that MB and AL volumes decreased from the foundress to the post-reproductive, late mother stage, is not typical of other insects studied. In two species of harvester ant queens, total brain volume, optic lobe volume, and, in one species, MB calyx volume, decreased after the foundress phase46. In the brains of foragers that became replacement reproductives in the queenless ant, Harpegnathos saltator, all measured brain areas showed decreased volume47,48. In both studies, the results were interpreted in terms of the queens reducing the metabolic maintenance costs of expensive neural tissue when they required less neural investment after establishing a nest underground with workers to forage and care for the brood. Once the queens had workers, they no longer required the navigation ability to forage, nor received the visual stimuli of doing so46,47. If C. calcarata females no longer require as much brain tissue after reproducing and rearing offspring to adulthood, they may similarly reduce investment in neural tissue. However, unlike ant foundress-turned-queens, C. calcarata late mothers still need to forage. They provide their newly emerged daughters with additional nutrition that increases their chances of surviving the following winter35. However, late mothers are near the end of their life: in a previous study, 93% died by September35.
In other species studied, age- and experience-related enlargement of the MB or other areas of the brain does not revert. In honeybee workers that are overwintering (and thus not foraging) there is no reduction in MB volume33. Likewise, in honeybee foragers that were manipulated to revert back to nursing behavior (workers typically nurse first, then forage), there was no reduction of MB volume back to lower, nurse-typical levels33. Previous studies have found that while ants and honeybees may show behavioral and physiological signs of senescence, there are not volumetric changes in the brain associated with senescence49,50. Workers of the ant Camponotus floridanus even showed continued MB volume increases up to six months of age7,32. However, all of these studies cited above were conducted on workers of eusocial species, which are not post-reproductive, because workers do not reproduce. Workers instead accrue indirect fitness by helping the colony reproduce throughout their lives. The only studies to examine the development of brain structures of solitary or small-colony bees either used individuals still engaged in reproduction15,18,23,28 or tropical species with no distinct end to the reproductive season27,31. Likewise, studies demonstrating experience-based enlargement of the MB in Lepidoptera also did not include post-reproductive individuals13,14,51. In Drosophila, changes in individual neurons and number of synapses, but not overall MB volume, are associated with senescence52. We hypothesize that C. calcarata life history may prioritize reproduction, resulting in reduced investment in neural structures late in life. Further studies of other solitary insects are required to test this hypothesis.
Effects of social interactions
In the only other species of Ceratina bees studied to date, C. australensis, reproductive females showed experience dependent expansion of the MB calyces, including a positive correlation of both wing wear and ovarian development with MB calyx volume15. In our study, we found little effect of ovarian development on C. calcarata brain structure, except for a correlation between OL volume and ovary development in foundresses. In another interesting difference between the two Ceratina species, C. australensis showed additional MB calyx enlargement associated with social dominance (in C. australensis, some females nest with a subordinate sister). This is opposite to what we found in C. calcarata, where late summer mothers with newly emerged daughters in their nest had smaller MBs than early season foundresses. This suggests that the nature of sociality in the two species (semisocial, with a dominance hierarchy between sisters in C. australensis, and subsocial, with maternal care of daughters in the nest in C. calcarata) imposes fundamentally different cognitive demands. Given that both species have sequenced genomes53,54, are in separate subgenera of a genus with a well-established phylogeny55,56, exhibit a range of social behaviors and are experimentally tractable57,58,59,60, Ceratina bees could be a productive system for studying the effects of development, reproduction, and social interactions on neural investment.
Data availability
The data that support the findings of this study are openly available in figshare.com https://doi.org/10.6084/m9.figshare.19620615.
References
Fahrbach, S. E. & Van Nest, B. N. Synapsin-based approaches to brain plasticity in adult social insects. Curr. Opin. Insect. Sci. 18, 27–34 (2016).
Fahrbach, S. E. Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 51, 209–232 (2006).
Zars, T. Behavioral functions of the insect mushroom bodies. Curr. Opin. Neurobiol. 10, 790–795 (2000).
Amador-Vargas, S., Gronenberg, W., Wcislo, W. T. & Mueller, U. Specialization and group size: Brain and behavioural correlates of colony size in ants lacking morphological castes. Proc. Biol. Sci. 282, 20142502 (2015).
Fahrbach, S. E., Moore, D., Capaldi, E. A., Farris, S. M. & Robinson, G. E. Experience-expectant plasticity in the mushroom bodies of the honeybee. Learn. Mem. 5, 115–123 (1998).
Farris, S. M., Robinson, G. E. & Fahrbach, S. E. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404 (2001).
Gronenberg, W., Heeren, S. & Hölldobler, B. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011–2019 (1996).
Ismail, N., Robinson, G. E. & Fahrbach, S. E. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc. Natl. Acad. Sci. U.S.A. 103, 207–211 (2006).
Krofczik, S., Khojasteh, U., De Ibarra, N. H. & Menzel, R. Adaptation of microglomerular complexes in the honeybee mushroom body lip to manipulations of behavioral maturation and sensory experience. Dev. Neurobiol. 68, 1007–1017 (2008).
Kühn-Bühlmann, S. & Wehner, R. Age-dependent and task-related volume changes in the mushroom bodies of visually guided desert ants Cataglyphis bicolor. J. Neurobiol. 66, 511–521 (2006).
Maleszka, J., Barron, A. B., Helliwell, P. G. & Maleszka, R. Effect of age, behaviour and social environment on honey bee brain plasticity. J. Comp. Physiol. A 195, 733–740 (2009).
Molina, Y. & O’Donnell, S. Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev. Neurobiol. 68, 950–959 (2008).
Montgomery, S. H. & Merrill, R. M. Divergence in brain composition during the early stages of ecological specialization in Heliconius butterflies. J. Evol. Biol. 30, 571–582 (2017).
Montgomery, S. H., Merrill, R. M. & Ott, S. R. Brain composition in Heliconius butterflies, posteclosion growth and experience-dependent neuropil plasticity. J. Comp. Neurol. 524, 1747–1769 (2016).
Rehan, S. M., Bulova, S. J. & O’Donnell, S. Cumulative effects of foraging behavior and social dominance on brain development in a facultatively social bee (Ceratina australensis). Brain Behav. Evol. 85, 117–124 (2015).
Seid, M. A. & Wehner, R. Ultrastructure and synaptic differences of the boutons of the projection neurons between the lip and collar regions of the mushroom bodies in the ant Cataglyphis albicans. J. Comp. Neurol. 507, 1102–1108 (2008).
Stieb, S. M., Muenz, T. S., Wehner, R. & Rössler, W. Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Dev. Neurobiol. 70, 408–423 (2010).
Withers, G. S., Fahrbach, S. E. & Robinson, G. E. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238 (1993).
Withers, G. S., Day, N. F., Talbot, E. F., Dobson, H. E. & Wallace, C. S. Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria. Dev. Neurobiol. 2, 73–82 (2008).
Withers, G. S., Fahrbach, S. E. & Robinson, G. E. Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees. J. Neurobiol. 26, 130–144 (1995).
Heisenberg, M., Heusipp, M. & Wanke, C. Structural plasticity in the Drosophila brain. J. Neurosci. 15, 1951–1960 (1995).
Seid, M. A., Harris, K. M. & Traniello, J. F. Age-related changes in the number and structure of synapses in the lip region of the mushroom bodies in the ant Pheidole dentata. J. Comp. Neurol. 488, 269–277 (2005).
Hagadorn, M. A., Johnson, M. M., Smith, A. R., Seid, M. A. & Kapheim, K. M. Experience, but not age, is associated with volumetric mushroom body expansion in solitary alkali bees. J. Exp. Biol. 224, 238899 (2021).
Molina, Y. & O’Donnell, S. Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav. Evol. 70, 137–144 (2007).
O’Donnell, S., Donlan, N. & Jones, T. Developmental and dominance-associated differences in mushroom body structure in the paper wasp Mischocyttarus mastigophorus. Dev. Neurobiol. 67, 39–46 (2007).
O’Donnell, S., Bulova, S. J., DeLeon, S., Barrett, M. & Fiocca, K. Caste differences in the mushroom bodies of swarm-founding paper wasps: implications for brain plasticity and brain evolution (Vespidae, Epiponini). Behav. Ecol. Sociobiol. 71, 116 (2017).
Jaumann, S., Seid, M. A., Simons, M. & Smith, A. R. Queen dominance may reduce worker mushroom body size in a social bee. Dev. Neurobiol. 2, 2 (2019).
Pahlke, S., Jaumann, S., Seid, M. A. & Smith, A. R. Brain differences between social castes precede group formation in a primitively eusocial bee. Sci. Nat. 106, 49 (2019).
Durst, C., Eichmüller, S. & Menzel, R. Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behav. Neural Biol. 62, 259–263 (1994).
Jones, B. M., Leonard, A. S., Papaj, D. R. & Gronenberg, W. Plasticity of the worker bumblebee brain in relation to age and rearing environment. Brain Behav. Evol. 82, 250–261 (2013).
Smith, A. R., Seid, M. A., Jimenez, L. C. & Wcislo, W. T. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae). Proc. Biol. Sci. 277, 2157–2163 (2010).
Seid, M. A. & Junge, E. Social isolation and brain development in the ant Camponotus floridanus. Sci. Nat. 103, 1–6 (2016).
Fahrbach, S. E., Farris, S. M., Sullivan, J. P. & Robinson, G. E. Limits on volume changes in the mushroom bodies of the honey bee brain. J. Neurobiol. 57, 141–151 (2003).
Rehan, S. M. & Richards, M. H. Nesting biology and subsociality in Ceratina calcarata (Hymenoptera: Apidae). Can. Entomol. 142, 65–74 (2010).
Mikát, M., Franchino, C. & Rehan, S. M. Sociodemographic variation in foraging behavior and the adaptive significance of worker production in the facultatively social small carpenter bee Ceratina calcarata. Behav. Ecol. Sociobiol. 71, 135 (2017).
Molina, Y., Harris, R. M. & O’Donnell, S. Brain organization mirrors caste differences, colony founding and nest architecture in paper wasps (Hymenoptera: Vespidae). Proc. R. Soc. B: Biol. Sci. 276, 3345–3351 (2009).
Sheehan, Z. B., Kamhi, J. F., Seid, M. A. & Narendra, A. Differential investment in brain regions for a diurnal and nocturnal lifestyle in Australian Myrmecia ants. J. Comp. Neurol. 527, 1261–1277 (2019).
O’Donnell, S. et al. A test of neuroecological predictions using paperwasp caste differences in brain structure (Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 68, 529–536 (2014).
Grob, R., Fleischmann, P. N., Grübel, K., Wehner, R. & Rössler, W. The role of celestial compass information in Cataglyphis ants during learning walks and for neuroplasticity in the central complex and mushroom bodies. Front. Behav. Neurosci. 11, 226 (2017).
McKenzie, S. K., Fetter-Pruneda, I., Ruta, V. & Kronauer, D. J. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl. Acad. Sci. U. S. A. 113, 14091–14096 (2016).
Fiala, J. C. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 (2005).
Mueller, U. G. & Wolf-Mueller, B. A method for estimating the age of bees: Age-dependent wing wear and coloration in the Wool-Carder bee Anthidium manicatum (Hymenoptera: Megachilidae). J. Insect Behav. 6, 529–537 (1993).
Michener, C. D. The Social Behavior of the Bees: A Comparative Study (Harvard University Press, 1974).
Rehan, S. M. & Richards, M. H. Reproductive aggression and nestmate recognition in a subsocial bee. Anim. Behav. 85, 733–741 (2013).
Kapheim, K. M. et al. Physiological variation as a mechanism for developmental caste-biasing in a facultatively eusocial sweat bee. Proc. R. Soc. B: Biol. Sci. 279, 1437–1446 (2012).
Julian, G. E. & Gronenberg, W. Reduction of brain volume correlates with behavioral changes in queen ants. Brain Behav. Evol. 60, 152–164 (2002).
Gronenberg, W. & Liebig, J. Smaller brains and optic lobes in reproductive workers of the ant Harpegnathos. Naturwissenschaften 86, 343–345 (1999).
Penick, C. A. et al. Reversible plasticity in brain size, behaviour and physiology characterizes caste transitions in a socially flexible ant (Harpegnathos saltator). Proc. R. Soc. B: Biol. Sci. 288, 20210141 (2021).
Giraldo, Y. M., Patel, E., Gronenberg, W. & Traniello, J. F. Division of labor and structural plasticity in an extrinsic serotonergic mushroom body neuron in the ant Pheidole dentata. Neurosci. Lett. 534, 107–111 (2013).
Wolschin, F., Munch, D. & Amdam, G. V. Structural and proteomic analyses reveal regional brain differences during honeybee aging. J. Exp. Biol. 212, 4027–4032 (2009).
Van Dijk, L. J., Janz, N., Schäpers, A., Gamberale-Stille, G. & Carlsson, M. A. Experience-dependent mushroom body plasticity in butterflies: Consequences of search complexity and host range. Proc. R. Soc. B: Biol. Sci. 284, 20171594 (2017).
Koch, S. C., Nelson, A. & Hartenstein, V. Structural aspects of the aging invertebrate brain. Cell Tissue Res. 383, 1–17 (2021).
Rehan, S. M., Glastad, K. M., Lawson, S. P. & Hunt, B. G. The genome and methylome of a subsocial small carpenter bee Ceratina calcarata. Genome Biol. Evol. 8, 1401–1410 (2016).
Rehan, S. M. et al. Conserved genes underlie phenotypic plasticity in an incipiently social bee. Genome Biol. Evol. 10, 2749–2758 (2018).
Rehan, S. & Schwarz, M. A few steps forward and no steps back: long-distance dispersal patterns in small carpenter bees suggest major barriers to back-dispersal. J. Biogeogr. 42, 485–494 (2015).
Groom, S. V. & Rehan, S. M. Climate-mediated behavioural variability in facultatively social bees. Biol. J. Linn. Soc. 125, 165–170 (2018).
Withee, J. R. & Rehan, S. M. Cumulative effects of body size and social experience on aggressive behaviour in a subsocial bee. Behaviour 153, 1365–1385 (2016).
Withee, J. R. & Rehan, S. M. Social aggression, experience, and brain gene expression in a subsocial bee. Integr. Comp. Biol. 57, 640–648 (2017).
Steffen, M. A. & Rehan, S. M. Genetic signatures of dominance hierarchies reveal conserved cis-regulatory and brain gene expression underlying aggression in a facultatively social bee. Genes Brains Behav. 2, 2 (2019).
Cook, C. N., Lawson, S. P., Brent, C. S. & Rehan, S. M. Biogenic amines shift during the pre-reproductive to reproductive transition in the small carpenter bee Ceratina calcarata. Apidologie 50, 90–99 (2019).
Acknowledgements
This work was supported by NSF Grant #17-1028536545 to ARS and an NSERC Discovery Grant to SMR.
Author information
Authors and Affiliations
Contributions
S.J., S.M.R. and A.R.S. designed the study and wrote the manuscript. S.M.R. collected all bees. S.J. and K.S. dissected bees, performed confocal microscopy and measured brain areas from the confocal micrographs. A.R.S. measured wing wear and ovary development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jaumann, S., Rehan, S.M., Schwartz, K. et al. Reduced neural investment in post-reproductive females of the bee Ceratina calcarta. Sci Rep 12, 8256 (2022). https://doi.org/10.1038/s41598-022-12281-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12281-7
- Springer Nature Limited