Abstract
Binge eating is increasingly prevalent among adolescents and young adults and can have a lasting harmful impact on mental and physical health. Mechanistic insights suggest that aberrant reward-learning and biased cognitive processing may be involved in the aetiology of binge eating. We therefore investigated whether recently developed approaches to catalyse brief interventions by putatively updating maladaptive memory could also boost the effects of cognitive bias modification training on binge eating behaviour. A non-treatment-seeking sample of 90 binge eating young adults were evenly randomised to undergo either selective food response inhibition training, or sham training following binge memory reactivation. A third group received training without binge memory reactivation. Laboratory measures of reactivity and biased responses to food cues were assessed pre-post intervention and bingeing behaviour and disordered eating assessed up to 9 months post-intervention. The protocol was pre-registered at https://osf.io/82c4r/. We found limited evidence of premorbid biased processing in lab-assessed measures of cognitive biases to self-selected images of typical binge foods. Accordingly, there was little evidence of CBM reducing these biases and this was not boosted by prior ‘reactivation’ of binge food reward memories. No group differences were observed on long-term bingeing behaviour, caloric consumption or disordered eating symptomatology. These findings align with recent studies showing limited impact of selective inhibition training on binge eating and do not permit conclusions regarding the utility of retrieval-dependent memory ‘update’ mechanisms as a treatment catalyst for response inhibition training.
Similar content being viewed by others
Introduction
Binge Eating disorder (BED) is on the rise in young adults1. BED is notable due to its high prevalence across genders (estimated 1–4%2). It can be disabling due to its high comorbidity with anxiety3 and depression4 and it is statistically associated with physical health risk factors5 such as excess adiposity6 diabetes7 and metabolic syndrome8. Current therapies for BED are typically CBT or combined pharmacotherapy-based, and may effectively reduce binge frequency9. However, these therapies have high long-term relapse rates10, with only a minority achieving remission following treatment11. BED thus constitutes an enormous financial and healthcare burden within the EU12. Phenomenological similarities to, and high comorbidity with, substance use disorders suggest that binge/over-eating may share some underlying neurobiological and psychological aetiology with addiction13,14,15,16. Indeed the framing of these disorders as types of ‘food addiction’13 although controversial, is increasingly prevalent17,18. While ‘food addiction’ has been criticised for providing an incomplete account of binge eating (see Refs.19,20, for a discussion) with alternative (though not incompatible) mechanisms such as negative affect, dietary restraint21 and beliefs also playing a role22, most authors acknowledge an important contributory role of reward and motivational mechanisms in predisposing to binge eating in the modern food environment. As such, insights into maladaptive reward processes from the field of addiction (and novel strategies targeting these) might also be usefully applied in binge eating, offering new opportunities for prevention and intervention.
Aberrant reward processing is thought to be a core ‘transdiagnostic’ mechanism in addiction and binge eating aetiology23,24,25. Under this model, heightened reward responses to binge food-related ‘cues’ trigger automatic food-seeking (approach), ‘hedonic hunger’, preoccupation with food16, craving and maladaptive consumption behaviour. Indeed, patterns of craving and eating for reward enhancement distinguish binge-eating individuals from weight-equivalent healthy control populations23.
These reward responses are thought to be learned, rather than innate. Binged-on foods are almost universally ‘highly palatable foods’ (HPFs; highly processed foods with high caloric density and high fat/sugar macronutrient profile26), which produce reward and hedonic responses via sharp increases in ventral striatal dopamine27, and endorphin signalling17, but comparatively low satiety. These properties promote overconsumption (far exceeding homeostatic requirements) and support associative learning about sensory cues (tastes, textures, smells and visual qualities) that predict HPF reward, conferring high ‘addictive potential’ to HPFs28,29 and imbuing these cues with high salience and incentive properties30. HPF cues can thus elicit attentional capture31,32 and automatic motor ‘approach’ responses when encountered33,34,35, in a similar manner to drug-related stimuli in substance-use disorders (SUDs)36. Theoretically, automatic approach and motivational processes elicited by HPF cues require opponent top-down inhibition of responses to reward cues to over-ride impulsive–compulsive consumption, but this is thought to be impaired in binge eating37. High impulsivity and reduced inhibitory control capacity may thus conspire to support binge eating behaviour38.
Response inhibition training (RIT), a sub-type of ‘cognitive bias modification’(CBM) , broadly aims to retrain automatic behavioural biases to eating cues, and might improve outcomes in binge-eating individuals23,39. Response inhibition training may improve food-specific inhibitory control and reduce cue-induced motor activation by overriding prepotent ‘cue → go’ tendencies40 with inhibitory ‘cue → no-go’ responses. It is typically implemented via a ‘Go/No-go’ task, in which food cues (e.g. HPF images) are consistently paired with ‘no-go’ responses41. This has been found to reduce chocolate craving42, weight and overeating in ‘normal weight’, ‘overweight’43 and ‘obese’ individuals (primary researchers’ own terms)44. It has been suggested to be particularly effective in those with high BMI, who desire to lose weight, supporting a role of prepotent action biases in excessive consumption, and of motivation in ameliorating these41. However, most evidence from single-session laboratory studies on RIT is in healthy or ‘overweight controls’ and effects may be modest in disordered eating populations45 particularly when clinical endpoints are used45,46. In these populations, the comparatively brief nature of RIT may be insufficient to counteract the years of maladaptive learning that has ingrained ‘go’ biases to HPFs.
Recent successes in the fear and addiction literature suggest it may be possible to surmount this short-lived efficacy by using maladaptive learning reminders to catalyse brief learning-based interventions. The effects of these reminders are typically attributed to reconsolidation-update; a ‘housekeeping’ mechanism for memory maintenance47. Reconsolidation putatively serves to selectively strengthen, weaken or update memories by incorporating new information, dependent on the prediction of predict salient outcomes by the memory trace. The reconsolidation process follows (and requires) the retrieval-dependent destabilisation of memories. If novel cue-response associations are presented or trained while memories are labile, or if reconsolidation is pharmacologically halted48, memories may putatively be either modified49,50 or weakened. Since food ‘go’ biases are learned (and therefore stored in memory ), if the reward associations between binge food cues and HPF reward can be destabilised, subsequent RIT may directly update cue-response relationships, greatly catalysing its efficacy. This approach has demonstrated lasting efficacy (at least 9 months) when applied to experimental ‘ultra-brief’ behavioural treatment modalities (exposure51, counterconditioning50, cognitive reappraisal52).
We therefore sought to examine whether single-session RIT to binge food cues could reduce subsequent response biases to HPF, binge frequency and symptomatology in a group of young-adult, sub-clinical binge eating individuals and whether this effect could be similarly boosted by a ‘reminder’ of maladaptive learning prior to training, consistent with a reconsolidation-update mechanism. This population was targeted as they displayed clear bingeing behaviour, but were not currently receiving treatment, with which the current experimental approach might interfere, conveying higher risk of iatrogenic harm. They also represent an important target group in their own right, in which low-intensity interventions such as this might play an important preventative role.
Methods
Participants and design
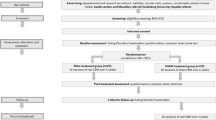
The study design and analysis was pre-registered on the Open Science Framework on 02/03/2018 (https://osf.io/82c4r/) and ISRCTN on 23/01/2019 (https://doi.org/10.1186/ISRCTN13262256). Young adults (aged 18–25) with sub-clinical bingeing behaviour (≥ 1 binge/month and Binge Eating Scale (BES) score > 17) were assigned using block randomisation to three groups in a single-blind, randomised experimental study: Groups were: Binge Memory reactivation + Response Inhibition Training (BMR + RIT), No memory reactivation + RIT (NR + RIT), or BMR + ‘sham’ RIT (BMR + sham), all N = 30 (total randomised N = 90). These groups allowed us to assess effects of RIT per se and via putative reconsolidation-update. Full inclusion, exclusion and randomisation protocols and power calculation are detailed in the Supplementary Information. All procedures were reviewed and approved by the University College London Research Ethics Committee.
Materials
Subjective measures
Binge eating symptoms were assessed via the BES53 (score ≥ 17 required for participation); and Eating Disorder Examination Questionnaire (EDE-Q54,55). The Yale Food Addiction Scale (Y-FAS) was used to assess addictive-like responses to food56. Susceptibility to food craving was assessed using the Power of Food Scale57 and general and state craving in response to HPFs by the food craving questionnaire-trait (FCQ-T) and state forms, respectively (FCQ-S)58. As depressed mood is comorbid with binge eating, we measured differences in recent depression with the Beck Depression Inventory (BDI59). Impulsivity; a putative predictor of binge behaviour, was indexed using the Barratt Impulsiveness Scale (BIS60).
A calendar-based self-report Timeline-Follow-Back measure was used to measure subjective binge frequency61, where participants reported the incidence of subjective binges; defined as ‘eating an unusually large amount of food with the subjective feeling of loss-of-control’. This was confirmed by completion of a daily food diary via the MyFitnessPal app. Participants were asked to log everything they consumed for one week prior to session 1 (baseline), from session 2 to session 3 (post-intervention) and post-session-3 (follow-up). From this, total daily calories, carbohydrates, fats and sugars were calculated.
HPF Cue reactivity and ‘taste test’
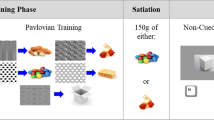
The procedure is outlined in detail in the Supplementary Information. Briefly, ‘pleasantness’, ‘desire to eat’ and ‘likelihood of bingeing on’ was assessed for 18 HPF and 18 LPF images on a 0–100 scale. From this task, individualised HPF and LPF images (four of each) were selected per- participant, for later use in the visual probe and Go/No-Go tasks based on highest and lowest reward reactivity ratings. Prior to image rating, participants selected a preferred HPF snack food item from a ‘menu' and were told they would eat this after rating some food images, in a sham ‘taste test’. The selected food was placed in front of the participant and visible during the ratings of all food images and at the end the picture rating, was itself rated for ‘desire to eat’ and predicted ‘enjoyment’ pre-consumption and its taste attributes, true ‘enjoyment’ and ‘wanting more’, post-consumption. The food was consumed according to on screen prompts requiring participants to ‘pick up food’, ‘prepare to eat’ and ‘eat the food’.
Go/No-Go Task
Response bias to binge foods was both assessed and retrained via a Go/No-Go task, adapted from Houben and Jansen42 and following previous research38,62. Full task details are given in the Supplementary Information and Ref.63. An ‘assessment version’ of the task was used in Sessions 1 and 3 and a ‘modification version’ on Session 2 (‘intervention' session). Task parameters were identical in both versions except HPF binge foods were paired with ‘No-go’ responses and LPF images paired with ‘Go’ responses on 100% trials in the ‘modification’ version. The ‘sham’ version of the Go/No-Go task on session 2 was simply the ‘assessment’ version; with parity between requirement for Go- or No-go responses for all stimulus types (HPF binge food, LPF or filler). Assessed indices of response bias were error rates, median reaction times, sensitivity (d-prime) and response bias (criterion C), indexing bias to ‘go’ to images regardless of response requirement42.
Visual probe
Eye-tracking in a dot-probe task was used to assess attentional bias to the self-selected LPF and HPF stimuli. All food images were paired with matched non-food images and dwell time and first fixation latency were calculated as indices of sustained and automatic attention, respectively. Details in Supplementary Information.
Binge memory retrieval and no-retrieval control
Participants in the BMR + RIT and BMR + sham groups underwent Binge Memory Retrieval (BMR) which followed a procedure parallel to those we have used successfully in previous studies on maladaptive reward memory reconsolidation48,64. The BMR procedure was introduced to the participants as a repeat of the session one ‘taste test’ (i.e. cue reactivity) task. Again, participants selected their favourite food from the ‘menu’ and were instructed that they would consume this after rating images. The presented images were the participant’s four highest-rated ‘binge cues’. They then rated their predicted enjoyment and ‘desire to eat’ their selected food. Following this, the on-screen consumption prompts read as before. The final prompt, however, read ‘Stop, put food down’ at which point the food was taken away. Participants were thus prevented from consuming their anticipated food reward, putatively engendering a cognitive prediction error.
Participants in the NR condition followed the same procedure as BMR, except: (1) the binge food cues were replaced with the lowest-rated LPF food images from the cue reactivity task (2) Instead of selecting their favourite HPF from the menu, participants were given a non-binge LPF (celery sticks) and told they would eat this after rating food images. Thereafter, the image and food ratings and prompt screens were identical to the BMR procedure, including the prediction error procedure. The NR procedure was designed to match the BMR as closely as possible without (re)activating binge food reward memory.
Procedure
After screening, participants attended three lab sessions and (remotely) provided follow-up data on four additional occasions (+ 2 week, 3 months, 6 months, 9 months). Prior to lab sessions they fasted from solid food (4 h) and abstained from caffeine (2 h). All lab sessions were conducted between 1 and 5 p.m. Written informed consent was given at the start of Session 1, following eligibility screening. The full procedure is outlined in detail in the Supplementary Information.
Session 1
Baseline demographic, questionnaire, biological (including blood glucose, blood pressure, weight & height for BMI calculation) and eating-related measures were obtained (see supplement for full list). In addition, state measures of food craving (FCQ) and hunger (hunger ruler) were assessed followed by the cue reactivity procedure and the assessment version of the Go/No-Go task. Finally, they completed the visual probe task.
Session 2 (session 1 + 48 h)
After repeating the biological and state measures from session 1, participants then completed the BMR or NR procedure as appropriate to their random group allocation. As with our previous studies48,49, following the BMR or NR procedure, participants completed high-load working memory tasks (prose recall from the Rivermead battery and digit span forwards and backwards), to ensure cognitive disengagement from the food cues. Following completion of these ‘distractor’ tasks (~ 5 min), participants began the ‘RIT’ or ‘sham’ version of the Go/No-Go task, followed by FCQ-state and ‘hunger ruler’.
Session 3 (session 2 + 7 days)
The session 3 procedure was identical to Session 1, except the participants did not complete the BIS, BIS/BAS or BDI scale.
Follow-up
At 2 weeks, 3, 6 and 9 months following Session 3, participants remotely completed the BES, EDE-Q, Y-FAS, TLFB of binges, TFEQ, PFS and rated each image used in the initial cue reactivity assessment task on the same metrics as in-lab.
Statistical approach
In-lab continuous measures (cue reactivity rating data, Go/No-Go reaction times, oculomotor attentional bias and state questionnaire measures) were assessed with 2 [Session:Session 1 (pre-manipulation) v. Session 3 (post-manipulation)) × 3 [BMR + RIT, BMR + sham, NR + RIT] × mixed ANOVA. Power calculation was based on this model (see Supplementary Information for full data handling protocols, sample size calculation data and randomisation). For analysis of cue reactivity and Go/No-Go RT data, a factor of Cue Type (HPF, LPF, non-food filler) was also modelled. For error rate and accuracy data in the Go/No-Go task, generalized estimating equations with a loglinear link function were used due to the approximate Poisson distribution of the count data. For long-term follow-up data, linear mixed models (LMMs; for continuous, normally distributed data) and generalized linear mixed models (GLMMs; binge count data) were used, incorporating effects of Group, Time point (baseline, post-manipulation, 2 weeks, 3 months, 6 months and 9 months) and their interaction. Signal detection metrics criterion C (i.e. ‘g bias’ and \({d}^{^{\prime}}\) were calculated for the Go/No-Go task) and analysed with LMMs and gamma GLMM (following inspection of data distribution). For tests of baseline trait, biometric and demographics variables, where group differences were not hypothesised, the false-discovery rate (FDR65) adjusted alpha level was applied. Post-hoc tests following omnibus tests were adjusted using the Sidak correction. Data were collected by LS and EC and analysed blind by RKD, using a code generated by SKK.
Ethical approval
The authors assert that all procedures contributing to this work were approved by and comply with University College London Research Ethics Committee’s ethical standards on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. ISRCTN Registration Identifier: ISRCTN13262256. Open Science Framework Pre-registration: https://osf.io/82c4r/.
Results
Descriptive statistics for key variables across groups are given in Table 1. Groups were very similar on assessed demographic variables, being typically in their early 20 s and in higher education. BES scores verified subjective binge-eating status and the PFS, TFEQ and FCQ indicated relatively high reactivity to food, emotional/uncontrolled eating and food craving indicating the sample displayed robust maladaptive reward responses to food. There was a trend for greater BMI in BMR + RIT than the other groups, due to three individuals with particularly high BMI (~ 37). There was also a trend for a difference (BMR + Sham > BMR + RIT) in the uncontrolled eating subscale of the TFEQ. Neither of these differences approached significance at FDR-corrected alpha. Groups were otherwise similar on baseline variables.
Short-term effects of RIT and BMR (in-lab measures)
Pre-manipulation, Go/No-Go task commission errors (False Alarms) were greater for both types of food stimuli (LPF and binge) than non-food stimuli. See Supplementary Information for full analyses. Error rates were examined across Group, Session (pre-manipulation vs post-manipulation), Stimulus Types (Binge, LPF, non-food filler) and Error Type (misses and false alarms). In line with analysis of baseline data, main effects of Stimulus Type (χ2(2) = 82.194, p < 0.001), Error Type (false alarms > misses): χ2(1) = 6.404, p = 0.011 and their interaction (χ2(2) = 13.013, p = 0.001) were found. The four-way interaction of Group, Stimulus Type, Error Type and Session was also significant. A three-way Stimulus Type × Session × Error Type interaction was present in all groups, although simple effects within each Group showed a change in response to Binge food stimuli only in BMR + RIT (see Table 2, top). At baseline, BMR + RIT showed significantly more false alarms than misses to binge food images (χ2(1) = 18.043, p < 0.001), however this was abolished post-training (χ2(1) = 1.222, p = 0.269).
To qualify this effect, Session × Stimulus Type interactions were assessed within each Group and Error Type (see Table 2, bottom). This showed a significant increase in binge-food ‘misses’ from session 1 to session 3 in BMR + RIT, but a significant decrease in misses (i.e. greater response to binge food) in BMR + Sham, indicating potential worsening of approach bias in this group. In NR + RIT, there was a significant decrease in false alarms on binge food ‘no-go’ trials and a decrease in false alarms to filler images.
Signal detection measures
Criterion C
A 3 (Group) × 2 (Session: pre-manipulation, post-manipulation) × Stimulus Type (Binge, LPF, filler) factorial linear mixed model with bootstrapped parameter estimates found main effects of Stimulus Type [F(2,450) = 3.59, p = 0.028] and a Group × Session × Stimulus Type interaction [F(4,450) = 3.011, p = 0.018]. The 3-way interaction was investigated through examination of Session × Group interactions for each Stimulus Type. This revealed a Session*Group interaction for binge images only.
In BMR + Sham, there was a significant worsening of response bias to food, reflected in a reduction in C for binge images from session 1 to session 3 [F(1,90) = 6.14,p = 0.015]. In BMR + RIT, there was a significant reduction in bias to binge images (increase in C towards 0) [F(1,90) = 4.635, p = 0.034]. In NR + RIT there was no statistically significant change [F(1,90) = 3.153,p = 0.079]. This is possibly evidence of a beneficial response in BMR + RIT, although it should be noted that this group showed the greatest bias to binge images on Session 1, indicating potential baseline dependency effects. This effect is shown in Fig. 1.
Changes in commission bias (Criterion C) across study sessions. Reductions in binge food commission (‘go’ bias) were seen in BMR + RIT, along with a shift in bias towards non-food images in NR + RIT. BMR + Sham showed an unfavourable shift in bias towards binge images, in line with a worsening of ‘go bias’ due to sham training. Bars are group mean ± SEM. *p < 0.05.
D prime (d′)
As with overall accuracy, d′ scores were highly skewed (z > 4 in most cases), indicating ceiling-level performance with regards to go/no-go signal sensitivity. For this reason, d′ scores were analysed using a gamma generalized linear mixed model, including factors of Group, Stimulus Type and Session factorially. This yielded a main effect of Stimulus Type only [F(2,522) = 4.124, p = 0.016], indicating lower d′ scores (reflecting greater false alarm rate) to binge food images vs. non-food filler images [t(522) = 2.783, p = 0.017], but no difference between HPF and LPF stimuli [t(522) = 0.766, p = 0.444].
Reaction time data
At baseline, median reaction times on (correct) ‘Go’ trials indicated an effect of Stimulus Type [F(2,174) = 8.447, p < 0.001, η2p = 0.089], that was invariant across groups [Stimulus Type × Group interaction: F(4,174) = 1.948, p = 0.105, η2p = 0.043]. Responses were faster to both types of food images (HPF and LPF) than non-food filler images [Helmert F(1,87) = 14.82, p < 0.001, η2p = 0.146], but not different between HPF and LPF images [Helmert F(1,87) = 0.089, p = 0.766, η2p = 0.001]. Thus there was an overall faster response to food images in the study sample, but not specifically to HPF ‘binge’ foods. A general speeding of responses between sessions 1 and 3 indicated practice effects [F(1,87) = 32.643, p < 0.001, η2p = 0.273], but there were no interactions nor group effects.
Oculomotor attentional bias (visual probe)
Dwell time assessment of HPF/binge food vs LPF food images found no evidence for differential sustained attention to binge-food images above any food image per se [main effect of image type F(1,85) = 2.79, p = 0.099, η2p = 0.032]. Equally, this did not vary pre-post manipulation [Session × Image Type F(1,85) = 0.7, p = 0.792, η2p=0.001] or across Groups [Session × Image Type × Group F(2,85) = 0.43, p = 0.65, η2p = 0.01]. Dwell time on long-latency trials (2000 ms) incorporates early automatic and later conscious control of visual attention. Indeed, significantly reduced latencies to first fixation on binge food images was observed (a measure of automatic attentional capture) vs LPF images [main effect of image type [F(1,85) = 27.508, p < 0.001, η2p = 0.245. Combined with the lack of difference in dwell time, this suggested that following initial (automatic) attentional capture, participants deployed effortful visual avoidance strategies to disengage attention from binge food images.
Food craving questionnaire (state)
Session (1 vs 3) × Time (pre vs. post cue reactivity) × Group ANOVA yielded a reduction in general food craving from Session 1 to Session 3 [F(1,82) = 4.058, p = 0.047, η2p = 0.047], that did not significantly differ between groups. The desire subscale showed a Session × Time × Group interaction [F(2,82) = 4.273, p = 0.017, η2p = 0.094]. This was found to be driven by a decrease in food desire across sessions in BMR + Sham at the post taste-test time point [F(1,82) = 7.173, p = 0.007, η2p = 0.086]. Conversely, a decrease in pre taste-test desire across sessions was seen in NR + RIT [F(1,82) = 6.498, p = 0.013, η2p = 0.073]. There was a reduction in the control subscale from session 1 to 3 [F(1,82) = 4.372, p = 0.04, η2p = 0.051], that did not differ between groups. The same was found for the hunger subscale [F(1,82) = 8.067, p = 0.006, η2p = 0.09]. No significant change was observed on the relief or reinforcement subscales.
Cue reactivity
Ratings of ‘likelihood to binge on’ depicted food images (HPF vs LPF/Session 1 vs. Session 3) showed a main effect of image type (HPF > LPF: F(1,85) = 564.630, p < 0.0005, η2p = 0.869), validating the use of HPF and LPF images as representing binge foods and non-binge foods, respectively). A modest Group × Session interaction was observed [F(2,85) = 3.931, p = 0.023, η2p = 0.085]. While there were no group differences on Session 1, on Session 3 (post-manipulation), both BMR + RIT [t(57) = 2.996, p = 0.011, d = 0.78] and NR + RIT [t(59) = 2.74, p = 0.022, d = 0.71] reported lower likelihood of bingeing on any depicted food than BMR + Sham. A marginal Session × Group interaction was also observed for food images’ impact on urge to eat ratings [F(2,85) = 3.167, p = 0.047, η2p = 0.069]. BMR + RIT reported lower ‘urge to eat’ than BMR + Sham on both sessions, however BMR + Sham showed lower urge to eat than NR + RIT on session 3 [t(59) = 2.89, p = 0.015, d = 0.75]. This was largely due to an increase in desire to eat ratings in NR + RIT from session 1 to session 3 [F(1,85) = 4.037, p = 0.48, η2p = 0.045].
Long-term disorder-relevant outcomes
Binge Eating Scale
The mixed modelling approach for the BES was supported by significant variance in intercepts (σ2 = 37.315, Z = 5.519, p < 0.001). A significant effect of Time (baseline (session 1), post-manipulation (session 3), 2 weeks, 3 months, 6 months, 9 months) was observed, indicating reduction in symptom severity across the study, but no effects of Group nor interaction (see Table 3). Despite significant variance in the Time effect: (σ2 = 8.178, SE = 3.617, Z = 2.261, p = 0.024), modelling Time as a random effect worsened BIC- assessed model-fit (3069.464 → 3072.779) and did not alter interpretation of any model terms. Bayes Factors (see supplement for calculation) provided substantial evidence in favour of the null hypothesis of no Group × Time effect (BF01 = 83). Bayes Factors calculated at follow-up time points for ANOVA across groups and for Welch’s t-tests contrasting RIT vs. sham (with the alternative hypothesis of lower BES scores in the RIT conditions), similarly provided evidence in favour of no differences post-intervention, but the latter were uninformative at subsequent time-points owing to reduced sample size. BF01s for these contrasts are given in Table 3.
ED symptomatology, craving and food addiction
FCQ total scores also reduced across Time from baseline to all subsequent time points [F(5,107.26) = 22.719, p < 0.001]. However, the Group × Time interaction was non-significant [F(10,107.351) = 0.759, p = 0.667]. Despite significant variance in the Time slopes (z = 3.178, p = 0.001), treating Time as a random effect worsened overall model fit. Similarly, negative binomial GLMM on YFAS symptom count scores showed an effect of Time [F(4,133) = 2.468, p = 0.048], indicating a reduction in food addiction-like symptoms from baseline to all follow-up time points (all ts ≥ 2.066, ps ≤ 0.039). The uncontrolled eating subscale of the TFEQ paralleled these effects, with significant reductions across time from Baseline to all subsequent time points [F(5,104.368) = 7.663, p < 0.001]. However, no significant Group × Time interaction was observed [F(10,104.382) = 1.2, p = 0.299]. Measures of disordered eating symptomatology were thus highly consistent in their pattern and supported a lack of Group effects.
Binge frequency
Poisson GLMM found a significant main effect of Time was found [F(5,84) = 16.149, p < 0.001]. This represented a reduction in binge episodes from baseline to all subsequent time points in all groups. No significant effects of Group [F(2,94) = 0.028, p = 0.972] or Group × Time interaction were found [F(10,85) = 0.862, p = 0.572]. The same pattern of results was found when modelling mean daily binge calories (using a gamma GLMM with log link). The intervention thus had no differential impact on bingeing behaviour (Table 4). Bayes factors favoured the null in one-way ANOVA at each time point and favoured no difference, or were inconclusive, for t-tests between RIT and sham (see Table 4).
Discussion
Learned cognitive biases have been posited to be an important factor in maintaining binge eating behaviour66 and a prime target for therapeutic intervention. This study sought to examine the possible augmentation of therapeutic efficacy of food response inhibition training (RIT) via putative ‘reconsolidation-update’ mechanisms in sub-clinical binge eating young adults. Participants generally showed robust reductions across the spectrum of maladaptive binge behaviours assessed. However, we found very little evidence for beneficial effects of RIT on either short-term indices of response biases (Go/No-Go and visual probe and cue reactivity), nor any clinically-relevant measures of eating disorder symptomatology (binge episodes, BES, YFAS). Equally, a retrieval procedure designed to elicit memory destabilisation prior to bias retraining produced minimal augmentation of subsequent RIT effects.
Despite evident bingeing behaviour and cognitive symptomatology, our sample displayed extremely high performance accuracy on the Go/No-Go task, indicating relatively little in the way of premorbid response inhibition deficits to binge cues and possibly restricting the potential impact RIT a priori . Via signal detection analysis, we observed significant, albeit modest, ‘go’ biases to all food stimuli (both HPF/binge foods and LPF/non-binge foods) and automatic visual attentional capture by HPFs in eye-tracking metrics. We found greater reductions in response bias on the Go/No-Go task in BMR + CBM, but insufficient evidence that these differences were substantively related to eating behaviour.
While promising short-to-medium-term effects of food inhibitory control training have previously been seen in laboratory studies in ‘healthy volunteers’43,67,68 and ‘obese’ individuals (primary researchers’ own terms)69, both this and recent research has observed modest effects in the majority of tested longer-term clinical endpoints and eating behaviour in disordered eating groups46,70,71,72,73. These inconsistencies may be due to different effects across (dis)ordered eating populations, focus on short-term (lab-based) vs. lasting effects and training parameters and control procedures, which have been shown to be key determinates of food response inhibition train effects45.
We adapted the Go/No-Go task which effectively reduced chocolate ‘go’ bias and consumption in previous research42. It is possible that the greater diversity of high-palatability food (HPF) stimuli used here were less evocative of response biases than chocolate-only stimuli, producing more heterogeneous responses. However, the HPF stimuli in our study were individualised based on idiosyncratic ratings of the most rewarding images from a pool of food images with high normative ratings for reward value74. Reactivity to these images should therefore be as high as could be expected within the bounds of an experimental setting. Eye-tracking data confirmed that HPF stimuli were salient, robustly inducing automatic visual orienting75 to a greater extent than low palatability foods, an index predictive of actual food intake76.
A more compelling explanation for the disparate findings is the inflation of previous studies’ effects by suboptimal choice of (or lack of) control for inhibitory training procedures34. Earlier studies on RIT in chocolate consumption employed a ‘control’ condition that pairs chocolate images with ‘go’ responses. This ‘go control’ is not inert, in that it may increase approach bias to chocolate images and maximise the apparent effect of RIT by artificially inflating the difference between conditions. Indeed, studies using such ‘opposing control’ conditions tend to show significant effects, whereas those using true ‘sham’ training, (50/50 Go/No-Go) do not78,79 but see Ref, an effect verified experimentally by Adams et al.45. As ‘food → go’ training may worsen overeating symptoms, it is not a clinically viable option as a control condition. For further rationale on for the sham CBM used here, see the Supplementary Information. Despite this, some studies have found positive effects with appropriate ‘sham’ controls.
Most studies on RIT employ immediate or 24-h post tests and it is possible that the 1-week delay between training and test we used prevented us from observing any immediate effects of training. However, we have observed effects in harmful drinkers from a similarly brief, single post-retrieval interventions that have been evident at 1-week and at least 9-months afterwards. If RIT effects are only observable in laboratory measures and at short latencies, we must question the comparative clinical utility of such an approach.
Clearly, null findings with a specific form of CBM (RIT) here do not preclude possible effects of other CBM modalities, such as approach bias modification, which, at least within the domain of AUD, have shown more consistent clinical effects. It is possible that alternative forms of CBM would have been more effective than the RIT used in the current study. The relative efficacy of these different modalities in changing maladaptive eating behaviour remains an open question in need of assessment. However, an examination of experimental evidence published since pre-registering this study and collecting the data (https://osf.io/hjtw3) indicates that this pattern of inconsistent findings is not specific to RIT, but is reflected in the broader literature examining CBM in binge and over-eating46,71,80,81, calling us to question the key moderators and potential therapeutic impact of cognitive bias modification. More robust effects of CBM generally, have been found for alcohol use disorders82, suggesting effects may be reward-domain specific. Indeed, although the authors of a recent narrative review concluded favourably for CBM across reward domains78, evidence for its efficacy in modifying eating behaviour has been questioned by authors of primary studies79 who note inconsistent findings and inappropriate CBM control groups. As a whole, therefore, the field would benefit from more consistent and well-controlled task design, larger randomised controlled studies with longer-term follow-up and direct assessment of the relative efficacy of different CBM modalities across different reward domains.
Limitations
The aim of this study was to assess whether RIT efficacy could be catalysed by conducting retraining following ‘reactivation’ of maladaptive food reward memory, as we have shown for behavioural and drug interventions48,50. We did not find evidence of such effects, aside from in short-lived Go/No-Go task performance interpreting this as a reconsolidation-update effect would be tenuous. However, demonstrating therapeutic enhancement via maladaptive memory reminders is dependent upon a memory-targeting intervention having a minimum of standalone efficacy. Since CBM was largely ineffective, even in the short term (in-lab) measures of responding collected here, we are unable to make any conclusions as to whether food reward memories were successfully destabilised our retrieval procedure nor whether this could confer additive benefit in longer-term clinical outcomes to a standalone behavioural therapy for binge eating. Multiple sessions of training could be used, although one of the great appeals of a putative reconsolidation-based therapy is its single-shot nature.
Binge-eating individuals frequently already engage in compensatory strategies to regulate their weight and minimise binge episodes, including effortful inhibition of food approach, and avoidance of ‘trigger’ foods. Our eye tracking data support this notion. The complex relationship that binge eating individuals have with binge foods thus entails reward and approach, but also avoidance, self-criticism and shame84 following bingeing. If binge-eating individuals are already well-practiced in trigger food avoidance strategies, the potential for added efficacy of brief avoidance training may have been limited a priori. Identifying target sub-groups with high levels of baseline response bias may yield greater effects of retraining. While we have found minimal evidence in the current study to recommend RIT as a clinical intervention in binge eating, given the relative ease of its implementation (e.g. via smartphone apps), limited potential for harm when constructed correctly and potential to orient more attention to one’s eating behaviours and related cognitions, there may be a rationale to recommend pursuing RIT approaches in these groups.
Our study sample were not receiving treatment and binge eating behaviour was primarily assessed via self-report instruments, which may be considered sub-optimal. However, it was not our intention to diagnose binge eating in this study and we focussed upon adolescent sub-clinical binge eaters as a group in whom preventative, low intensity interventions might be usefully employed. The existence of the relevant disordered eating behaviours was further triangulated against other disordered eating measures and food logs. Regarding these, one reviewer noted that MyFitnessPal usage is prevalent among disordered eating populations85 and on pro-eating disorder forums, questioning the ethics of it use in the current setting. While there is no current evidence for a causal link between MyFitnessPal usage and eating disorders and the reductions in eating disorder symptomatology across all groups in the current study suggest it was not a cause of harm, future studies may instead wish to use recovery-focussed apps, such as Recovery Record.
Strengths
We employed a highly rigorous randomised, pre-registered design including more appropriate control procedures than some previous studies, and comprehensive assessment of both short-term target cognitive processes and long-term eating behaviour and disorder symptomatology, with a follow- up period considerably longer than prior research. This allows us to fairly comprehensively reject the possibility of lasting intervention efficacy over a clinically-relevant timeframe. Doing so, we found no evidence for a lasting beneficial effect of inhibitory control training, either alone or when combined with pre-training maladaptive memory retrieval.
Data availability
Study data are available upon request from Ravi Das.
References
Goldschmidt, A. B., Wall, M. M., Zhang, J., Loth, K. A. & Neumark-Sztainer, D. Overeating and binge eating in emerging adulthood: 10-Year stability and risk factors. Dev. Psychol. 52, 475–483 (2016).
Marzilli, E., Cerniglia, L. & Cimino, S. A narrative review of binge eating disorder in adolescence: Prevalence, impact, and psychological treatment strategies. Adolesc. Health. Med. Ther. 9, 17–30 (2018).
Rosenbaum, D. L. & White, K. S. The relation of anxiety, depression, and stress to binge eating behavior. J. Health Psychol. 20, 887–898 (2015).
Araujo, D. M. R., Santos, G. F. D. S. & Nardi, A. E. Binge eating disorder and depression: A systematic review. World J. Biol. Psychiatry 11, 199–207 (2010).
Mitchell, J. E. Medical comorbidity and medical complications associated with binge-eating disorder. Int. J. Eat. Disord. 49, 319–323 (2016).
Kessler, R. C. et al. The prevalence and correlates of binge eating disorder in the World Health Organization world mental health surveys. Biol. Psychiatry 73, 904–914 (2013).
Johnson, J. G., Spitzer, R. L. & Williams, J. B. W. Health problems, impairment and illnesses associated with bulimia nervosa and binge eating disorder among primary care and obstetric gynaecology patients. Psychol. Med. 31, 1455–1466 (2001).
Tanofsky-Kraff, M. et al. Children’s binge eating and development of metabolic syndrome. Int. J. Obes. 36, 956–962 (2012).
Wilson, G. T. & Shafran, R. Eating disorders guidelines from NICE. Lancet 365, 79–81 (2005).
Eddy, K. T. et al. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J. Clin. Psychiatry 78, 184–189 (2017).
Hilbert, A. et al. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J. Consult. Clin. Psychol. 87, 91–105 (2019).
Schmidt, U. et al. Eating disorders: The big issue. Lancet Psychiatry 3, 313–315 (2016).
Smith, D. G. & Robbins, T. W. The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biol. Psychiatry 73, 804–810 (2013).
Davis, C. Compulsive overeating as an addictive behavior: Overlap between food addiction and binge eating disorder. Curr. Obes. Rep. 2, 171–178 (2013).
Davis, C. A commentary on the associations among ‘food addiction’, binge eating disorder, and obesity: Overlapping conditions with idiosyncratic clinical features. Appetite 115, 3–8 (2017).
May, J., Andrade, J., Kavanagh, D. J. & Hetherington, M. Elaborated intrusion theory: A cognitive-emotional theory of food craving. Curr. Obes. Rep. 1, 114–121 (2012).
Fortuna, J. L. The obesity epidemic and food addiction: Clinical similarities to drug dependence. J. Psychoact. Drugs 44, 56–63 (2012).
Hebebrand, J. et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 47, 295–306 (2014).
Ziauddeen, H. & Fletcher, P. C. Is food addiction a valid and useful concept?. Obes. Rev. 14, 19–28 (2013).
Wiss, D. A. & Avena, N. M. Food addiction, binge eating, and the role of dietary restraint: Converging evidence from animal and human studies. In Binge Eating 193–209 (Springer, 2020).
Linardon, J. The relationship between dietary restraint and binge eating: Examining eating-related self-efficacy as a moderator. Appetite 127, 126–129 (2018).
Burton, A. L. & Abbott, M. J. Processes and pathways to binge eating: Development of an integrated cognitive and behavioural model of binge eating. J. Eat. Disord. 7, 1–9 (2019).
Leslie, M., Turton, R., Burgess, E., Nazar, B. P. & Treasure, J. Testing the addictive appetite model of binge eating: The importance of craving, coping, and reward enhancement. Eur. Eat. Disord. Rev. 26, 541–550 (2018).
Wilson, D. R., Loxton, N. J., O’Shannessy, D., Sheeran, N. & Morgan, A. Similarities and differences in revised reinforcement sensitivities across eating disorder subtypes. Appetite 133, 70–76 (2019).
Fairburn, C. G., Cooper, Z. & Shafran, R. Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behav. Res. Ther. 41, 509–528 (2003).
Zilberter, T. Food addiction and obesity: Do macronutrients matter?. Front. Neuroenergetics 10, 7 (2012).
Gómez-A, A., Shnitko, T. A., Caref, K. L., Nicola, S. M. & Robinson, D. L. Stimuli predicting high-calorie reward increase dopamine release and drive approach to food in the absence of homeostatic need. Nutr. Neurosci. 108, 1–10 (2020).
Schulte, E. M., Avena, N. M. & Gearhardt, A. N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One 10, e0117959 (2015).
Gearhardt, N. A., Davis, C., Kuschner, R. & Brownell, D. K. The addiction potential of hyperpalatable foods. Curr. Drug Abus. Rev. 4, 140–145 (2011).
Berridge, K. C. ‘Liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 97, 537–550 (2009).
Popien, A., Frayn, M., von Ranson, K. M. & Sears, C. R. Eye gaze tracking reveals heightened attention to food in adults with binge eating when viewing images of real-world scenes. Appetite 91, 233–240 (2015).
Schmidt, R., Lüthold, P., Kittel, R., Tetzlaff, A. & Hilbert, A. Visual attentional bias for food in adolescents with binge-eating disorder. J. Psychiatr. Res. 80, 22–29 (2016).
Wiers, R. W. et al. Cognitive motivational processes underlying addiction treatment. In Addiction (eds. Kopetz, C. E. & Lejuez, C. W.) 201–236 (Psychology Press, 2016).
Schmitz, F. & Svaldi, J. Effects of bias modification training in binge eating disorder. Behav. Ther. 48, 707–717 (2017).
Kavanagh, D. J., Andrade, J. & May, J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychol. Rev. 112, 446 (2005).
Townshend, J. & Duka, T. Attentional bias associated with alcohol cues: Differences between heavy and occasional social drinkers. Psychopharmacology 157, 67–74 (2001).
Turton, R., Chami, R. & Treasure, J. Emotional eating, binge eating and animal models of binge-type eating disorders. Curr. Obes. Rep. 6, 217–228 (2017).
Kakoschke, N., Kemps, E. & Tiggemann, M. Combined effects of cognitive bias for food cues and poor inhibitory control on unhealthy food intake. Appetite 87, 358–364 (2015).
Treasure, J., Leslie, M., Chami, R. & Fernández-Aranda, F. Are trans diagnostic models of eating disorders fit for purpose? A consideration of the evidence for food addiction. Eur. Eat. Disord. Rev. 26, 83–91 (2018).
Stice, E., Lawrence, N. S., Kemps, E. & Veling, H. Training motor responses to food: A novel treatment for obesity targeting implicit processes. Clin. Psychol. Rev. 49, 16–27 (2016).
Veling, H., van Koningsbruggen, G. M., Aarts, H. & Stroebe, W. Targeting impulsive processes of eating behavior via the internet. Effects on body weight. Appetite 78, 102–109 (2014).
Houben, K. & Jansen, A. Chocolate equals stop. Chocolate-specific inhibition training reduces chocolate intake and go associations with chocolate. Appetite 87, 318–323 (2015).
Lawrence, N. S. et al. Training response inhibition to food is associated with weight loss and reduced energy intake. Appetite 95, 17–28 (2015).
Martinsen, K. D. et al. Prevention of anxiety and depression in school children: Effectiveness of the transdiagnostic EMOTION program. J. Consult. Clin. Psychol. 87, 212–219 (2019).
Adams, R. C., Lawrence, N. S., Verbruggen, F. & Chambers, C. D. Training response inhibition to reduce food consumption: Mechanisms, stimulus specificity and appropriate training protocols. Appetite 109, 11–23 (2017).
Turton, R. et al. To Go or Not to Go: A proof of concept study testing food-specific inhibition training for women with eating and weight disorders. Eur. Eat. Disord. Rev. 26, 11–21 (2018).
Lee, J. L. C. Reconsolidation: Maintaining memory relevance. Trends Neurosci. 32, 413–420 (2009).
Das, R. K. et al. Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nat. Commun. 10, 5187 (2019).
Das, R. K., Lawn, W. & Kamboj, S. K. Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation. Transl. Psychiatry 5, e645–e645 (2015).
Gale, G. et al. Long-term behavioural rewriting of maladaptive drinking memories via reconsolidation-update mechanisms. Psychol. Med. 108, 1–11 (2020).
Xue, Y.-X. et al. Effect of selective inhibition of reactivated nicotine-associated memories with propranolol on nicotine craving. JAMA Psychiat. 74, 224–232 (2017).
Hon, T., Das, R. K. & Kamboj, S. K. The effects of cognitive reappraisal following retrieval-procedures designed to destabilize alcohol memories in high-risk drinkers. Psychopharmacology 233, 851–861 (2016).
Gormally, J., Black, S., Daston, S. & Rardin, D. The assessment of binge eating severity among obese persons. Addict. Behav. 7, 47–55 (1982).
Stice, E., Telch, C. F. & Rizvi, S. L. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol. Assess. 12, 123–131 (2000).
Stice, E., Fisher, M. & Martinez, E. Eating disorder diagnostic scale: Additional evidence of reliability and validity. Psychol. Assess. 16, 60 (2004).
Gearhardt, A. N., Corbin, W. R. & Brownell, K. D. Preliminary validation of the Yale food addiction scale. Appetite 52, 430–436 (2009).
Lowe, M. R. et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 53, 114–118 (2009).
Rodríguez, S. et al. Adaptation of the food-craving questionnaire trait for the assessment of chocolate cravings: Validation across British and Spanish Women. Appetite 49, 245–250 (2007).
Beck, A. T., Steer, R. A. & Carbin, M. G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 8, 77–100 (1988).
Patton, J. H., Stanford, M. S. & Barratt, E. S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774 (1995).
Sobell, L. C. & Sobell, M. B. Timeline follow-back. In Measuring Alcohol Consumption 41–72 (Springer, 1992). https://doi.org/10.1007/978-1-4612-0357-5_3.
Meule, A. & Platte, P. Attentional bias toward high-calorie food-cues and trait motor impulsivity interactively predict weight gain. Health Psychol. Open 3, 2055102916649585 (2016).
Houben, K., Nederkoorn, C., Wiers, R. W. & Jansen, A. Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 116, 132–136 (2011).
Das, R. K., Gale, G., Hennessy, V. & Kamboj, S. K. A Prediction error-driven retrieval procedure for destabilizing and rewriting maladaptive reward memories in hazardous drinkers. J. Vis. Exp. 131, e56097–e56097. https://doi.org/10.3791/56097 (2018).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Voon, V. Cognitive biases in binge eating disorder: The hijacking of decision making. CNS Spectr. 20, 566–573 (2015).
Jones, A. et al. Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite 97, 16–28 (2016).
Allom, V., Mullan, B. & Hagger, M. Does inhibitory control training improve health behaviour? A meta-analysis. Health Psychol. Rev. 10, 168–186 (2016).
Stice, E., Yokum, S., Veling, H., Kemps, E. & Lawrence, N. S. Pilot test of a novel food response and attention training treatment for obesity: Brain imaging data suggest actions shape valuation. Behav. Res. Ther. 94, 60–70 (2017).
Preuss, H., Pinnow, M., Schnicker, K. & Legenbauer, T. Improving Inhibitory Control Abilities (ImpulsE)—A promising approach to treat impulsive eating?. Eur. Eat. Disord. Rev. 25, 533–543 (2017).
Giel, K. E., Speer, E., Schag, K., Leehr, E. J. & Zipfel, S. Effects of a food-specific inhibition training in individuals with binge eating disorder—Findings from a randomized controlled proof-of-concept study. Eat. Weight Disord. Stud. Anorexia Bulim. Obes. 22, 345–351 (2017).
Chami, R. et al. Targeting binge eating in bulimia nervosa and binge eating disorder using inhibitory control training and implementation intentions: A feasibility trial. Psychol. Med. https://doi.org/10.1017/S0033291720002494 (2020).
Di Rosa, E. et al. Exploring changes in event-related potentials after a feasibility trial of inhibitory training for bulimia nervosa and binge eating disorder. Front. Psychol. https://doi.org/10.3389/fpsyg.2020.01056 (2020).
Blechert, J., Meule, A., Busch, N. A. & Ohla, K. Food-pics: An image database for experimental research on eating and appetite. Front. Psychol. 5, 15037–15042 (2014).
Volkow, N. D., Wang, G. J., Fowler, J. S., Tomasi, D. & Telang, F. The capture of attention by entirely irrelevant pictures of calorie-dense foods. Proc. Natl. Acad. Sci. U.S.A. 108, 15037–15042 (2011).
Folkvord, F., Anschütz, D. J., Wiers, R. W. & Buijzen, M. The role of attentional bias in the effect of food advertising on actual food intake among children. Appetite 84, 251–258 (2015).
Schumacher, S. E., Kemps, E. & Tiggemann, M. Bias modification training can alter approach bias and chocolate consumption. Appetite 96, 219–224 (2016).
Kakoschke, N., Kemps, E. & Tiggemann, M. Approach bias modification training and consumption: A review of the literature. Addict. Behav. 64, 21–28 (2017).
Becker, D., Jostmann, N. B. & Holland, R. W. Does approach bias modification really work in the eating domain? A commentary on Kakoschke et al (2017). Addict. Behav. 77, 293–294 (2018).
Brockmeyer, T., Hahn, C., Reetz, C., Schmidt, U. & Friederich, H.H.-C. Approach bias modification in food craving—A proof-of-concept study. Eur. Eat. Disord. Rev. 23, 352–360 (2015).
Brockmeyer, T. et al. Approach bias modification training in bulimia nervosa and binge-eating disorder: A pilot randomized controlled trial. Int. J. Eat. Disord. 52, 520–529 (2019).
Rinck, M., Wiers, R. W., Becker, E. S. & Lindenmeyer, J. Relapse prevention in abstinent alcoholics by cognitive bias modification: Clinical effects of combining approach bias modification and attention bias modification. J. Consult. Clin. Psychol. 86, 1005–1016 (2018).
Eberl, C. et al. Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best?. Dev. Cogn. Neurosci. 4, 38–51 (2013).
Serpell, L., Amey, R. & Kamboj, S. K. The role of self-compassion and self-criticism in binge eating behaviour. Appetite 144, 104470 (2020).
Levinson, C. A., Fewell, L. & Brosof, L. C. My Fitness Pal calorie tracker usage in the eating disorders. Eat. Behav. 27, 14–16 (2017).
Funding
This work was supported by the Medical Research Council [Grant number: MR/R004919/1].
Author information
Authors and Affiliations
Contributions
R.K.D., U.S., R.W. and S.K.K. secured funding for the research, R.K.D and S.K.K. designed the research protocol, E.C., L.S. and G.P. collected and prepared the data, R.K.D. analysed the data and wrote the manuscript, U.S., S.K.K. and R.W. edited the manuscript and advised on study design. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das, R.K., Cawley, E.A., Simeonov, L. et al. The effects of response inhibition training following binge memory retrieval in young adults binge eaters: a randomised-controlled experimental study. Sci Rep 12, 9281 (2022). https://doi.org/10.1038/s41598-022-12173-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12173-w
- Springer Nature Limited