Abstract
Deep vein thrombosis (DVT) refers to the abnormal coagulation of blood in a deep vein. Recently, some studies have found that metabolites are related to the occurrence of DVT and may serve as new markers for the diagnosis of DVT. In this study, we used the GWAS summary dataset of blood metabolites and DVT to perform a large-scale genetic correlation scan of DVT and blood metabolites to explore the correlation between blood metabolites and DVT. We used GWAS summary data of DVT from the UK Biobank (UK Biobank fields: 20002) and GWAS summary data of blood metabolites from a previously published study (including 529 metabolites in plasma or serum from 7824 adults from two European population studies) for genetic correlation analysis. Then, we conducted a causal study between the screened blood metabolites and DVT by Mendelian randomization (MR) analysis. In the first stage, genetic correlation analysis identified 9 blood metabolites that demonstrated a suggestive association with DVT. These metabolites included Valine (correlation coefficient = 0.2440, P value = 0.0430), Carnitine (correlation coefficient = 0.1574, P value = 0.0146), Hydroxytryptophan (correlation coefficient = 0.2376, P value = 0.0360), and 1-stearoylglycerophosphoethanolamine (correlation coefficient = − 0.3850, P value = 0.0258). Then, based on the IVW MR model, we analysed the causal relationship between the screened blood metabolites and DVT and found that there was a suggestive causal relationship between Hydroxytryptophan (exposure) and DVT (outcome) (β = − 0.0378, se = 0.0163, P = 0.0204). Our study identified a set of candidate blood metabolites that showed a suggestive association with DVT. We hope that our findings will provide new insights into the pathogenesis and diagnosis of DVT in the future.
Similar content being viewed by others
Introduction
Deep vein thrombosis (DVT) refers to the abnormal coagulation of blood in a deep vein. It leads to the obstruction of blood reflux and causes lower extremity oedema and even pulmonary embolism1,2. In addition, DVT is an important complication of several inherited and acquired diseases, but it can also occur spontaneously3. Currently, there are many methods for the diagnosis of DVT, such as d-dimer and ultrasound (US) imaging, that have been adopted to aid in the diagnosis of DVT pathways4. Recently, some studies have found that metabolites are related to the occurrence of DVT and may be new markers for the diagnosis of DVT5.
The metabolome is defined as the collection of metabolites and small molecules that are involved in cell metabolism. They are produced in cells and can be divided into many categories6. Approximately 50% of the total phenotypic variation in metabolite levels is due to SNP, but estimates of heritability vary by metabolite class7. Genomic and metabolomic analyses of common SNPs in human metabolism have successfully identified the metabolites affected by genetics8. The elucidation of the genetic mechanism of metabolism may provide new therapeutic targets or new biomarkers for disease diagnosis9. Among them, metabolism in human blood is controlled by different degrees of genetic effects, complex regulatory effects and nongenetic effects10.
The genetic control of metabolite levels and their impact on human health is evident in inborn metabolic errors. In these errors, rare SNPs disrupt individual genes and then lead to extreme and ultimately toxic levels of the related metabolites8. Genome-wide association studies with metabolomics (mGWAS) that use population-scale metabolomics and genotypic data can systematically study the less obvious effects of more common and less harmful SNPs on human metabolism. This was demonstrated by Gieger et al. in the first mGWAS11.
Genetic correlation is an important population parameter that can describe the genetic relationships between two traits12,13,14. Using summary data from genome-wide association studies (GWAS), LDSC can screen for thousands of traits simultaneously and find genetic correlations between them13. Mendelian randomization (MR) refers to studies in observational epidemiology that use SNP to make causal inferences about risk factors for disease and health-related outcomes15,16. MR analysis presents a valuable tool, especially when randomized controlled trials to examine causality are not feasible and observational studies provide biased associations because of confounding or reverse causality. These issues are addressed by using genetic variants as instrumental variables for the tested exposure: the alleles of this exposure-associated genetic variant are randomly allocated and not subject to reverse causation17. Previous studies have used LDSC and MR to analyse the association between inflammation pathway and suicide and found that IL-6 signalling is associated with suicide18. Thus, we hope to use LDSC and MR analysis to further dissect the association between blood metabolites and DVT.
In this study, for the first time, we used a large-scale GWAS summary dataset of blood metabolites and DVT to perform a genetic correlation scan of DVT and blood metabolites to explore the genetic relationship between blood metabolites and DVT. Our study has the potential to provide new insights into the genetic mechanisms, diagnosis and treatment of DVT.
Methods
GWAS summary datasets of DVT
The GWAS summary data of DVT used in this study were obtained from the UK Biobank (UK Biobank fields: 20002)19. The DVT cases in the UK Biobank were defined based on self-reported diagnosis. The UK Biobank was a large prospective cohort study involving approximately 500,000 people aged 37 to 76 years (99.5% aged 40 to 69 years) from across the UK. This cohort included 9059 DVT patients and 443,205 control cases20. The UK Biobank has received ethical approval from the Northwest Multicentre Research Ethics Committee and the informed consent of all participants. All participants provided a range of information on their health status, demographics and lifestyle through questionnaires and interviews19. Detailed information on the samples, imputation and genotyping can be found in previously published studies19.
GWAS summary datasets of human blood metabolites
A previously published large-scale GWAS dataset of human blood metabolites was used here10. The study sample included 529 metabolites in the plasma or serum from 7824 adults from two European population studies10. More than half of the 529 metabolites (N = 333, 63%) can be chemically identified as 8 metabolic groups (amino acids, carbohydrates, cofactors and vitamins, energy, fat, nucleotides, peptides and xenobiotics)10. After strict quality control, there were 486 subsets of metabolites available for genetic analysis, including 309 known metabolites and 177 unknown metabolites10. Detailed descriptions of the quality control, sample characteristics, research design and statistical analysis can be found in previously published studies10.
Statistical analysis
Referring to the methods recommended by the developers13,14 and previous studies21, we used LDSC software (v1.0.0; https://github.com/bulik/ldsc) to analyse the genetic correlation between each blood metabolite and DVT. The basic principle of the LDSC method is to estimate the deviation between the χ2 test statistics of an SNP and its expected values directly from the GWAS summary data under the null hypothesis of no association22. The study used the European LD score, which was calculated by the developers from 1000 genomes23. After correcting for multiple testing, the significance threshold of this study should be P < 9.45 × 10−5 (0.05/529 = 9.45 × 10−5). Since few metabolites reached the significance level after multiple test corrections, P < 0.05 was adopted as the suggested significance level.
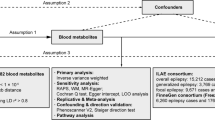
MR analysis can be used to evaluate the causal relationship between blood metabolites and DVT. The MR technique uses SNPs related to modifiable traits/exposures as tools to detect causal associations among the outcomes15. In an MR test, three key assumptions must be met: (1) the SNP is directly associated with the exposure; (2) the SNP is not related to factors known to obscure the connection between the exposure and the outcome; and (3) the SNP has no effect on outcome. The inverse-variance weighted (IVW) method uses a meta-analysis approach to combine the Wald ratio estimates of the causal effect obtained from different SNPs and to provide a consistent estimate of the causal effect of the exposure on the outcome when each of the SNPs satisfies the assumptions of an instrumental variable24. Egger regression is a tool to detect small study bias in meta-analysis and can be adapted to test for bias from pleiotropy. The slope coefficient from Egger regression provides an estimate of the causal effect25. The weighted median estimate provides a consistent estimate of causal effects, even if up to 50 percent of the analytical information comes from SNP of ineffective IVs26. In addition, MR-Egger and weighted median can be used as sensitivity analysis. Particularly, MR-Egger and weighted median could provide a more valid MR estimates if their assumptions are met and if multiple SNPs are pleiotropic. To test whether there was a weak instrumental variable bias, namely genetic variants selected as instrumental variables had a weak association with exposure, we calculated the F statistic (F = R2(n − k − 1)/k(1 − R2); R2, variance of exposure explained by selected instrumental variables, and we got the value of R2 in MR Steiger directionality test; n, sample size; and k, number of instrumental variables). If the F statistic is much greater than 10 for the instrument-exposure association, the possibility of weak instrumental variable bias is small.
In the absence of pleiotropy, the IVW estimator is the gold standard method. The main analysis was IVW, and the sensitivity analysis was the Leave-one-out test. When the effect SNP of the metabolite is more than 3, we test the MR results by Leave-one-out test. We used the Wald estimator when there is only one instrument available, the IVW method when at least two instruments were selected, and the IVW, the MR-Egger, and the weighted median methods with more than 3 instruments.
We used the MR basic platform (http://app.mrbase.org/) to analyse the causal relationship between the screened blood metabolites and DVT. After correcting for multiple testing, the significance threshold of this study should be P < 0.005 (0.05/9 = 0.005). Since few metabolites reached the significance level after multiple test corrections, P < 0.05 was adopted as the suggested significance level.
All methods were performed in accordance with the relevant guidelines and regulations (for example—Declarations of Helsinki).
Results
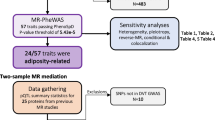
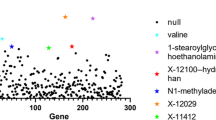
In the first stage, genetic correlation analysis identified 9 suggestive blood metabolites that were significantly associated with DVT (Fig. 1, Table 1), including Valine, Carnitine, Hydroxytryptophan, 1-stearoylglycerophosphoethanolamine, X-11317, X-11550, X-12465, X-12644, and X-13741. The LDSC results of those that do not reach the level of significance are shown in the Supplementary Table S1 and Supplementary Table S2.
Detailed flowchart of the result. We analyzed the genetic correlation between DVT and 529 blood metabolites by LDSC, and found that 9 blood metabolites may had genetic correlation with DVT. Then the causal relationship between these 9 blood metabolites and DVT was analyzed by MR, and it was found that 2 blood metabolites had causal correlation with DVT.
We conducted instrumental variable screening strictly in accordance with the three core assumptions of Mendelian randomization analysis. First, SNP must be strongly correlated with exposure factor, so we screened SNPs with P < 5 × 10−8, R2 = 0.001, KB = 10,000. We used the PhenoScanner platform to exclude SNPs associated with DVT and risk factors associated with DVT to show that there is no horizontal pleiotropy in our analysis from a biological point of view. Based on the IVW MR model, we analysed the causal relationship between the screened blood metabolites and DVT and found that there was a suggestive inverse causal relationship between Hydroxytryptophan (exposure) and DVT (outcome) (Table 2, Fig. 2). In addition, we also found that there was a suggestive inverse causal relationship between X-12644 (exposure) and DVT (outcome) (Table 2, Fig. 3). The Egger intercept did not deviate significantly from zero (intercept = 0.0021, SE = 0.0017, P = 0.423). Thus, there was no evidence for unbalanced pleiotropy, which would suggest that the IVW estimates were unbiased. However, the results of the MR–Egger and weighted median methods did not support this conclusion (MR–Egger: β = − 0.1452, se = 0.0889, P = 0.3498; weighted median: β = − 0.0265, se = 0.0146, P = 0.0694). In addition, the difference between Q and Q′ (Q − Q′ = 0.2327) is not sufficiently extreme under a χ12 distribution, which means that the MR–Egger model does not fit our data better than the IVW model. Because some metabolites included fewer instruments, we evaluated the instruments included in Table 3. According to the above results, we found that the F values of the instrumental variables included in X-12644 and Hydroxytryptophan were all more than 10, which effectively reduce the likelihood of weak instrument bias. In addition, sensitivity analysis was performed on the results (leave-one-out test). As the other 7 blood metabolites were included in fewer instruments, the leave-one-out test could not be used to test them. Only Carnitine and X-12644 were subjected to the leave-one-out method for sensitivity analysis. The results are shown in Fig. 4. According to the result of the leave-one-out test, we found that the MR analysis of Carnitine was stable, while the MR analysis of X-12644 was not. (Fig. 4).
Then, we performed a reverse MR analysis. However, when DVT was used as the exposure variable, we found no causal relationship between DVT and 9 blood metabolites, as shown in Table 4.
Discussion
DVT is a disease that is affected by many factors and is caused by the interaction of a series of acquired and hereditary risk factors27. Major hereditary thrombotic diseases include a lack of natural anticoagulants, antithrombin and proteins C and S in plasma28. The clinical diagnosis of DVT mainly depends on clinical symptoms, d-dimer levels and ultrasonic examination29,30. Although the plasma level of d-dimer is highly sensitive, its deletion may help to exclude DVT31. However, d-dimer levels are easily altered by cancer, surgery and other factors, so its specificity and positive predictive value are very low32. Therefore, more appropriate biomarkers are needed to conduct appropriate risk assessment for the occurrence of DVT to improve the sensitivity of DVT diagnosis, timely treatment or avoid invasive surgery. As an important part of systems biology33, metabonomics has been widely used in the study of pathogenesis-related diagnostic and prognostic biomarkers by detecting endogenous small molecule compounds in biological samples34,35. Recent studies have found that glycolysis, purines and redox-related metabolites may contribute to the discovery of fresh venous thrombosis, and changes in metabolites may affect the formation of venous thrombosis5. Therefore, we hope to provide new ideas for the diagnosis of DVT and provide insights into the genetic mechanisms of DVT by studying the relationship between blood metabolites and DVT.
To study the relationship between blood metabolites and DVT, we carried out genetic correlation analysis based on the GWAS summary data of blood metabolites and DVT. We found that 9 blood metabolites were genetically correlated with DVT, including 4 known metabolites and 5 unknown metabolites. Then, we analysed the identified blood metabolites and DVT by MR and found that there was an inverse causal relationship between the two blood metabolites and DVT. To our knowledge, this is the first time that a large-scale genetic association between blood metabolites and DVT has been assessed, and our findings may greatly expand the biological knowledge associated with DVT.
Valine, also known as 2-amino-3-methylbutyric acid, is a branched-chain amino acid. It is one of the eight essential amino acids and sugar-producing amino acids of the human body36. It can promote normal growth, repair tissue, regulate blood sugar and provide the necessary energy37. A mutation in the gene for Factor XIII that leads to a Valine-leucine exchange has been reported to be protective against DVT38. Factor XIII (FXIII) is a transglutaminase found in plasma and platelets39. During thrombus formation, activated FXIII cross-links fibrin, which promotes thrombus stability39. Fujimura et al. found that serum samples from patients with DVT showed high levels of Valine40. FXIII cross-links fibrin when thrombin is activated. The activation of thrombin releases activated peptides. A common polymorphism (Valine to leucine variant at residue 34, V34L) that is located in the activating peptide has been found to be associated with the prevention of thrombosis41. However, the specific mechanism by which Valine participates in the prevention of thrombi is still unclear and requires further experimental research.
Carnitine is considered a conditionally essential nutrient because of its importance in human physiology42. The anticoagulant effect of Carnitine is related to the regulation of prostaglandin formation by its derivatives, which can stimulate prostacyclin production43. The cytoprotective and vasodilator effects of prostacyclin are well known. It has been shown that l-Carnitine supplementation reduces serum CRP and plasma fibrinogen levels in haemodialysis patients44. Supplementation with l-Carnitine reduces inflammatory substances, such as CRP, IL-6, and TNF-α, and increases oxidative stress levels45. Deguchi et al. confirmed that there is a low level of acyl Carnitine in the plasma of patients with venous thromboembolism. They also showed that acyl Carnitine can act as an anticoagulant because of its ability to bind and inhibit Xa factor46. Our study found a genetic correlation between Carnitine and DVT, which agrees with these previous studies. Moreover, the genetic association between DVT and Carnitine found in our study provides a new direction for further research on how Carnitine affects the pathogenesis of DVT.
For the results of the LDSC, DVT was found to be genetically correlated with Valine and Carnitine. We hold the opinion that these results should be interpreted as Valine and Carnitine being genetically correlated with DVT. However, these two metabolisms may not lead to deep vein thrombosis, and specific directions should be explained by referring to relevant literature. Through a literature search, we found no evidence that Valine and Carnitine may be involved in increasing the incidence of DVT. Instead, we found that these two metabolites are involved in anticoagulation, which needs to be stated.
5-Hydroxytryptophan (5-HTP) is a kind of amino acid. It can be used as a precursor of serotonin (serotonin, 5-HT) in the human body (and then as a precursor of melatonin)47. According to clinical studies, taking 5-HTP can significantly improve the mood of patients with depression48,49. Studies have found that serotonergic antidepressants have a weak anticoagulant effect50. In addition, selective serotonin reuptake inhibitors (SSRIs) inhibit the formation of tight clots of platelets in vitro, which indicates that SSRIs have a direct antithrombotic or fibrinolytic effect51. Serotonin stored in platelets accounts for more than 99% of the total serotonin concentration in the human body. After blood vessel injury and platelet activation, serotonin is released into the bloodstream and binds to specific receptors. It thereby promotes vasoconstriction and platelet aggregation and facilitates haemostasis52. Serotonin reuptake into platelets involves a serotonin transporter that is blocked by the SSRI, thereby inhibiting serotonin reuptake into platelets53. This inhibition in turn reduces the likelihood of platelet agglutination. This then reduces the formation of platelet thrombosis, which in turn increases the risk of bleeding53,54. We found that DVT and 5-HTP were genetically correlated, and 5-HTP and DVT demonstrated reverse causality. However, no studies have determined how 5-HTP is involved in the pathogenesis of DVT. In the analysis results of the LDSC, we found that hydroxyl tryptophan and DVT have a strong genetic correlation, but in patients with DVT, the hydroxyl tryptophan levels may be higher than normal. This is not just because of the disease itself but also because of genetic factors. Thus, it cannot explain why Hydroxytryptophan causes DVT. In contrast, MR analysis found that Hydroxytryptophan was negatively correlated with DVT. Our study provides a new idea for the involvement of 5-HTP in the pathogenesis and diagnosis of DVT.
According to our study, another 6 blood metabolites were found to be genetically correlated with DVT, and among them, X-12644 had a reverse causal relationship with DVT. However, we have not found any studies on the correlation between these blood metabolites and DVT or blood coagulation.
To the best of our knowledge, this is the first large-scale genetic correlation analysis of the blood metabolic groups and DVT. Because we used GWAS gene data, the results are not easily affected by environmental confounding factors. Furthermore, we not only studied the genetic correlation between DVT and blood metabolites but also determined the causal relationship. Of course, some limitations of this research should be noted. First, the significance threshold should be P < 9.45 × 10−5 after multiple test correction. Unfortunately, according to our results, there was no significant genetic correlation at this threshold. Since all blood metabolites identified in this study are suggested to be associated with DVT, the results should be interpreted carefully. Furthermore, it should be noted that the purpose of this study was to evaluate the genetic correlation between blood metabolites and DVT and to scan for new candidate blood metabolites associated with DVT. In this study, further basic research is needed to confirm our findings and to clarify the potential biological mechanism of the observed link between blood metabolites and DVT. Finally, the GWAS summary data of this study are all from European origin. Therefore, we should be careful to apply our research results to other ethnic groups.
Conclusion
In short, by using the LDSC method, we conducted a large-scale analysis to investigate the genetic correlation between blood metabolites and DVT and verified the causal relationship by MR analysis. Our study identified a set of suggestive candidate blood metabolites that showed an association with DVT. We hope that our findings will provide new insights into the pathogenesis and diagnosis of DVT in the future and serve as a basic resource for understanding the genetic mechanism of the effects of blood metabolites on DVT.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The GWAS dataset of DVT is available at GeneATLAS website, http://geneatlas.roslin.ed.ac.uk/. The GWAS dataset of human blood metabolites: http://metabolomics.helmholtz-muenchen.de/gwas/gwas_server/shin_et_al.metal.out.tar.gz.
Abbreviations
- DVT:
-
Deep vein thrombosis
- US:
-
Ultrasound
- LDSC:
-
Linkage disequilibrium score regression
- GWAS:
-
Genome-wide association study
- MR:
-
Mendelian randomization
- IVW:
-
Inverse-variance weighted
- FXIII:
-
Factor XIII
- 5-HTP:
-
5-Hydroxytryptophan
- SSRIs:
-
Selective serotonin reuptake inhibitors
References
Kyrle, P. A. & Eichinger, S. Deep vein thrombosis. Lancet 365, 1163–1174. https://doi.org/10.1016/s0140-6736(05)71880-8 (2005).
Di Nisio, M., van Es, N. & Büller, H. R. Deep vein thrombosis and pulmonary embolism. Lancet 388, 3060–3073. https://doi.org/10.1016/s0140-6736(16)30514-1 (2016).
Lensing, A. W., Prandoni, P., Prins, M. H. & Büller, H. R. Deep-vein thrombosis. Lancet 353, 479–485. https://doi.org/10.1016/s0140-6736(98)04298-6 (1999).
Bernardi, E. & Camporese, G. Diagnosis of deep-vein thrombosis. Thromb. Res. 163, 201–206. https://doi.org/10.1016/j.thromres.2017.10.006 (2018).
Maekawa, K. et al. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: Potential markers for early deep vein thrombosis. Thromb. Res. 177, 136–144. https://doi.org/10.1016/j.thromres.2019.03.011 (2019).
Patti, G. J., Yanes, O. & Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 13, 263–269. https://doi.org/10.1038/nrm3314 (2012).
Nicholson, G. et al. Human metabolic profiles are stably controlled by genetic and environmental variation. Mol. Syst. Biol. 7, 525. https://doi.org/10.1038/msb.2011.57 (2011).
Kastenmüller, G., Raffler, J., Gieger, C. & Suhre, K. Genetics of human metabolism: An update. Hum. Mol. Genet. 24, R93-r101. https://doi.org/10.1093/hmg/ddv263 (2015).
Kuehnbaum, N. L. & Britz-McKibbin, P. New advances in separation science for metabolomics: Resolving chemical diversity in a post-genomic era. Chem. Rev. 113, 2437–2468. https://doi.org/10.1021/cr300484s (2013).
Shin, S. Y. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550. https://doi.org/10.1038/ng.2982 (2014).
Gieger, C. et al. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet. 4, e1000282. https://doi.org/10.1371/journal.pgen.1000282 (2008).
Vonberg, F. W. & Bigdeli, T. B. Genetic correlation between schizophrenia and epilepsy. JAMA Neurol. 73, 125–126. https://doi.org/10.1001/jamaneurol.2015.3480 (2016).
Lee, J. J., McGue, M., Iacono, W. G. & Chow, C. C. The accuracy of LD Score regression as an estimator of confounding and genetic correlations in genome-wide association studies. Genet. Epidemiol. 42, 783–795. https://doi.org/10.1002/gepi.22161 (2018).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295. https://doi.org/10.1038/ng.3211 (2015).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. JAMA 318, 1925–1926. https://doi.org/10.1001/jama.2017.17219 (2017).
Sekula, P., Del Greco, M. F., Pattaro, C. & Kottgen, A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27, 3253–3265. https://doi.org/10.1681/ASN.2016010098 (2016).
Smith, G. D. & Ebrahim, S. “Mendelian randomization”: Can genetic epidemiology contribute to understanding environmental determinants of disease?. Int. J. Epidemiol. 32, 1–22. https://doi.org/10.1093/ije/dyg070 (2003).
Kappelmann, N. et al. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: A genetic correlation and 2-sample mendelian randomization study. JAMA Psychiatr. 78, 161–170. https://doi.org/10.1001/jamapsychiatry.2020.3436 (2021).
Canela-Xandri, O., Rawlik, K. & Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 50, 1593–1599. https://doi.org/10.1038/s41588-018-0248-z (2018).
Cox, N. UK Biobank shares the promise of big data. Nature 562, 194–195. https://doi.org/10.1038/d41586-018-06948-3 (2018).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241. https://doi.org/10.1038/ng.3406 (2015).
Shi, H., Kichaev, G. & Pasaniuc, B. Contrasting the genetic architecture of 30 complex traits from summary association data. Am. J. Hum. Genet. 99, 139–153. https://doi.org/10.1016/j.ajhg.2016.05.013 (2016).
Anney, R. J. L. et al. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 8, 21. https://doi.org/10.1186/s13229-017-0137-9 (2017).
Pierce, B. L. & Burgess, S. Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 178, 1177–1184. https://doi.org/10.1093/aje/kwt084 (2013).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. https://doi.org/10.1093/ije/dyv080 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. https://doi.org/10.1002/gepi.21965 (2016).
Rosendaal, F. R. Venous thrombosis: A multicausal disease. Lancet 353, 1167–1173. https://doi.org/10.1016/s0140-6736(98)10266-0 (1999).
Rosendaal, F. R. & Reitsma, P. H. Genetics of venous thrombosis. J. Thromb. Haemostasis 7(Suppl 1), 301–304. https://doi.org/10.1111/j.1538-7836.2009.03394.x (2009).
Line, B. R. Pathophysiology and diagnosis of deep venous thrombosis. Semin. Nucl. Med. 31, 90–101. https://doi.org/10.1053/snuc.2001.21406 (2001).
Kruger, P. C., Eikelboom, J. W., Douketis, J. D. & Hankey, G. J. Deep vein thrombosis: Update on diagnosis and management. Med. J. Aust. 210, 516–524. https://doi.org/10.5694/mja2.50201 (2019).
Goldhaber, S. Z. & Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 379, 1835–1846. https://doi.org/10.1016/s0140-6736(11)61904-1 (2012).
Wells, P. S. et al. Evaluation of d-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 349, 1227–1235. https://doi.org/10.1056/NEJMoa023153 (2003).
Nicholson, J. K. & Lindon, J. C. Systems biology: Metabonomics. Nature 455, 1054–1056. https://doi.org/10.1038/4551054a (2008).
Rinschen, M. M., Ivanisevic, J., Giera, M. & Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 20, 353–367. https://doi.org/10.1038/s41580-019-0108-4 (2019).
Laíns, I. et al. Metabolomics in the study of retinal health and disease. Prog. Retin. Eye Res. 69, 57–79. https://doi.org/10.1016/j.preteyeres.2018.11.002 (2019).
Bishop, C. A., Schulze, M. B., Klaus, S. & Weitkunat, K. The branched-chain amino acids Valine and leucine have differential effects on hepatic lipid metabolism. FASEB J. 34, 9727–9739. https://doi.org/10.1096/fj.202000195R (2020).
Chen, M., Shi, C., Zhao, J., Gao, Z. & Zhang, C. Application and microbial preparation of d-Valine. World J. Microbiol. Biotechnol. 32, 171. https://doi.org/10.1007/s11274-016-2119-z (2016).
Catto, A. J. et al. Association of a common polymorphism in the factor XIII gene with venous thrombosis. Blood 93, 906–908 (1999).
Byrnes, J. R. & Wolberg, A. S. Newly-recognized roles of factor XIII in thrombosis. Semin. Thromb. Hemost. 42, 445–454. https://doi.org/10.1055/s-0036-1571343 (2016).
Cao, J. et al. Comparison of the serum metabolic signatures based on (1)H NMR between patients and a rat model of deep vein thrombosis. Sci. Rep. 8, 7837. https://doi.org/10.1038/s41598-018-26124-x (2018).
Anwar, R., Gallivan, L., Edmonds, S. D. & Markham, A. F. Genotype/phenotype correlations for coagulation factor XIII: Specific normal polymorphisms are associated with high or low factor XIII specific activity. Blood 93, 897–905 (1999).
Steiber, A., Kerner, J. & Hoppel, C. L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 25, 455–473. https://doi.org/10.1016/j.mam.2004.06.006 (2004).
Ezgü, F. S. et al. Intracranial venous thrombosis after hypoxic-ischemic brain insult in two newborns: Could low serum Carnitine levels have contributed?. Nutr. Neurosci. 7, 63–65. https://doi.org/10.1080/1028415042000194612 (2004).
Hakeshzadeh, F., Tabibi, H., Ahmadinejad, M., Malakoutian, T. & Hedayati, M. Effects of l-Carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren. Fail. 32, 1109–1114. https://doi.org/10.3109/0886022x.2010.510617 (2010).
Fathizadeh, H. et al. The effects of l-Carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 19, 1879–1894. https://doi.org/10.1007/s40200-020-00627-9 (2020).
Deguchi, H. et al. AcylCarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood 126, 1595–1600. https://doi.org/10.1182/blood-2015-03-636761 (2015).
Bader, M. Serotonylation: Serotonin signaling and epigenetics. Front. Mol. Neurosci. 12, 288. https://doi.org/10.3389/fnmol.2019.00288 (2019).
Jacobsen, J. P. R., Krystal, A. D., Krishnan, K. R. R. & Caron, M. G. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: Clinical and preclinical rationale. Trends Pharmacol. Sci. 37, 933–944. https://doi.org/10.1016/j.tips.2016.09.001 (2016).
Javelle, F. et al. Effects of 5-Hydroxytryptophan on distinct types of depression: A systematic review and meta-analysis. Nutr. Rev. 78, 77–88. https://doi.org/10.1093/nutrit/nuz039 (2020).
Hoirisch-Clapauch, S. & Nardi, A. E. Antidepressants: Bleeding or thrombosis?. Thromb. Res. 181(Suppl 1), S23-s28. https://doi.org/10.1016/s0049-3848(19)30362-7 (2019).
Maurer-Spurej, E. Serotonin reuptake inhibitors and cardiovascular diseases: A platelet connection. Cell. Mol. Life Sci. 62, 159–170. https://doi.org/10.1007/s00018-004-4262-1 (2005).
Li, N., Wallén, N. H., Ladjevardi, M. & Hjemdahl, P. Effects of serotonin on platelet activation in whole blood. Blood Coagul. Fibrinolysis 8, 517–523. https://doi.org/10.1097/00001721-199711000-00006 (1997).
Halperin, D. & Reber, G. Influence of antidepressants on hemostasis. Dialogues Clin. Neurosci. 9, 47–59. https://doi.org/10.31887/DCNS.2007.9.1/dhalperin (2007).
Hergovich, N. et al. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin. Pharmacol. Ther. 68, 435–442. https://doi.org/10.1067/mcp.2000.110456 (2000).
Funding
This work was supported by the National Natural Science Foundation of China (82072432, 81772410).
Author information
Authors and Affiliations
Contributions
P.L., J.W.X., S.Q.C. and P.X. wrote the main manuscript text and F.Z., K.X. and W.S.J. prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, P., Xu, J., Cheng, S. et al. Large-scale genetic correlation scanning and causal association between deep vein thrombosis and human blood metabolites. Sci Rep 12, 7888 (2022). https://doi.org/10.1038/s41598-022-12021-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12021-x
- Springer Nature Limited