Abstract
It is unknown whether the thinner slice reconstruction has added value relative to 3 mm reconstructions in predicting major adverse cardiac events (MACEs). This retrospective study included 550 asymptomatic individuals who underwent cardiac CT. Coronary artery calcium (CAC) scores and severity categories were assessed from 1.5 and 3 mm scans. CAC scores obtained from 1.5 and 3 mm scans were compared using Wilcoxon signed-rank tests. Cox proportional hazard models were developed to predict MACEs based on the degree of coronary artery stenosis on coronary CT angiography and the presence of CAC on both scans. Model performances were compared using the time-dependent ROC curve and integrated area under the curve (iAUC) methods. The CAC scores obtained from 1.5 mm scans were significantly higher than those from 3 mm scans (median, interquartile range 4.5[0–71] vs. 0[0–48.4]; p < 0.001). Models showed no difference in predictive accuracy of the presence of CAC between 1.5 and 3 mm scans (iAUC, 0.625 vs. 0.672). In conclusion, CAC scores obtained from 1.5 mm scans are significantly higher than those from 3 mm scans, but do not provide added prognostic value relative to 3 mm scans.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Coronary artery calcium (CAC) scores obtained from electrocardiogram (ECG)-gated computed tomography (CT) scans have been established as strong predictors of risk for cardiac events in asymptomatic individuals1,2. The absence of CAC is associated with a low risk of coronary mortality and morbidity, particularly in asymptomatic individuals3,4,5. The 2010 American Heart Association cardiac CT appropriateness criteria judged CAC scoring as appropriate in asymptomatic individuals with intermediate risk for coronary heart disease (Framingham risk score, 10–20%)6. CAC scoring also offers guidance on interventions to prevent cardiovascular disease in asymptomatic individuals. Recently, the American College of Cardiology/American Heart Association published guidelines for measuring CAC scores in adults at intermediate risk [a 10-year atherosclerotic cardiovascular disease (ASCVD) risk range of ≥ 7.5% to < 20%] or borderline risk (a 1-year ASCVD risk of 5% to < 7.5%) when risk-based decisions for preventive statin therapy were uncertain7. When a CAC score is 1–99, then statin therapy is favored; when ≥ 100, it is indicated.
The standard CT scan protocol for CAC scoring is ECG-gated, non-contrast CT at a 2.5 to 3 mm slice thickness, using 120 kVp. However, with advanced CT scanners (e.g., second- or third-generation 128- or 256-detector-row dual-source instruments), image reconstruction with thinner slices (1–1.5 mm) is available without additional radiation exposure. Previous studies reported that a small amount of CAC could be depicted with higher sensitivity using thinner slice thickness reconstructions than the standard 3 mm slice thickness8,9,10,11. However, detecting the presence of small calcifications in thinner slices did not significantly increase the value of CAC for diagnosing the presence of obstructive stenosis11. Moreover, the relative prognostic value of obtaining CAC scores with thinner slice reconstruction has not been determined.
Consequently, this study was performed to evaluate whether obtaining CAC scores from 1.5 mm slice reconstructions has a better prognostic value for predicting major adverse cardiovascular events (MACEs) than using 3 mm slice reconstructions.
Results
Baseline clinical characteristics
Of 550 subjects, 316 (57.5%) were men, and the mean age was 59.4 ± 10.6 years. The clinical characteristics of the patient population are presented in Supplementary Table 1. The mean body mass index of the subjects was 24.0 ± 3.4 kg/m2 (range, 15.6 to 65.0 kg/m2). Diabetes mellitus, hypertension, and dyslipidemia were prevalent in 10.9%, 16.6%, and 53.5% of the study participants, respectively, and 16.7% were current smokers.
Interreader agreement of CAC scoring
CAC scoring demonstrated high interreader agreement on both 1.5 mm and 3 mm slice thickness scans (ICC 0.99 for both; κ value for risk category 0.951 and 0.988, respectively).
CAC scoring from 1.5 and 3 mm slice thickness scans
CAC scores obtained from 1.5 mm scans (IQR, 0–71; median, 4.5) were significantly higher than those from 3 mm scans (IQR, 0–48.4; median, 0, p < 0.001; Fig. 1). Image noise with 3 mm slice thickness reconstruction in the ascending aorta (mean ± SD, 20.1 ± 3.9) was significantly lower, as compared to 1.5 mm slice thickness reconstruction (mean ± SD, 27.4 ± 5.5, p < 0.001). CAC scores from 1.5 and 3 mm slice thickness scans showed excellent agreement (ICC = 0.998). The mean difference and 95% LOA between 1.5 and 3 mm slice thickness scans were 10.1 and 67.6 to − 47.5, respectively.
An 80-year-old male with 2-vessel CAD and moderate CAC on 1.5 mm (CAC score, 354.8) and 3 mm (CAC score, 243.0) slice thickness reconstructions. Images from a 1.5 mm slice thickness (a) and 3 mm slice thickness (b) at the corresponding scan level are shown. Calcified plaques in the left anterior descending artery (white arrow), left circumflex artery (white arrowhead), and distal right coronary artery (black arrow) are more evident in the 1.5 mm slice thickness reconstruction. The patient underwent revascularization by PCI after 272 days.
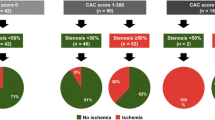
The agreement of CAC severity categories between 1.5 and 3 mm slice thickness scans was good (weighted κ, 0.758; 95% CI, 0.715–0.800). However, the distribution of CAC severity was significantly different between 1.5 and 3 mm slice thickness scans (p < 0.001; Table 1). The proportion of subjects with non-zero CAC score was significantly higher on 1.5 mm scans than on 3 mm scans (p < 0.001; Table 1). Among the 301 subjects with zero CAC scores from 3 mm slice thickness scans, 117 were reclassified as having non-zero CAC scores from 1.5 mm slice thickness scans. In 135 of 550 participants, more severe CAC categories were obtained from 1.5 mm scans than 3 mm scans. Two patients had more severe CAC categories from 3 mm scans than 1.5 mm scans (one subject categorized as having mild severity was re-categorized as moderate, and one subject with moderate severity was re-categorized as severe; Supplementary Fig. 1).
The associations of CAD extent with the severity of CAC score in 1.5 and 3 mm slice thickness scans are shown in Table 2. A higher degree of CAD was significantly associated with increasing CAC severity category on 1.5 and 3 mm slice thickness scans (p < 0.001 for all).
Volume and density scoring from 1.5 and 3 mm slice thickness scans
The volume scores obtained from 1.5 mm scans (IQR, 0–58.4; median, 5.39) were significantly higher than those from 3 mm scans (IQR, 0–37.8; median, 0, p < 0.001). The density scores obtained from 1.5 mm scans (IQR, 1.2–1.8; median, 1.61) were also significantly higher than those from 3 mm scans (IQR, 3.2–4; median, 3.71, p < 0.001). The volume scores from 1.5 and 3 mm slice thickness scans showed excellent agreement (ICC = 0.991, 95% CI, 0.989–0.992, p < 0.001). However, the density scores from 1.5 and 3 mm slice thickness scans showed poor agreement (ICC = 0.083, 95% CI, − 0.177–0.285, p = 0.248).
The correlation for the severity of coronary artery stenosis in relation to the volume score and density score showed ⍴ = 0.624, 0.465 for 1.5 mm (p < 0.001) and 0.645 and 0.35 (p < 0.001) for 3 mm, respectively.
Association of clinical and CT variables with MACEs
During the mean follow-up period of 1339.8 ± 626.9 days, a total of 14 MACEs (2.6%) occurred, including one unstable angina, four nonfatal myocardial infarctions requiring hospitalization, and nine episodes of revascularizations > 90 days after the index CCTA. Subjects who experienced MACEs had higher CAC scores and volume scores from 1.5 and 3 mm slice thickness scans and more frequent obstructive CAD findings with CCTA than patients who did not experience MACEs (Table 3, p < 0.05 for all). Density scores were not significantly different between groups. Significant differences were observed in CAC severity categories between those who did and did not experience MACEs only in analyses of 3 mm slice thickness images (p = 0.026 for 3 mm and p = 0.075 for 1.5 mm).
The univariable Cox regression analysis showed that characteristics of current smoking, obstructive CAD, and the presence of CAC in 3 mm scans were significantly associated with MACEs (all p < 0.05; Table 4). However, there was no graded relationship between increasing CAC and CAD severities and subsequent MACEs. (Supplementary Table 2).
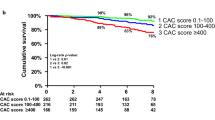
The results of time-dependent ROC curve analyses over the entire follow-up period are shown in Fig. 2 and Supplementary Fig. 1. Values for iAUC were 0.787 for Model 1, 0.625 for Model 2, and 0.672 for Model 3.
Predictive accuracy of three different models for MACEs: iAUC by follow-up time. There were no statistically significant differences among the three models. (a) Model 1: CAD extent. (b) Model 2: the presence of CAC with 1.5 mm slice thickness. (c) Model 3: the presence of CAC with 3 mm slice thickness.
Discussion
Our study demonstrated that CAC scores obtained from CT scans reconstructed with a 1.5 mm slice thickness yielded were significantly higher than those obtained from 3 mm scans. Additionally, the CAC severity category was reclassified in approximately one-quarter of cases. However, CAC scores obtained from 1.5 mm scans did not add value for predicting MACEs relative to scores obtained from 3 mm scans.
Our results are consistent with previous studies that reported an increase in CAC scores with thinner slices8,9,10,11. In our study, 117 of 301 (38.9%) participants with negative CAC scores from 3 mm scans were reclassified as having positive CAC scores from 1.5 mm scans. Thinner slice thickness may improve the detection of calcified plaques in coronary arteries by decreasing partial volume effects, which may cause small, attenuated calcifications to be underestimated or ignored. Agatston CAC scoring depends on the size and peak voxel attenuation of a calcified lesion12, so detecting small voxels can result in higher scores from larger calcium sizes and higher peak attenuation.
Even though higher CAC scores are obtained from thinner CT scan reconstructions, the prognostic value of CAC scoring from thinner scans remains undetermined. Han et al. reported a prognostic significance of subtle calcified plaques on CCTA among individuals whose CAC scores were zero and suggested modifying CAC image acquisition protocols and/or scoring methods to improve the detection of subtle CAC13. Accordingly, we hypothesized that thinner slice imaging could detect CAC with higher sensitivity and have better prognostic value for predicting MACEs than conventional 3 mm scans.
However, our study shows that the presence of CAC in 1.5 mm scans did not improve the prediction of subsequent MACEs in asymptomatic individuals. We propose that this result can be explained in two ways. First, we included asymptomatic subjects at low risk of CAD with a low incidence rate (2.6%) for MACEs, so the prognostic value of using thin-slice CT scans could not be sufficiently demonstrated. An association between stepwise increases in the risk for subsequent MACEs and higher CAC scores and CAC severity was not clearly observed in our population, because the rate of MACEs in patients with severe CAC or 3-vessel disease was low or zero, in contrast to previous studies1,14,15,16,17. Therefore, we chose binary variables to categorize CAC and CAD severities (absence vs. presence of CAC and no CAD or non-obstructive CAD vs. obstructive CAD) for the prediction models, and the presence of CAC and obstructive CAD were significantly associated with a higher risk of MACEs in the univariable analysis. Second, thin‐slice reconstruction detects small calcifications better but also causes more image noise, which may bias the detection of CAC. Moreover, it is not always feasible to distinguish small calcified lesions from spurious lesions caused by image noise on non-contrast examination, even when using CCTA. Therefore, excessive image noise can mimic small calcified lesions, increasing false-positive detections that increase the CAC score.
Our study results may suggest that adjusting reconstruction parameters such as thinner slice thickness to detect small calcified lesions may not be necessary because the prognostic significance is unclear. However, further study is necessary to investigate whether 1.5 mm slice thicknesses provide added prognostic value in various populations, such as symptomatic individuals. In addition, our data increase our understanding of the clinical significance of incidentally detected CAC in non-ECG gated CT scans, where reconstruction at slice thicknesses of 1–1.5 mm is commonly applied. A recent meta-analysis reported that the agreement of CAC severities assessed by ECG-gated and non-ECG-gated CT scans was excellent; however, scan acquisition and reconstruction parameters, such as slice thickness and reconstruction kernel, could significantly affect the results18. Moreover, the prognostic significance of CAC detected on chest CT scans for lung cancer screening may differ according to the slice thickness, and CAC observed in thinner slice data may not have a better prognostic value, as shown in a previous study and our results19.
Interestingly, the CAC score values in two participants were higher in 3 mm reconstruction images than 1.5 mm scans (Supplementary Fig. 2). One reason could be because the thinner slice reconstructions resulted in better spatial resolution (smaller voxels) that reduced the partial volume effect and decreased the overestimation of calcified plaques. We hypothesize that increasing the CT image noise lowers the CAC scores in these cases. Although image noise can mimic small calcified lesions, increasing image noise can also decrease the CAC score because higher noise means that fewer voxels will be above the 130 HU threshold because of the higher variability in the values20.
There are limitations to this study. First, this study was conducted at a single center with asymptomatic individuals. The retrospective nature of this study is also associated with selection bias. Therefore, the results of this study may not be generalizable to all patients with CAD. Second, the overall incidence of MACEs during the follow-up period was low (14/550, 2.6%) and driven mainly by revascularization (9/14, 64.3%). Consequently, the ability to compare the predictive accuracy for MACEs among the three models was limited. Additional studies are needed to evaluate the prognostic value of CAC scoring from thinner slice reconstructions in symptomatic populations with higher rates of MACEs.
Materials and methods
Study population
This retrospective study has been approaved by the Institutional Review Board of Severance Hospital, Yonsei University Health System, Seoul, Korea (4–2021-0785). The requirement for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Severance Hospital.
We retrospectively enrolled 818 asymptomatic individuals who consecutively underwent cardiac CT for health check-ups at our institution between September 2013 and December 2018. In our institution, images for CAC scoring are produced from cardiac CT scans conducted at health check-ups and reconstructed with two slice thicknesses: 1.5 and 3 mm. Subjects were excluded if they had a prior history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) (n = 27), did not have a complete set of 1.5 and 3 mm reconstructions (n = 123), or had a follow-up interval of less than one year (n = 118). A total of 550 individuals [mean age, 59.4 ± 10.6 years (range, 23–84); 316 men, 234 women] were included in the analysis.
CT image acquisition
All cardiac CT scans were performed with second- or third-generation 128- or 256-detector-row dual-source CT scanners (SOMATOM Definition Flash or SOMATOM Definition Force, Siemens Healthineers, Forchheim, Germany). The image acquisition protocol used when obtaining each scan was the one recommended by the Society of Cardiovascular Computed Tomography6. Non-contrast CT images were acquired with prospective ECG-gated sequential scanning with a tube voltage of 120 kV and a tube current of 50 mAs for CAC scoring. The scan range extends from the angle of the carina to below the cardiac apex. A complete cardiac non-contrast coronary artery image was acquired during a single inspiration and breath-hold at 70% or 35% of the R-R interval, depending on the heart rate. Image reconstruction was performed using a medium sharp kernel (B35f.), specifically designed to enhance calcification detection. The whole volume was reconstructed in non-overlapping data sets of 1.5 mm and 3 mm slice thickness from the acquired raw data. Contrast-enhanced coronary computed tomography angiography (CCTA) was performed during an inspiratory breath-hold with the use of prospective ECG gating. If a patient’s resting heart rate was ≥ 65 beats/min, oral metoprolol was administered 30 min before the CCTA examination. Sublingual nitroglycerin (0.3–0.6 mg) was administered immediately before contrast injection for maximal vasodilatation.
CT image analysis
CT image analysis was performed by two radiologists (Y.J.S and S.Y.K) with 13 and 3 years of experience in cardiac CT, respectively. On non-contrast-enhanced calcium scoring scans, CAC scores were measured on 1.5 and 3 mm slice images using the Agatston method, which defines the calcific lesion on CT with a threshold of 130 Hounsfield units (HU) and an area ≥ 1 mm212. CAC scores were evaluated using commercial software (AVIEW CAC, Coreline Soft, Seoul, Korea). Firstly, coronary lesions with attenuation > 130 HU and area ≥ 1 mm2 were automatically color-coded. Secondly, regions of interest showing calcium attenuation were manually selected. Lastly, the total CAC score was calculated as the sum of the individual lesion scores. We classified the results from each subject into four categories based on CAC score: none (score = 0), mild (scroe 1–100), moderate (score 101–400), and severe (score > 400)21. In addition to CAC score, a volume score was also calculated on 1.5 and 3 mm datasets. We used a formula using CAC scores and the volume scores to create CAC density score. The formula was: CAC score/area score = density score22.To evaluate interreader agreement of manual CAC scoring, 100 (18.2%) CT scans were randomly selected for 1.5 and 3 mm thickness values, with even distribution over the CAC severity categories (25 cases in each category).
Image noise was measured on 1.5 and 3 mm slice images, which was defined as the standard deviation (SD) of the measured pixel values in HU within circular ROIs in the ascending aorta on slice image at level of left main coronary artery. The degree of coronary luminal stenosis was classified by the maximal luminal diameter stenosis observed on any plane of CCTA. Stenosis was graded as minimal (≤ 25%), mild (26–50%), or significant (> 50%)23,24.
Clinical outcome
Clinical follow-up data were collected by reviewing electronic medical records to identify MACEs: cardiac death, nonfatal myocardial infarction, unstable angina requiring hospitalization, and revascularization either by PCI or CABG > 90 days from the index CCTA. The dates of MACE occurrence and last follow-up visit were recorded.
Statistical analysis
The mean, SD, and median values were calculated for CAC scores, volume scores, and density scores obtained with the 1.5 and 3 mm datasets. The Wilcoxon signed ranks test was applied to determine the statistical significance of differences between the CAC scores, volume socres, density cores, and image noise on 1.5 and 3 mm slice thicknesses. Interreader agreement of CAC scores and the category of CAC severity was evaluated using the intraclass correlation coefficient (ICC) and weighted κ statistics. The agreement of CAC scores between the 1.5 and 3 mm reconstructions was evaluated using the ICC and Bland–Altman analysis with a 95% limit of agreement (LOA). The agreement of the category of CAC severity between the two slice thicknesses was assessed using weighted κ statistics. The correlation between the volume score, density score and the severity of coronary artery stenosis were assessed with Spearman’s rank correlation coefficient (⍴). When comparing the groups with and without MACEs, chi-square or McNemar tests were used for categorical variables, and the Mann–Whitney U test was used for continuous variables. Cox regression analyses were used to identify prognostic factors of MACEs using hazard ratios (HRs) and 95% confidence intervals (CIs). We developed three models to assess the added value of 1.5 mm slice thickness CAC data in risk prediction. Model 1 includes the coronary artery disease (CAD) extent on CCTA (no or non-obstructive CAD vs. obstructive CAD). Model 2 includes the presence of CAC from 1.5 mm slice thickness images (absence vs. presence of CAC). Model 3 includes the presence of CAC from 3 mm slice thickness images (absence vs. presence of CAC). The discriminatory function of each model was evaluated using the time-dependent receiver operating characteristic (ROC) curve method, and the predictive accuracy of the models was calculated using the integrated area under the curve (iAUC). The iAUC is a weighted average of the AUC across a follow-up period, and it measures the predictive prognosis of a model during follow-up. A higher iAUC indicates a better predictive prognosis. A p value of < 0.05 was considered to be statistically significant. All analyses were performed with SAS (version 9.2, SAS Institute Inc., Cary, NC, USA).
Conclusion
In conclusion, CT scans reconstructed at a 1.5 mm slice thickness scan can detect small CAC with higher sensitively than conventional 3 mm scans. However, the presence of CAC in 1.5 mm scans has no added value for predicting subsequent MACEs in asymptomatic individuals.
Data availability
The data set analysed during the current study are not publicly available due to medical confidentiality but are available from the first author on reasonable request summarized form pending the approval of the IRB.
References
Detrano, R. et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 358, 1336–1345. https://doi.org/10.1056/NEJMoa072100 (2008).
Budoff, M. J. et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 49, 1860–1870. https://doi.org/10.1016/j.jacc.2006.10.079 (2007).
Blaha, M. et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc. Imaging 2, 692–700. https://doi.org/10.1016/j.jcmg.2009.03.009 (2009).
Sarwar, A. et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc. Imaging 2, 675–688. https://doi.org/10.1016/j.jcmg.2008.12.031 (2009).
Oudkerk, M. et al. Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Eur. Radiol. 18, 2785–2807. https://doi.org/10.1007/s00330-008-1095-6 (2008).
Taylor, A. J. et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 56, 1864–1894. https://doi.org/10.1016/j.jacc.2010.07.005 (2010).
Arnett, D. K. et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140, e596–e646. https://doi.org/10.1161/cir.0000000000000678 (2019).
Aslam, A. et al. Assessment of isotropic calcium using 0.5-mm reconstructions from 320-row CT data sets identifies more patients with non-zero Agatston score and more subclinical atherosclerosis than standard 3.0-mm coronary artery calcium scan and CT angiography. J. Cardiovasc. Comput. Tomogr. 8, 58–66. https://doi.org/10.1016/j.jcct.2013.12.007 (2014).
van der Bijl, N. et al. Assessment of coronary artery calcium by using volumetric 320-row multi-detector computed tomography: Comparison of 0.5 mm with 3.0 mm slice reconstructions. Int. J. Cardiovasc. Imaging 26, 473–482. https://doi.org/10.1007/s10554-010-9581-8 (2010).
Mühlenbruch, G. et al. The accuracy of 1- and 3-mm slices in coronary calcium scoring using multi-slice CT in vitro and in vivo. Eur. Radiol. 17, 321–329. https://doi.org/10.1007/s00330-006-0332-0 (2007).
Urabe, Y. et al. Identifying small coronary calcification in non-contrast 0.5-mm slice reconstruction to diagnose coronary artery disease in patients with a conventional zero coronary artery calcium score. J. Atheroscler. Thromb. 23, 1324–1333. https://doi.org/10.5551/jat.35808 (2016).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832. https://doi.org/10.1016/0735-1097(90)90282-t (1990).
Han, D. et al. Prognostic significance of subtle coronary calcification in patients with zero coronary artery calcium score: From the CONFIRM registry. Atherosclerosis 309, 33–38. https://doi.org/10.1016/j.atherosclerosis.2020.07.011 (2020).
Wang, R. et al. Higher coronary artery calcification score is associated with adverse prognosis in patients with stable angina pectoris. J. Thorac. Dis. 9(3), 582–589. https://doi.org/10.21037/jtd.2017.02.84 (2017).
Mortensen, M. B. et al. CAD severity on cardiac CTA identifies patients with most benefit of treating LDL-cholesterol to ACC/AHA and ESC/EAS targets. JACC Cardiovasc. Imaging 13, 1961–1972. https://doi.org/10.1016/j.jcmg.2020.03.017 (2020).
Greenland, P., LaBree, L., Azen, S. P., Doherty, T. M. & Detrano, R. C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 291, 210–215. https://doi.org/10.1001/jama.291.2.210 (2004).
Taylor, A. J. et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: Mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J. Am. Coll. Cardiol. 46, 807–814. https://doi.org/10.1016/j.jacc.2005.05.049 (2005).
Kim, J. Y., Suh, Y. J., Han, K. & Choi, B. W. Reliability of coronary artery calcium severity assessment on non-electrocardiogram-gated CT: A meta-analysis. Korean J. Radiol. 22, 1034–1043. https://doi.org/10.3348/kjr.2020.1047 (2021).
Christensen, J. L. et al. Impact of slice thickness on the predictive value of lung cancer screening computed tomography in the evaluation of coronary artery calcification. J. Am. Heart Assoc. 8, e010110. https://doi.org/10.1161/jaha.118.010110 (2019).
Li, Q. et al. Impact of reconstruction algorithms and gender-associated anatomy on coronary calcium scoring with CT: An anthropomorphic phantom study. Acad. Radiol. 23, 1470–1479. https://doi.org/10.1016/j.acra.2016.08.014 (2016).
Perk, J. et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 33, 1635–1701. https://doi.org/10.1093/eurheartj/ehs092 (2012).
Criqui, M. H. et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 311, 271–278. https://doi.org/10.1001/jama.2013.282535 (2014).
Leipsic, J. et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 8, 342–358. https://doi.org/10.1016/j.jcct.2014.07.003 (2014).
Min, J. K. et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J. Am. Coll. Cardiol. 50, 1161–1170. https://doi.org/10.1016/j.jacc.2007.03.067 (2007).
Acknowledgements
JungBok Lee (Department of Medical Statistics, Asan Medical Center, University of Ulsan College of Medicine) kindly provided statistical advice for this manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (No. 2021R1A2C4002195).
Author information
Authors and Affiliations
Contributions
Conceptualization: S.Y.K., Y.J.S., H.-J.L., Y.J.K.; Data curation: S.Y.K., Y.J.S.; Formal analysis: S.Y.K., Y.J.S.; Funding acquisition: Y.J.S., H.-J.L., Y.J.K.; Investigation: S.Y.K., Y.J.S., H.-J.L., Y.J.K.; Methodology: S.Y.K., Y.J.S., H.-J.L., Y.J.K.; Project administration: Y.J.S., H.-J.L., Y.J.K.; Resources: S.Y.K., Y.J.S., H.-J.L., Y.J.K.; Software: Y.J.S., H.-J.L., Y.J.K.; Supervision: Y.J.S., H.-J.L., Y.J.K.; Validation: Y.J.S., H.-J.L., Y.J.K.; Visualization: Y.J.S., H.-J.L., Y.J.K.; Writing-original draft: S.Y.K., Y.J.S.; Writing-review and editing: S.Y.K., Y.J.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.Y., Suh, Y., Lee, HJ. et al. Prognostic value of coronary artery calcium scores from 1.5 mm slice reconstructions of electrocardiogram-gated computed tomography scans in asymptomatic individuals. Sci Rep 12, 7198 (2022). https://doi.org/10.1038/s41598-022-11332-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11332-3
- Springer Nature Limited