Abstract
Natural killer/T-cell lymphoma (NKTCL) in children and adolescents is a rare type of T/NK cell neoplasms. The aim of the present study was to analyze the clinicopathological and genetic features of this rare entity of lymphoma. We evaluated the clinical, histopathological and molecular features of 22 young people with NKTCL, including 15 males and 7 females, with a median age of 15 years. The results revealed that the nasal site was the most involved region while non-nasal sites were observed in 27.3% out of all cases. The tumor cells were composed of small‑sized to large cells and 19 (86.4%) cases exhibited coagulative necrosis. The neoplastic cells in all patients were positive for CD3 and the cytotoxic markers. Nineteen (86.4%) cases were positive for CD56. Reduced expression of CD5 was observed in all available cases. CD30 was heterogeneously expressed in 15 (75.0%) cases. All 22 patients were EBV positive. Seven (36.8%) out of all the 19 patients during the follow-up died of the disease, and the median follow‑up period was 44 months. Moreover, patients treated with radiotherapy/chemotherapy showed significantly inferior OS compared with the untreated patients. High mutation frequencies were detected including KMT2C (5/5), MST1 (5/5), HLA-A (3/5) and BCL11A (3/5), which involved in modifications, tumor suppression and immune surveillance. These results suggest that NKTCL in children and adolescents exhibits histopathological and immunohistochemical features similar to the cases in adults. Active treatment is necessary after the diagnosis of NKTCL is confirmed. Furthermore, genetic analyse may provide a deep understanding of this rare disease.
Similar content being viewed by others
Introduction
Natural killer/T-cell lymphoma (NKTCL) is a major type of natural killer and T cells neoplasm, which is considered to be an aggressive disease with distinct clinical and histopathological features, characterized by Epstein–Barr virus (EBV) infection. The incidence of this disease is higher in Asia, Mexico, and South America than in Western countries1,2. In China, NKTCL is the second most common type of lymphoma following diffuse large B-cell lymphoma (DLBCL)3. Although NKTCL can occur in children and adolescents, studies with large samples are not available due to the low incidence of NKTCL in this age group4,5,6,7. Recently, Hang reported 17 NKTCL cases from 2012 to 2014 in which the patients were younger than 17 years of age and having an occupancy of 6% (17/286) of the total sample size during the same period8. In a few retrospective analyses of pediatric patients with NKTCL, it has been shown that these patients have some clinical features in common such as male and female distribution were equal or having a slight male predominancy and B-symptoms were often observed with the involvement of non-nasal sites, including the skin, central nervous system, testis, and lungs5,6,7,8. However, the molecular feature of young patients with NKTCL has not been well-described in these studies and genetic difference between adults and children still remains unknown. In this study, we reviewed the clinicopathologic and genetic features of young patients with NKTCL at our institution in China. The present study may provide a better understanding of the pathophysiological aspects of this rare entity of lymphoma.
Materials and methods
Patient selection
Biopsied tissues of patients with NKTCL between May 2012 and March 2019 were identified at the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). All patients were diagnosed according to the World Health Organization classification (WHO) criteria: the morphological and immunophenotypic characteristics of the tumor cells fulfilled the criteria of NKTCL, and all the cases were EBV positive. The criteria for the enrollment of patients in the present study were based on the following requirements: (1) the maximum age limit for the enrolled patients was eighteen years of age with no minimum age limit. (2) No previous history of chronic EBV-associated illness. (3) Patients with no previous history of treatment for lymphoma. All specimens were routinely processed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. This study was approved by the First Affiliated Hospital of Zhengzhou University and complies with the Declaration of Helsinki. And the consent obtained from all subjects included in the study and the children’s parents was both informed and written.
Immunohistochemistry
Immunohistochemistry was performed using 10% formalin-fixed paraffin-embedded tissues that were cut into 4-µm sections, followed by Envision method (a modified avidin‑biotin complex method) on an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA). Immunohistochemistry for CD20 (L26, ZSGB-BIO, Beijing, China), CD3 (SP7, Maixin Biotech Co., Ltd., Fuzhou, China), CD5 (SP19, Maixin Biotech Co., Ltd., Fuzhou, China), CD56 (MRQ42, Jiehao Biotech Co., Ltd., Shanghai, China), TIA-1 (2G9A1085, Maixin Biotech Co., Ltd., Fuzhou, China), granzyme B (EP230, ZSGB BIO, Beijing, China), CD30 (UMAB256, ZSGB BIO, Beijing, China), EBNA-2 (M83171, Fitzgerald, USA), and ki-67 (GM001, Gene Tech, Shanghai, China)) was performed (Supplementary Information).
In situ hybridization (ISH) for EBV
EBV RNA was detected using the Epstein‑Barr Virus Early RNA kit (cat. no. ISH‑5021; OriGene Technologies, Inc.), following the manufacturer's protocol. Briefly, 4–6-µm sections were cut from paraffin‑embedded tissues, deparaffinized with xylene at 37 °C for 10 min, rehydrated, predigested with proteinase K (OriGene Technologies, Inc.), and hybridized with DIG‑labeled RNA probe. Following washing, the reaction was accomplished using anti‑DIG horseradish peroxidase conjugate (OriGene Technologies, Inc.), followed by staining with the 3,3′‑diaminobenzidine substrate at 37 °C for 5 min.
Sequencing and data processing
Five out of 22 cases with formalin-embedded tissues were eligible for the next-generation sequencing (NGS) test. The criteria for selection as follows: (1) tumor tissues with sufficiently high DNA quality. (2) Formalin-embedded tissues within 5 years. (3) To avoid necrotic areas. Deep sequencing was performed on the Illumina HiSeq4000 platform (Shihe, Nanjing, China). The Genome Analysis Toolkit (GATK)9 was used to perform local realignments around indels and base quality reassurance. Single nucleotide variants (SNVs) and short insertions/deletions (indels) were identified using VarScan2 2.3.910 with the minimum variant allele frequency threshold set at 0.01 and the P value threshold for calling variants set at 0.05.
Statistical analysis
All analyses were performed using SPSS 17.0. Overall survival (OS) rate was defined as the duration between the date of diagnosis and the date of mortality or the last follow-up. Survival rate was analyzed using the Kaplan–Meier method with the log‑rank test. P < 0.05 was considered to be statistically significant.
Ethics approval
This study was approved by the First Affiliated Hospital of Zhengzhou University.
Consent to participate
And the consent obtained from all subjects included in the study and the children’s parents was both informed and written.
Consent for publication
All the authors agreed to publish our work on this journal.
Results
Clinical features
The clinical features of 22 patients with NKTCL are summarized in Table 1. Overall, there were 15 male and 7 female patients (male/female, 2.14:1) with a median age of 15 years and a range of 2–18 years. Among these cases, the nasal site was the most involved region, including the nasal cavity (54.5%, 12/22), nasopharynx (13.6%, 3/22), and maxillary sinus (4.5%, 1/22). Non-nasal sites including skin (9.1%, 2/22), lymph nodes (13.6%, 3/22), and testis (4.5%, 1/22) were observed in 6 (27.3%) out of 22 cases. B-symptoms were frequent (77.3%, 17/22), but BM involvement was detected in only 1 patient (5.0%, 1/20).
The serum lactate dehydrogenase level was elevated in almost half of the patients (59.1%, 13/22). Anemia (< 110 g/L) was observed in 36.4% (8/22) of the patients. Leucopenia (5–12 ×109/L) was observed in 45.5% (10/22) of the cases. Advanced Ann Arbor stage III /IV and high/intermediate IPI were observed in 72.7% (16/22) and 54.5% (12/22) of the patients, respectively.
Morphological findings
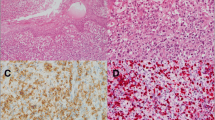
The pathological features of the 22 NKTCL patients are summarized in Table 2. Based on the cell size of tumor cells, NKTCL in the present study could be classified into four histological types: 6 patients with small cell type; 12 patients with medium-size cell type; 2 patients with large cell type; and 2 patients with pleomorphic cell type. Nineteen out of 22 (86.4%) cases exhibited various degrees of coagulative necrosis (Fig. 1A).
Pathologic features of NKTCL in children and adolescents, lymph nodes (case 21). (A) Lymphoid cells were medium-large in size with coagulative necrosis (HE ×200). Neoplastic cells were positive for (B) CD3 and (C) CD56 (×200). (D) CD5 negative was expressed in this case (×200). (E) The tumor cells showed focal positivity for CD30 (×200). (F) Lymphoid cells were EBV positive by ISH detection, > 100 per high-power field in the hot spot region (×200).
Immunohistochemical analysis and ISH for EBV.
The neoplastic cells in all patients were tested positive for the cytoplasmic CD3 expression (Fig. 1B) and the cytotoxic markers such as granzyme B and TIA-1. The tumor cells were CD56 (Fig. 1C) positive in most cases (86.4%, 19/22), while the other 3 cases were negative for the expression of CD56. Reduced expression of CD5 (Fig. 1D) was observed in all available cases, where 15 (83.3%) cases were focally positive, and 3 cases (16.7%) were negative. CD30 (Fig. 1E) was heterogeneously expressed in 15 out of 20 available cases. In addition, three types of CD30 + expression pattern were observed: sporadic (25.0%, 5 out of 20), focal (40.0%, 8/20), and diffuse (10.0%, 2/20). Proliferative activity of tumor cells was assessed by evaluating Ki-67 expression, ranging between 30 and 90%. All 22 patients were EBV-positive (Fig. 1F) according to ISH detection but negative for Epstein-Barr virus nuclear antigen (EBNA)-2.
Gene expression profiling
NGS was performed for 5 cases of NKTCL (Fig. 2). The mutations were arranged based on deleterious SNPs prediction (SIFT < 0.05 and Polyphen2-HDIV > 0.453) and tumor driver genes screening. The results revealed that the high recurrently mutated genes in this cohort were the KMT2C (5/5, 100%; 5 missense), MST1(5/5, 100%; 5 missense), followed by HLA-A (3/5,60%;3 missense), and BCL11A (3/5,60%;1 missense and 2 intron variant).
Treatment and follow-up
The treatment modalities and the clinical outcomes are summarized in Table 1. In brief, 5 patients underwent combined chemo-radiation therapy, 14 patients received chemotherapy or radiotherapy only, and 3 patients refused to receive any treatment. During the follow-up period ranging from 2 to 67 months, 3 patients lost contact information were excluded from the follow-up study. Seven (36.8%) out of all the 19 patients during the follow-up died of the disease, and the median follow‑up period was 44 months. In univariate analysis, patients treated with radiotherapy/chemotherapy showed significantly inferior OS compared with the untreated patients (P = 0.004). In multivariate analysis, region involved, B-symptoms, LDH level, stage, IPI score, and treatment had no prognostic correlation with the outcome (P > 0.05).
Discussion
In line with previous studies, the current study of 22 NKTCL in children and adolescents reported here also demonstrated that the male-to-female ratio was 2.14:1, which indicates that males are more susceptible than females, and the B-symptoms were also observed in most cases (73.3%). All 22 cases exhibited focal symptoms and revealed nasal cavity involvement or non-nasal involvement initially and gradually extended to other sites. Accordingly, 6 cases had the manifestations of non-nasal involvement. It is worthy to note that 3 cases revealed initial symptoms in the lymph nodes area, but subsequent imaging examination showed the spreading of the disease to the nasal sites, which could improve the level of those early diagnoses. The Ann Arbor staging system was used to evaluate the clinical stages of patients: stage I/II was observed in less than 30% of all cases, which is lower than that reported by the previous literature6,7,8,11. It may be presumably related to the restrictive standard of the enrolled group in our study. One of the diagnostic criteria of enrolled patients was multiple site involvement, so most patients in our study had been classified into stage III /IV.
According to the WHO criteria, patients with NKTCL usually exhibit prominent coagulative necrosis and angio-destructive growth pattern in terms of histopathological features. The cytological spectrum is broad with variable-sized cells. In the present study, the most common type of morphological pattern was MC type, which accounted for half of all patients. Most patients (86.4%) showed coagulative necrosis. The current study also revealed that all 22 cases exhibited immunohistochemical features similar to the cases observed in adults, having a positive expression of CD3 and the cytotoxic markers such as granzyme B and TIA-1. Most importantly, the tumor cells were CD56 positive in most cases. CD5 focal positive or negative were expressed in all available cases. It should be noted that 2 patients with NKTCL exhibited strong and diffuse CD30 immunoreactivity. In our previous study, we have reported that the tumor cells were positive for CD30 heterogeneously in most cases of NKTCL, and some cases were CD30 diffuse positive, and this may be confused with anaplastic large cell lymphoma12. In the present study, all 22 patients were EBNA-2 negative, as shown by immunohistochemistry analysis. EBV transiently runs a short lytic program and then predominantly establishes latent infection in the primary infection stage, and EBV-infected cells are EBNA-2 positive in type III latency pattern, i.e., infectious mononucleosis (IM) or lymphomas in immunocompromised people13. Nevertheless, NKTCL expresses latent membrane protein (LMP)-1 and EBNA-1 with latency II of EBV infection. EBNA-2 is supposed to be negative in this disease14,15. Therefore, EBNA-2 may be a useful marker for diagnosis and differential diagnosis of NKTCL.
NKTCL in children and adolescents may be particularly difficult to distinguish from other EBV-related disorders including IM and chronic active EBV infection (CAEBV) because these diseases share similar immunophenotypic markers like CD3, granzyme‑B, TIA‑1, and EBV. Zhou et al. reported 9 patients in China with EBV+ T cell lymphoid hyperplasia (TLH) in the upper aerodigestive tract, whose clinical symptoms were similar to those of IM, and all cases were initially misdiagnosed or misjudged as NKTCL16. The clinical symptoms may help to distinguish between NKTCL and other EBV-related disorders, including IM, EBV-TLH or CAEBV17,18. Besides, the current study demonstrated that patients with NKTCL tend to exhibit coagulative necrosis and reduced expression of CD5, which is very rare in other EBV-related disorders. Due to difficulty in evaluating clinical course of some cases in the early stages of this disease, early diagnosis has become a challenge to pathologists. Case 21 was initially misdiagnosed as EBV acute infection, but subsequent imaging studies demonstrated the involvement of multiple organs with symptoms of hemophagocytic syndrome. Later, the patient was eventually diagnosed as NKTCL. This disease should be diagnosed with sufficient clinical and pathological evidence, or else follow-up observation should be considered. Furthermore, CD30+ anaplastic large cell lymphoma should also be excluded as NKTCL as it could also exhibit heterogeneous expression of CD30. ISH for EBV may help to distinguish between the two diseases.
According to NGS, genes with high mutation frequencies in 5 cases of NKTCL were detected. These genes were KMT2C, MST1, HLA-A and BCL11A which are involved in epigenetic modifications, tumor suppression and immune surveillance19,20,21,22. However, inconsistent with the results of previous studies of NKTCL in adults23,24,25, no mutations in DDX3X and JAK-STAT pathway molecules, which are considered as recurrent mutations in NKTCL, were found in our study. The analysis of molecular signature genes based on the 5 cases may provide a deep understanding of this rare disease. Whether NKTCL in children and adolescents represents a specific type of NKTCL that has characteristic molecular features, requires further study on a larger sample size.
Although some unfavourable prognostic factors, including high LDH level and non-nasal involvement at an advanced stage are reported in NKTCL of adults, and similar studies have not been analyzed well in young patients for limited samples26,27,28,29. In the present study, 3 out of 7 cases which refused chemotherapy or radiotherapy died of this disease. Moreover, univariate analysis showed that cases without treatment were associated with a more unsatisfactory outcome, suggesting that active treatment is necessary after confirmed diagnoses of children and adolescents with NKTCL. However, no statistically significant association was found between clinical parameters and prognosis; therefore, further investigation with a large sample size is needed.
In conclusion, this study, to our knowledge, displays the clinicopathological and genetic features of this rare disease for the first time. The current study revealed that NKTCL in children and adolescents could involve non-nasal sites and exhibited histopathological and immunohistochemical features similar to the cases in adults. This disease is difficult to distinguish from EBV-related disorders in young people, including IM and CAEBV. Furthermore, active treatment is necessary after the diagnosis of NKTCL is confirmed.
Data availability
All data generated or analysed during this study are included in this published article.
References
Swerdlow, S. H., Campo, E., Harris, N. L. et al. (eds) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition) (IARC Press, 2017).
Locke, F. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20, 31–42. https://doi.org/10.1016/s1470-2045(18)30864-7 (2019).
Sun, J. et al. Distribution of lymphoid neoplasms in China: Analysis of 4,638 cases according to the World Health Organization classification. Am. J. Clin. Pathol. 138, 429–434. https://doi.org/10.1309/ajcp7yltqpusdq5c (2012).
Wilberger, A. & Liang, X. Primary nonanaplastic peripheral natural killer/T-cell lymphoma in pediatric patients-an unusual distribution pattern of subtypes. Pediatr. Dev. Pathol. 22, 128–136. https://doi.org/10.1177/1093526618807110 (2019).
Maciejka-Kemblowska, L. et al. Clinical features and treatment outcomes of peripheral T-cell lymphoma in children. A current data report from Polish Pediatric Leukemia/Lymphoma Study Group (PPLLSG). Adv. Med. Sci. 61, 311–316. https://doi.org/10.1016/j.advms.2016.03.002 (2016).
Kontny, U. et al. Non-anaplastic peripheral T-cell lymphoma in children and adolescents—a retrospective analysis of the NHL-BFM study group. Br. J. Haematol. 168, 835–844. https://doi.org/10.1111/bjh.13216 (2015).
Kobayashi, R. et al. Retrospective analysis of non-anaplastic peripheral T-cell lymphoma in pediatric patients in Japan. Pediatr. Blood Cancer 54, 212–215. https://doi.org/10.1002/pbc.22329 (2010).
Huang, Y., Xie, J., Ding, Y. & Zhou, X. Extranodal natural killer/T-cell lymphoma in children and adolescents: A report of 17 cases in China. Am. J. Clin. Pathol. 145, 46–54. https://doi.org/10.1093/ajcp/aqv010 (2016).
McKenna, A. et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. https://doi.org/10.1101/gr.107524.110 (2010).
Koboldt, D. et al. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576. https://doi.org/10.1101/gr.129684.111 (2012).
Mellgren, K. et al. Non-anaplastic peripheral T cell lymphoma in children and adolescents-an international review of 143 cases. Ann. Hematol. 95, 1295–1305. https://doi.org/10.1007/s00277-016-2722-y (2016).
Wang, G. et al. Prognostic significance of CD30 expression in nasal natural killer/T-cell lymphoma. Oncol. Lett. 13, 1211–1215. https://doi.org/10.3892/ol.2017.5592 (2017).
Murata, T. & Tsurumi, T. Switching of EBV cycles between latent and lytic states. Rev. Med. Virol. 24, 142–153. https://doi.org/10.1002/rmv.1780 (2014).
Quintanilla-Martínez, L. et al. Primary intestinal non-Hodgkin’s lymphoma and Epstein-Barr virus: High frequency of EBV-infection in T-cell lymphomas of Mexican origin. Leuk. Lymphoma 30, 111–121. https://doi.org/10.3109/10428199809050934 (1998).
Liu, A., Nakatsuka, S., Yang, W., Kojya, S. & Aozasa, K. Expression of cell adhesion molecules and chemokine receptors: Angioinvasiveness in nasal NK/T-cell lymphoma. Oncol. Rep. 13, 613–620 (2005).
Jianlan, X. et al. Acute Epstein-Barr virus-positive cytotoxic T cell lymphoid hyperplasia in the upper aerodigestive tract, mimicking extranodal natural killer/T cell lymphoma, nasal type. Virch. Arch. Int. J. Pathol. 474, 219–226. https://doi.org/10.1007/s00428-018-2498-7 (2019).
Ishii, T. et al. Clinical differentiation of infectious mononucleosis that is caused by Epstein-Barr virus or cytomegalovirus: A single-center case-control study in Japan. J. Infect. Chemother. 25, 431–436. https://doi.org/10.1016/j.jiac.2019.01.012 (2019).
Hue, S., Oon, M., Wang, S., Tan, S. & Ng, S. Epstein-Barr virus-associated T- and NK-cell lymphoproliferative diseases: An update and diagnostic approach. Pathology 52, 111–127. https://doi.org/10.1016/j.pathol.2019.09.011 (2020).
Chang, Y. et al. Activated hippo signal pathway inhibits cell proliferation and promotes apoptosis in NK/T cell lymphoma cells. Cancer Med. 8, 3892–3904. https://doi.org/10.1002/cam4.2174 (2019).
Polprasert, C. et al. Frequent mutations in HLA and related genes in extranodal NK/T cell lymphomas. Leuk. Lymphoma 62, 95–103. https://doi.org/10.1080/10428194.2020.1821011 (2021).
de Mel, S., Hue, S., Jeyasekharan, A., Chng, W. & Ng, S. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J. Hematol. Oncol. 12, 33. https://doi.org/10.1186/s13045-019-0716-7 (2019).
Shi, H. et al. BCL11A is oncogenic and predicts poor outcomes in natural killer/T-cell lymphoma. Front. Pharmacol. 11, 820. https://doi.org/10.3389/fphar.2020.00820 (2020).
Jiang, L. et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 47, 1061–1066. https://doi.org/10.1038/ng.3358 (2015).
Song, T. et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 132, 1146–1158. https://doi.org/10.1182/blood-2018-01-829424 (2018).
Xiong, J. et al. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell 37, 403-419.e406. https://doi.org/10.1016/j.ccell.2020.02.005 (2020).
Qi, S. et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk. Lymphoma 57, 2575–2583. https://doi.org/10.1080/10428194.2016.1180689 (2016).
Su, Y. et al. Extranodal NK/T-cell lymphoma, nasal type: Clinical features, outcome, and prognostic factors in 101 cases. Eur. J. Haematol. 101, 379–388. https://doi.org/10.1111/ejh.13126 (2018).
Allen, P. & Lechowicz, M. Management of NK/T-Cell Lymphoma, Nasal Type. J. Oncol. Pract. 15, 513–520. https://doi.org/10.1200/jop.18.00719 (2019).
Liu, Z. et al. Characteristics, prognostic factors, and survival of patients with NK/T-cell lymphoma of non-upper aerodigestive tract: A 17-year single-center experience. Cancer Res. Treat. 51, 1557–1567. https://doi.org/10.4143/crt.2018.681 (2019).
Acknowledgements
We sincerely thank the participants in this study.
Author information
Authors and Affiliations
Contributions
W.G.N. and L.W.C. designed the research and made the final version of the manuscript; all other authors contributed to data collection and/or data analysis for this study. All authors read and approved the fnal manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, G., Zhao, W., Zhang, XD. et al. A retrospective study on the clinicopathological and molecular features of 22 cases of natural killer/T-cell lymphoma in children and adolescents. Sci Rep 12, 7118 (2022). https://doi.org/10.1038/s41598-022-11247-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11247-z
- Springer Nature Limited
This article is cited by
-
Clinicopathological analysis of immunohistochemical CD47 and signal-regulatory protein-α expression in Extranodal Natural killer/T-cell lymphoma
Annals of Hematology (2024)
-
Novel target and treatment agents for natural killer/T-cell lymphoma
Journal of Hematology & Oncology (2023)