Abstract
Electromechanical-assisted gait training may be an effective intervention to promote motor recovery after brain injury. However, many studies still have difficulties in clarifying the difference between electromechanical-assisted gait training and conventional gait training. To evaluate the effectiveness of electromechanical-assisted gait training compared to that of conventional gait training on clinical walking function and gait symmetry of stroke patients. We randomly assigned patients with stroke (n = 144) to a control group (physical therapist-assisted gait training) and an experimental group (electromechanical gait training). Both types of gait training were done for 30 min each day, 5 days a week for 4 weeks. The primary endpoint was the change in functional ambulatory category (FAC). Secondary endpoints were clinical walking functions and gait symmetries of swing time and step length. All outcomes were measured at baseline (pre-intervention) and at 4 weeks after the baseline (post-intervention). FAC showed significant improvement after the intervention, as did clinical walking functions, in both groups. The step-length asymmetry improved in the control group, but that in the experimental group and the swing-time asymmetry in both groups did not show significant improvement. In the subgroup analysis of stroke duration of 90 days, FAC and clinical walking functions showed more significant improvement in the subacute group than in the chronic group. However, gait symmetries did not show any significant changes in either the subacute or the chronic group. Electromechanically assisted gait training by EXOWALK was as effective as conventional gait training with a physiotherapist. Although clinical walking function in the subacute group improved more than in the chronic group, gait asymmetry did not improve for either group after gait training.
Trial registration: KCT0003411 Clinical Research Information Service (CRIS), Republic of Korea.
Similar content being viewed by others
Introduction
In some people with disabilities, all or some motor functions of the lower limbs are significantly decreased because of brain lesions. Rehabilitation following brain injury from stroke can improve their walking efficiency and functional independence for activities of daily living1. For gait rehabilitation, clinicians prefer a repetitive approach with higher intensities of walking-practice programs2. Electromechanical-assisted gait training that requires repetitive tasks can improve the neuro-plasticity for motor learning with a focus on reorganization of brain tissue, resulting in better balance and a faster gait3.
In the 2020 Cochrane review, the people who received electromechanical-assisted gait training in combination with physiotherapy after stroke are more likely to achieve independent walking than people who receive gait training without these devices. Specifically, people in the first three months after stroke and those who are not able to walk seem to benefit most from this type of intervention4. However several studies reported that electromechanically assisted gait training can improve the gait function in patients with chronic stroke5,6. Accumulating evidence has suggested that high-intensity repetitive task-specific practice might be the most effective strategy for promoting motor recovery after a stroke7. Electromechanical-assisted gait training represents such a treatment option8.
Improvement of gait symmetry was achieved electromechanical gait training and related to impairment in balance9. According to a recent pilot study, the gait symmetry of patients with stroke came closer to the normal range after gait training with a Wearable Hip-Assist device for 4 weeks10. A recent review has indicated that there is still a need for well-designed, large-scale, multicenter studies to evaluate the benefits of electromechanical-assisted gait training for walking after stroke4.
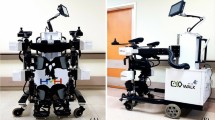
EXOWALK (HMH Co., HR-01, A67020.02, Grade2, South Korea) is an electromechanically assisted gait trainer that can provide stable and firm standing ability with little chance of falling (Fig. 1). It obviates the need for an additional cane or walker more than do currently popular exoskeletons. Such designs are user-friendly without needing a harness for weight support.
Many studies have investigated the effectiveness by clinical evaluation and still have found it difficult to clarify the difference between conventional and electromechanically assisted gait training11,12, sometimes because of having too few subjects13,14,15. In systematic reviews of electromechanically assisted gait training plus physiotherapy versus physiotherapy alone, there is still a need for large-scale and multicenter studies with good design after strokes4. Our purpose in this prospective study was to use a multi-center randomized design to investigate the effect of electromechanically assisted gait training using the EXOWALK, which provides repetitive training with normal symmetric gait on clinical walking function and gait symmetry of stroke patients.
Methods
This was a multicenter, randomized, prospective, and parallel-group study on the efficacy and safety of the electromechanical gait trainer EXOWALK. All enrolled subjects were patients with stroke. The following three clinical research centers participated in this trial: Dongguk University Ilsan Hospital, Chungnam National University Hospital, and Seoul National University Bundang Hospital. This research protocol was approved by each hospital as follows: Dongguk University Ilsan Hospital’s Institutional Review board (IRB No. DUIH 2018-08-026-001), Chungnam National University Hospital’s IRB (IRB No. CNUH 2018-09-033) and Seoul National University Bundang Hospital’s IRB (IRB No. B-1810/497-001). This study was registered at the Clinical Research Information Service (CRIS, KCT0003411; date of registration, 03/01/2019). We obtained informed consent from all participants and did all the research in accordance with the Declaration of Helsinki.
Given the data of patients who agreed to participate in this study, we screened to select eligible subjects based on the following inclusion and exclusion criteria. Inclusion criteria were:
-
(1)
those who had had a stroke,
-
(2)
those who had a score of 10 or more for the Mini-Mental State Examination (MMSE),
-
(3)
those who had a Modified Ashworth Scale (MAS) Grade 2 or lower, and
-
(4)
those who could stand alone.
Exclusion criteria were:
-
(1)
those with poor cognition that made it difficult for them to carry out instructions,
-
(2)
those with ataxia that caused unstable standing balance,
-
(3)
those with spasticity MAS Grade 3 or above,
-
(4)
those with severe leg arthritis, and
-
(5)
those with difficulty walking because of joint problems of the lower leg.
This was a randomized, controlled, single-blind trial. We assigned subjects into an experimental group or a control group by using a randomized allocation table for subjects who met the inclusion/exclusion criteria and who agreed to participate in this study. Randomization tables were created for each research organization. Randomization was done using a random-number generator computerized with a block randomization method in SAS version 9.4 (SAS institute, Inc., Cary, NC, USA). Outcome assessors were blinded for reducing the bias. Intervention and evaluation were done by different physiotherapists with five years or more of experience, to increase the reliability by minimizing the measurement error. At enrollment, we instructed patients not to reveal their allocation arm to the outcome assessor. The researcher who did the randomization and data analyses was not involved in any assessment or training.
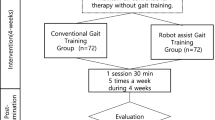
We prospectively enrolled 144 suitable patients from November 2018 to August 2020; each hospital registered 48 subjects. We assigned subjects into an experimental group or a control group. Patients in both groups were given 30 min of training per session, five times per week for 4 weeks. In addition, we did basic rehabilitation (neurodevelopmental treatment, exercise for range of motion and strengthening) for both groups. The experimental group received electromechanically assisted gait training with EXOWALK and the control group received conventional gait rehabilitation treatment by therapists. We recommended the patients in this study to receive the electromechanical exoskeleton-assisted gait training at a comfortable speed. Although the maximum safe velocity of this device was 2.3 km/h, we provided the gait training velocity under 1.8 km/h according to the initial evaluation to prevent fatigue. For subjects in the control group, the physiotherapist guided and walked the patient while assisting the subject on the side or the back.
Outcome measures
In this study, we measured and documented the demographic and clinical characteristics of subjects after screening. Demographic information included sex, date of birth, height, weight, and joint problems (or not). Clinical characteristics included the name of the diagnosis, the cause of the disability (brain infarction, cerebral hemorrhage), the paralyzed side (right, left), whether they could express intention (standard: MMSE 10 or higher), and the lower-limb spasticity score (standard: MAS grade 2 or lower).
The change in functional ambulatory category (FAC)16 from before to after gait training was the primary endpoint for evaluating the efficacy of electromechanical exoskeleton-assisted gait training. We evaluated the FAC at baseline (pre-intervention) and at 4 weeks after the baseline (post-intervention), by dividing the degree of need for assistance when walking from 1 to 6. FAC level ranged from Level 1 (‘nonfunctional’) to Level 6 (‘independent without help for non-level surfaces’).
The second endpoint had seven assessments. First, we used the RMI17 to evaluate motor skills. It consisted of 15 questions step by step, depending on the level, ranging from bed rotation to running. Each of the 15 questions scored 1 point (if yes) or 0 (if no). The sum was used as a result of the evaluation.
Second, we used walking velocity in 10 mWT18 to measure the speed during a 10-m walk. The unit was m/s (meter per second). Similarly, we evaluated walking capacity with a 6 mWT19 to measure the distance that one could walk for 6 min. The unit was m (meter).
The fourth item was MI20, which we evaluated as 1 to 99 points by measuring the lower-leg force level from the ankle to the knee. Assessment items consisted of three questions, each with a score of 0/9/14/19/25/33. The sum of scores was used as the result of the evaluation.
The fifth item was BBS21 to evaluate the balance ability with 0 to 56 points. Each of the 14 questions was scored 0 to 4 points. The sum of scores was used as the result of the evaluation.
The last two evaluation items were quantitative gait symmetry of swing time and step length measured by a dynamic foot-pressure device (HumanTrack; Rbiotech Co., Ltd., Seoul, Korea)22. Swing time was defined as the time obtained by subtracting previous toe-off time from heel-strike time. Step length is the direct distance between the point of initial contact of one foot and the point of initial contact of the opposite foot. We calculated the following ratios for gait symmetry23.
Swing-time symmetry = paretic swing time/nonparetic swing time.
Step-length symmetry = nonparetic step length/paretic step length.
For the patients who consented to additional evaluation, we did all measurements 4 weeks after the last intervention (follow-up). Physical content of clinical alteration was reported by auditors, practitioners, and patients at each visit. All indications, data of onset, and period were recorded.
Analysis
In a previous trial24, the mean change of FAC between pre- and post-intervention was 0.54 in the control group (conventional gait training with physiotherapist) and 1 in the test group. We expected the medical devices used in this work to achieve approximately 25% better performance, because we provided a longer intervention time than did the reference, and we assumed the change of the mean value to be 1.25. Thus, we assumed that the difference in change between the test medical device and the control (conventional gait training) group was 0.71, and that the largest value, 1.4, was needed for conservative access to the standard deviation. Thus, 65 participants were needed for each group to achieve 80% power at a significant level of 0.05. Considering a possible dropout rate of 10%, we chose the sample size as 144 (72 participants per group).
For demographic and clinical characteristics, categorical variables such as sex, joint problems, disability cause, paralyzed side, and lower extremity MAS scores are presented as frequency and percentage. They were analyzed for pre-homogeneity with chi-squired tests. Continuous variables are presented as mean, standard deviation (SD), and range of minimum and maximum (Min, Max). For height and weight satisfying normality, we analyzed pre-homogeneity using a Student's t-test. For age not satisfying normality, we used a Wilcoxon rank sum test.
All values of primary and secondary endpoints are presented as mean and SD (Mean ± SD). Although basic results of FAC were scored 1, 2, 3, 4, 5, and 6 on an ordinal scale, FAC is presented as mean and SD, and was one of the most popular tools for measuring ambulatory function25,26. Within each group, we analyzed the changes in the values of pre-intervention and post-intervention using a paired t-test if normality was satisfied or a Wilcoxon's signed rank test if not satisfied. In addition, for comparison between pre-intervention and post-intervention values of the test and control groups, we used a Student's t-test and a Wilcoxon's rank sum test (Table 2).
Stroke duration was the most important factor that affected results. Subgroup analysis compared variations between subjects with stroke durations of 90 days or less and those with 91 days or more (over 91 days) in the experimental group. For all results, we analyzed values of pre-intervention and post-intervention changes using a paired t-test if normality was satisfied and a Wilcoxon's signed rank test if normality was not. In order to compare pre-post changes between groups, we analyzed them by using a Student's t-test or a Wilcoxon's rank sum test, depending on whether normality was satisfied (Tables 3, 4). We did all statistical analyses using SAS version 9.4 or later. All statistical tests were two-sided, and the level of significance was set at p < 0.05.
Ethics approval and consent to participate
This research protocol was approved by the Institutional Review Board (IRB) of Dongguk University Ilsan Hospital, Chungnam National University Hospital, and Seoul National University Bundang Hospital. This study was performed following protocols approved by the IRB and included only patients who provided written informed consent.
Results
We included 144 subjects in this study. Of them, 104 completed gait training and outcome measures at 4 weeks after initiation of the intervention. In the experimental group, withdrawal before first evaluation for personal reasons (n = 2), withdrawal before the first treatment for personal reasons (n = 2), withdrawal after the first treatment for personal reasons (n = 4), and incomplete second evaluation (n = 9) occurred (Fig. 2). In the control group, there was withdrawal before first evaluation for personal reasons (n = 4), withdrawal before the first treatment for personal reasons (n = 2), withdrawal after the first treatment for personal reasons (n = 7), and incomplete second evaluation (n = 5) (Fig. 2). There were no significant differences in baseline characteristics between the control and experimental groups. All subjects could control their gait direction and speed. The mean MMSE was 24.81 ± 4.65 in the experimental group and 23.69 ± 5.17 in the control group. All subjects could ambulate with or without the assistance of another person (Table 1).
The mean FAC in the experimental group was 3.15 ± 1.39 before intervention (pre-intervention) and 4.22 ± 1.37 after the intervention (post-intervention) (Table 2). The mean FAC in the control group was 3.11 ± 1.29 pre-intervention and 4.20 ± 1.03 post-intervention. Between pre-intervention and post-intervention, the change in FAC showed significant improvement in both groups. However, the change in FAC did not differ between the two groups (Table 2). In the experimental group, the outcomes of clinical walking functions showed improvement after intervention, but those of quantitative gait symmetry did not. In the control group, the outcome of clinical waking functions and step-length asymmetry showed improvement, but that of swing-time asymmetry did not. However, the change of all secondary outcomes between two groups was not different (Table 2).
When each group was divided into two groups according to stroke duration of 90 days, the changes in clinical walking functions were greater in the below-90-days group than in the over-91-days group in both the control group (Table 3) and the experimental group (Table 4). However, changes in gait symmetries did not show any significant difference between the two groups (Tables 3, 4). Adverse events were not found during gait training in either group.
Discussion
Electromechanical gait-training devices have been developed; their effectiveness has been proven by clinical studies for stroke patients27. Gait rehabilitations with an electromechanical gait-training device can increase the length, intensity, and number of physiotherapy sessions28. For clinical effects of electromechanical-assisted gait training, Mehrholz et al. have demonstrated that it could improve post-stroke independent walking recovery when it is combined with physical therapy in patients suffering from a stroke4. It is effective for patients in the first three months after stroke and for those who are not able to walk5. In fact, there is growing evidence that the motor system is plastic following a stroke and that motor training can aid patients, particularly in the first 3 months29,30,31.
EXOWALK is an electromechanical exoskeleton-assisted gait-training device. It has a unique design that applies an exoskeleton in front of a robotic body and makes walking possible using a motorized wheel controlled by the patient. We used two randomized controlled trials (RCTs) to investigate the effect of electromechanically assisted gait training with EXOWALK on stroke patients31,32. The first study revealed that gait training for 30 min with EXOWALK was effective31. The stroke patients had confidence in their gait and desire to continue gait training. However, the effect declined with increasing stroke duration31. We found that, for them, electromechanically assisted gait training with EXOWALK was not superior to conventional physiotherapy32. We also found that electromechanically assisted gait training was as effective as conventional gait training, although its effect was better for those with less than 90 days of stroke duration. These results were the same as our previous studies33.
The difference in FAC change between pre-intervention and post-intervention was 1.09 ± 1.01 in the control group and 1.07 ± 0.82 in the experimental group. When we did various interventions of gait training including conventional treatments for stroke patients, FAC showed an improvement in a range of 0.3 to 1.034,35,36. In this study, changes in FAC and secondary outcomes were significant enough clinically. However, they declined in the over-91-days group. The Cochrane review by Mehrholz et al. revealed that electromechanically assisted training for walking after stroke did not improve the walking capacity or velocity4. In this study, walking capacity and velocity were improved after intervention in the below-90-days group. These were not related to walking symmetry.
Electromechanically assisted gait training is effective in patients with acute and sub-acute stroke, but not in those with chronic stroke, according to subgroup analysis of 461 participants in the chronic phase, defined as more than 90 days after stroke4. When the experimental group was divided into two groups in terms of stroke duration of 90 days, most outcomes of subacute patients showed improvements more than did those of chronic patients in both control and experimental groups. In this study, 87 participants among 104 total patients underwent the follow-up evaluation. Baseline characteristics of follow-up evaluation showed no significant differences between the control group and the experimental group. Most outcomes showed significant improvements that were maintained at follow-up evaluation (Fig. 3).
Gait asymmetry and different values of gait parameters were additional gait characteristics of fallers37. When the lower extremities have different levels of ability, gait asymmetry might be an element for improving the dynamic balance38. We found that the changes in gait symmetries did not show significant improvement after electromechanically assisted gait training intervention for 4 weeks. The balance improved clinically, which might be related to muscle power but not to gait asymmetry, as shown by a previous RCT that investigated the effects of electromechanically assisted gait training on step-length symmetry in subacute stroke patients with hemiplegia and showed no significant difference between the control (gait training with the physiotherapy) group and the experimental group (electromechanically assisted gait training)9. Asymmetry of gait is related to increases in energy expenditure, reduced balance control, and risk of unaffected limb injury39. As motor impairment occurs over time, there is an adapted gait pattern40. Electromechanical gait training can provide much repetitive training with a normal gait pattern. However, it cannot change gait asymmetry in both subacute and chronic patients.
Limitation
Since the inclusion criteria were for stroke patients who could stand alone, many patients who needed walking aids or assistance were registered. We did not evaluate their gait symmetry at the time of pre-intervention. Even though some of them could walk independently without walking aids or assistance, their measurements of gait symmetry post-intervention were not included in the result. Some old and chronic stroke patients had fixed deformity, and gait analysis was not relevant. Thus, there were few subjects with gait symmetry and more dropout patients than we expected; so the power of the result was small. We needed to estimate sample size more conservatively.
Conclusions
The clinical walking function was improved significantly after electromechanically assisted gait training, and the improvement was the same as with conventional gait training with a physiotherapist. The clinical walking function of subacute patients after 4 weeks of gait training was improved more than that of chronic patients. However, the gait asymmetry was not improved in either subacute or chronic stroke patients.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Reisman, D. S., Rudolph, K. S. & Farquhar, W. B. Influence of speed on walking economy poststroke. Neurorehabil. Neural Repair 23, 529–534. https://doi.org/10.1177/1545968308328732 (2009).
French, B. et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta-analysis. J. Rehabil. Med. 42, 9–14. https://doi.org/10.2340/16501977-0473 (2010).
Kwakkel, G., Kollen, B. & Lindeman, E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor. Neurol. Neurosci. 22, 281–299 (2004).
Mehrholz, J., Thomas, S., Kugler, J., Pohl, M. & Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 10, 6185. https://doi.org/10.1002/14651858.CD006185.pub5 (2020).
Bang, D. H. & Shin, W. S. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: A randomized controlled pilot trial. NeuroRehabilitation 38, 343–349. https://doi.org/10.3233/NRE-161325 (2016).
Aprile, I. et al. Efficacy of robotic-assisted gait training in chronic stroke patients: Preliminary results of an Italian bi-centre study. NeuroRehabilitation 41, 775–782. https://doi.org/10.3233/NRE-172156 (2017).
Langhorne, P., Coupar, F. & Pollock, A. Motor recovery after stroke: A systematic review. Lancet. Neurol. 8, 741–754. https://doi.org/10.1016/S1474-4422(09)70150-4 (2009).
Werner, C., Von Frankenberg, S., Treig, T., Konrad, M. & Hesse, S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: A randomized crossover study. Stroke 33, 2895–2901. https://doi.org/10.1161/01.str.0000035734.61539.f6 (2002).
Tanaka, N. et al. Effect of stride management assist gait training for poststroke hemiplegia: A single center, open-label, randomized controlled trial. J. Stroke Cerebrovasc. Dis. 28, 477–486. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.10.025 (2019).
Lee, H. J. et al. Training for walking efficiency with a wearable hip-assist robot in patients with stroke: A pilot randomized controlled trial. Stroke 50, 3545–3552. https://doi.org/10.1161/STROKEAHA.119.025950 (2019).
Peurala, S. H., Karttunen, A. H., Sjogren, T., Paltamaa, J. & Heinonen, A. Evidence for the effectiveness of walking training on walking and self-care after stroke: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 46, 387–399. https://doi.org/10.2340/16501977-1805 (2014).
Lee, H. Y., Park, J. H. & Kim, T. W. Comparisons between Locomat and Walkbot robotic gait training regarding balance and lower extremity function among non-ambulatory chronic acquired brain injury survivors. Medicine 100, e25125. https://doi.org/10.1097/MD.0000000000025125 (2021).
Conesa, L. et al. An observational report of intensive robotic and manual gait training in sub-acute stroke. J. Neuroeng. Rehabil. 9, 13. https://doi.org/10.1186/1743-0003-9-13 (2012).
Taveggia, G., Borboni, A., Mule, C., Villafane, J. H. & Negrini, S. Conflicting results of robot-assisted versus usual gait training during postacute rehabilitation of stroke patients: a randomized clinical trial. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation 39, 29–35. https://doi.org/10.1097/MRR.0000000000000137 (2016).
Kim, J. et al. Effects of robot-(Morning Walk((R))) assisted gait training for patients after stroke: A randomized controlled trial. Clin. Rehabil. 33, 516–523. https://doi.org/10.1177/0269215518806563 (2019).
Holden, M. K., Gill, K. M., Magliozzi, M. R., Nathan, J. & Piehl-Baker, L. Clinical gait assessment in the neurologically impaired Reliability and meaningfulness. Phys. Therapy 64, 35–40. https://doi.org/10.1093/ptj/64.1.35 (1984).
Collen, F. M., Wade, D. T., Robb, G. F. & Bradshaw, C. M. The rivermead mobility Index: A further development of the rivermead motor assessment. Int. Disabil. Stud. 13, 50–54. https://doi.org/10.3109/03790799109166684 (1991).
van Loo, M. A., Moseley, A. M., Bosman, J. M., de Bie, R. A. & Hassett, L. Inter-rater reliability and concurrent validity of walking speed measurement after traumatic brain injury. Clin. Rehabil. 17, 775–779. https://doi.org/10.1191/0269215503cr677oa (2003).
Guyatt, G. H. et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 132, 919–923 (1985).
Cameron, D. & Bohannon, R. W. Criterion validity of lower extremity Motricity Index scores. Clin. Rehabil. 14, 208–211. https://doi.org/10.1191/026921500675786655 (2000).
Berg, K. O., Wood-Dauphinee, S. L., Williams, J. I. & Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 83(Suppl 2), S7-11 (1992).
Cho, Y. S. et al. Evaluation of validity and reliability of inertial measurement unit-based gait analysis systems. Ann. Rehabil. Med. 42, 872–883. https://doi.org/10.5535/arm.2018.42.6.872 (2018).
Patterson, K. K. et al. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 89, 304–310. https://doi.org/10.1016/j.apmr.2007.08.142 (2008).
Watanabe, H., Tanaka, N., Inuta, T., Saitou, H. & Yanagi, H. Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: A randomized controlled pilot study. Arch. Phys. Med. Rehabil. 95, 2006–2012. https://doi.org/10.1016/j.apmr.2014.07.002 (2014).
Chang, M. C., Kim, D. Y. & Park, D. H. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. 8, 561–566. https://doi.org/10.1016/j.brs.2015.01.411 (2015).
Lee, S. H. et al. Feasibility and effects of newly developed balance control trainer for mobility and balance in chronic stroke patients: A randomized controlled trial. Ann. Rehabil. Med. 36, 521–529. https://doi.org/10.5535/arm.2012.36.4.521 (2012).
Peurala, S. H. et al. Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. J. Rehabil. Med. 41, 166–173. https://doi.org/10.2340/16501977-0304 (2009).
Bruni, M. F. et al. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J. Clin. Neurosci. 48, 11–17. https://doi.org/10.1016/j.jocn.2017.10.048 (2018).
Wolpert, D. M., Diedrichsen, J. & Flanagan, J. R. Principles of sensorimotor learning. Nat. Rev. Neurosci. 12, 739–751. https://doi.org/10.1038/nrn3112 (2011).
Mossberg, K. A. Reliability of a timed walk test in persons with acquired brain injury. Am. J. Phys. Med. Rehabil. 82, 385–390. https://doi.org/10.1097/01.PHM.0000052589.96202.BE (2003).
Marque, P., Gasq, D., Castel-Lacanal, E., De Boissezon, X. & Loubinoux, I. Post-stroke hemiplegia rehabilitation: Evolution of the concepts. Ann. Phys. Rehabil. Med. 57, 520–529. https://doi.org/10.1016/j.rehab.2014.08.004 (2014).
Nam, Y. G. et al. Effects of electromechanical exoskeleton-assisted gait training on walking ability of stroke patients: A randomized controlled trial. Arch. Phys. Med. Rehabil. 100, 26–31. https://doi.org/10.1016/j.apmr.2018.06.020 (2019).
Nam, Y. G. et al. Further effects of electromechanically assisted gait trainer (EXOWALK(R)) in patients with chronic stroke: A randomized controlled trial. J. Rehabil. Med. 52, 97. https://doi.org/10.2340/16501977-2723 (2020).
Pohl, M. et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: A single-blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clin. Rehabil. 21, 17–27. https://doi.org/10.1177/0269215506071281 (2007).
Hidler, J. et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil. Neural Repair 23, 5–13. https://doi.org/10.1177/1545968308326632 (2009).
Yoshikawa, K. et al. Gait training with hybrid assistive limb enhances the gait functions in subacute stroke patients: A pilot study. NeuroRehabilitation 40, 87–97. https://doi.org/10.3233/NRE-161393 (2017).
Ehrhardt, A. et al. Fall-related functional impairments in patients with neurological gait disorder. Sci. Rep. 10, 21120. https://doi.org/10.1038/s41598-020-77973-4 (2020).
Hendrickson, J., Patterson, K. K., Inness, E. L., McIlroy, W. E. & Mansfield, A. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Post. 39, 177–181. https://doi.org/10.1016/j.gaitpost.2013.06.022 (2014).
Jorgensen, L., Crabtree, N. J., Reeve, J. & Jacobsen, B. K. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: Bone adaptation after decreased mechanical loading. Bone 27, 701–707. https://doi.org/10.1016/s8756-3282(00)00374-4 (2000).
Wang, Y. et al. Persistent effect of gait exercise assist robot training on gait ability and lower limb function of patients with subacute stroke: A matched case-control study with three-dimensional gait analysis. Front. Neurorobot. 14, 42. https://doi.org/10.3389/fnbot.2020.00042 (2020).
Acknowledgements
This research was supported by a grant (Grant number: HI18C2324) of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Y.G.N. and B.S.K. made substantial contributions to the experimental design, data analysis, and drafting of the manuscript. S.K.B., N.J.B., C.Y.L. and M.J.K. made substantial contributions to the data collection, data analysis, and drafting of the manuscript. L.J.W. made substantial contributions to the development of the device, device maintenance during the clinical trial, and drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nam, Y.G., Ko, M.J., Bok, S.K. et al. Efficacy of electromechanical-assisted gait training on clinical walking function and gait symmetry after brain injury of stroke: a randomized controlled trial. Sci Rep 12, 6880 (2022). https://doi.org/10.1038/s41598-022-10889-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10889-3
- Springer Nature Limited