Abstract
The oral cavity is an entrance for respiratory viruses, such as influenza. Recently, saliva has been shown to exert both antimicrobial and antiviral activities. Thus, saliva may be a biological factor that contributes to the prevention of influenza infection. However, the actual salivary anti-influenza A virus (IAV) activity in individuals and its determinant factors are unknown. By assessing individual variations in salivary anti-IAV activity in 92 people using an established new high-throughput system in this study, we found that the anti-IAV activity varied widely between individuals and showed a significant positive correlation with protein-bound sialic acid (BSA) level (ρ = 0.473; p < 0.001). Furthermore, the anti-IAV activity of saliva with enzymatically reduced BSA content was significantly lower. These results indicate that BSA is a direct regulator of salivary anti-IAV activity and is a determinant of individual differences. Additionally, after comparing the anti-IAV activity across the groups by age, anti-IAV activity in young people (aged 5–19 years) were lower than in adults aged 20–59 years and elderly people aged 60–79 years. Our study suggests that BSA levels in saliva may be important in preventing influenza infection.
Similar content being viewed by others
Introduction
Influenza viruses are some of the most important human pathogens. Seasonal influenza viruses (types A and B) cause epidemics worldwide, with the World Health Organization estimating hundreds of million infections and approximately 250,000–500,000 deaths per year1,2, leading to substantial social and economic burdens worldwide3. Several preventive and curative strategies exist against influenza, including vaccination and therapeutic agents, such as neuraminidase inhibitors and adamantanes4. However, influenza viruses evolve rapidly, which enables them to escape immunity induced by prior infections or vaccination; moreover, those viruses are transmitted efficiently from human to human via respiratory droplets. Recently, there has been substantial concern about the possibility of a pandemic of a novel influenza strain 5, with reports of an increasing resistance of influenza A viruses to neuraminidase inhibitors and adamantanes6. Thus, new approaches are needed to effectively prevent and treat influenza infections.
The oral cavity is one of the entry sites for respiratory viruses. Saliva plays an essential role in maintaining the integrity of human health. It provides lubrication and protection and buffering action and clearance in the oral cavity, maintains tooth integrity, and aids taste and digestion7. A previous study indicated that hyposalivation may be a risk factor for acute respiratory infection8, suggesting that saliva may contribute to the role of innate immunity in the early stages of infection. Saliva induces both antimicrobial and antiviral activities9. Additionally, multiple soluble factors in saliva have potent inhibitory activity against influenza A viruses (IAVs), including sialic acid-containing mucin 5 B (MUC5B), salivary scavenger receptor cysteine-rich glycoprotein-340 (gp-340), alpha-2-microglobulin (A2M), and lectins (surfactant protein D [SP-D])10,11,12,13,14,15,16,17,18. Two main mechanisms for the anti-IAV activity of these factors have been proposed. MUC5B, gp-340, and A2M acts as classic γ inhibitors, mediating the inhibition of IAV by presenting a sialic acid ligand that binds to the viral hemagglutinin (HA), thereby preventing HA attachment to target cells12,13,14,15. In contrast, SP-D acts as a classic β inhibitor by binding the lectin carbohydrate recognition domain (CRD) to specific oligosaccharides on the head of the viral HA16,17,18. It has been shown that salivary anti-IAV activity is primarily due to sialic-acid-containing proteins rather than lectin13,19. As the first line of defense, salivary antiviral factors are likely to play an important role in protection against respiratory infections. However, since previous studies on salivary anti-IAV activity mentioned above have only been conducted using purifying salivary proteins and small size analyses, the actual salivary anti-IAV activity of each individual, and its determinant factors are unknown. To solve this problem, high-throughput evaluation systems are required to evaluate multiple samples simultaneously. In this study, we constructed a new high-throughput system to evaluate salivary anti-IAV activity using neuraminidase activity derived from influenza virus as an index, and we investigated individual variations in salivary anti-IAV activity and its determinant factors.
Materials and methods
Participants
In Study 1 of the individual differences in salivary anti-IAV activity, 92 healthy voluntary participants aged 20–59 years (67 men and 25 women; mean age ± S.D., 36.9 ± 9.9 years) were recruited using snowball sampling. This trial was registered with the University Hospital Medical Information Network (ID: R000028439 UMIN000024720, URL: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_his_list.cgi?recptno=R000028439). In Study 2 of age-related changes in saliva, 240 participants aged 5–79 years (60 young people, 30 boys and 30 girls aged 5–19 years; 120 adults, 60 men and 60 women aged 20–59 years; and 60 elderly people, 30 men and 30 women aged 60–79 years), who registered using a monitor at a contractor (Research and Development, Inc.) were recruited. Participants in Study 2 did not overlap with those in Study 1. In these studies, the exclusion criteria were as follows: (1) patients with serious disease; (2) those going to a hospital regularly and under regular pharmaceutical treatment; (3) those who were pregnant or expecting pregnancy; (4) those who were physically unable to complete the study-related questionnaires; (5) those with scratches in the oral cavity; and (6) those considered unsuitable to participate in the study by the study physician. After receiving a full explanation of the study, all participants and their legal guardians provided written informed consent.
Sample collection and treatment
Saliva samples were collected in a quiet room during the morning, between 09:00 and 10:00, and at least 60 min after eating. Subjects were asked to accumulate saliva in their mouths and spit into a sterile plastic dish at the desired time or indicated time, and saliva samples were collected for 5 min in Study 1 or 10 min in Study 2. The saliva secretion rate (mL/min) was calculated by dividing the amount of saliva collected at the time of collection. Whole saliva samples were centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatant was collected and stored at − 80 °C until use. Frozen saliva samples were thawed and vigorously mixed by vortexing prior to the assay.
Cells and virus
Madin–Darby canine kidney (MDCK) cells were cultured in minimum essential medium (MEM; Sigma-Aldrich Co. LLC, St. Louis, MO) supplemented with 5% (v/v) heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich Co.) and 50 µg/mL gentamicin at 37 °C in humidified air containing 5% CO2. Influenza A virus strains [A/Puerto Rico/8/34 (H1N1)] and [A/Memphis/1/1971 (H3N2)] were propagated at 37 °C using MDCK cells in serum-free medium (SFM; Thermo Fisher Scientific Japan K.K., Kanagawa, Japan) supplemented with 2 µg/mL acetylated trypsin (Sigma-Aldrich Co.) and 50 µg/mL gentamicin.
Anti-IAV activity assays of saliva sample
MDCK cells (4 × 104 cells/well) were cultured in a 96-well plate at 37 °C for 24 h. The saliva samples were diluted eight-fold in phosphate-buffered saline (PBS). Fifty microliters of each saliva sample was mixed with 50 µL of the virus dilutions at a multiplicity of infection (MOI) of 0.5 in PBS and 100 µL of 2 × SFM at room temperature, and the 180 µL mixture was immediately applied to the MDCK cells. After incubation at 37 °C for 30 min, the MDCK cells were washed with PBS to remove the saliva sample and the virus that was not adsorbed on the cells, and incubated in 100 μL of SFM at 37 °C for 16–18 h for virus multiplication. Thirty microliters of the MDCK cell supernatant was mixed with 20 µL of 0.25 mM 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (4MU-Neu5Ac; Funakoshi) and incubated at 37 °C for 30 min. The enzyme reaction for 4MU-Neu5Ac was stopped by adding 200 µL of 100 mM sodium carbonate buffer (pH 10.7). Fluorescence from the sialidase reaction of virus NA was quantified using a microplate reader (Infinite 200 multi-mode Reader, Tecan, Männedorf, Switzerland) with excitation and emission wavelengths of 355 nm and 460 nm, respectively, for 4-metylumbelliferone (4MU). The relative sialidase activity in each sample was calculated by setting the sialidase activity to 100% when PBS was used instead of saliva. Finally, anti-IAV activity was defined by deducting the relative sialidase activity from 100%.
Hemagglutination inhibition (HI) assay
Hemagglutinin (HA), which is present on the membrane surface of influenza viruses, binds to erythrocytes and causes erythrocyte aggregation. We evaluated the inhibitory activity of saliva on the binding of HA to erythrocytes. To remove the nonspecific inhibitory activity of saliva-induced hemagglutination, 100 µL saliva samples were mixed with 200 µL of guinea pig erythrocytes and incubated at 4 °C for 2 h. The mixture was centrifuged at 2,000g for 10 min, and the supernatant (pretreated saliva sample) was used for the HI assay. The PR8 IAV strain was adjusted to 4 hemagglutinin units (HAU) per 25 µL PBS using a pre-hemagglutinin assay. In 96-well plates, a two-fold serial dilution of the pretreated saliva sample was mixed with 25 µL of 4-HAU PR8 IAV strain, followed by the addition of 50 µL of 0.7% guinea pig red blood cells and left at 4 °C for 2 h. The HI titer was calculated as the reciprocal of the highest dilution that produced complete hemagglutination inhibition.
Sialic acid assay
The concentrations of total sialic acid (TSA) and free sialic acid (FSA) in saliva were measured using a sialic acid assay kit (Bioassay Systems). The concentration of protein-bound sialic acid (BSA) was then calculated by subtracting the concentration of FSA from the concentration of TSA.
Salivary proteome analysis
For ultrafiltration, 300 µL of saliva sample was mixed with 200 µL of 100 mM Tris–HCl buffer (pH 8.5; Nippon Gene Co., Ltd.) in an Amicon Ultra-0.5 Centrifugal Filter Unit (3 kDa; Merck Millipore Ltd), and the mixture was spun down at 14,000g for 30 min at 4 °C. The trapped saliva on the filter was filtered three times using 400 µL of Tris–HCl buffer. Finally, the filter on a new tube was spun down at 1,000g for 2 min at 4 °C, and the sample for proteome analysis was collected. The total protein concentration of each sample was determined using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific Inc.) and the saliva sample was prepared with equal concentrations of proteins from each individual sample. The saliva sample containing 100 µg protein in Tris–HCl buffer (90 µL in total) was mixed with 5 µL of 100 mM dl-dithiothreitol (MP Biomedicals, LLC) for the reduction solution and heated at 56 °C for 45 min. The mixture was added to 5 µL of 111 mg/mL iodoacetamide (FUJIFILM Wako Pure Chemical Corporation) and incubated at 37 °C for 30 min in shade for alkylation. The mixture was then mixed with 50 µL of 0.1 µg/µL trypsin solution (Promega Corporation) and incubated at 37 °C for 16 h. The reaction solution was added to a final concentration of 0.3% (v/v) of 10% trifluoroacetic acid (FUJIFILM Wako Pure Chemical Corporation) to stop the reaction, and then subjected to LC–MS/MS analysis. All reagents were diluted with Tris–HCl buffer.
Five microliters of the purified peptides were injected into a high-performance liquid chromatography system (ACQUITY UPLC: Waters) connected to a triple-quadrupole mass spectrometer (TSQ Vantage; Thermo Fisher Scientific) with an ion source of electrospray ionization in multiple reaction monitoring (MRM) mode. Chromatographic separation was performed on a Cadenza CD-C18 column (150 × 1 mm, 3 µm; Imtakt) by binary gradient elution at a flow rate of 0.05 mL/min. Eluent A was 0.1% formic acid in water, while acetonitrile containing 0.1% formic acid was used as eluent B. Ion source (ESI) parameters were optimized as follows: spray voltage, 400 V; vaporizer temperature, 100 °C; sheath gas pressure, 40 Arb; auxiliary gas pressure, 5 Arb; and capillary temperature, 250 °C. The peak areas for each target peptide were calculated using Xcalibur (Quan Browser) software (Thermo Scientific).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of secretory IgA (sIgA), defensin alpha 1, neutrophil (DEFa1), and LL-37 in saliva were measured using competitive ELISA kits (sIgA; Immundiagnostik GmbH, DEFa1; CLOUD-CLONE CORP., LL-37; Hycult Biotech Inc.) in accordance with the manufacturer’s instructions.
Statistical analysis
Results from multiple experiments are expressed as means ± standard deviation (S.D.) and all data were analyzed using IBM SPSS Statistics Version 24 (IBM Japan, Ltd., Tokyo, Japan). Relationships between the two sets of data were analyzed using Spearman's rank correlation. The statistical significance of differences between groups was determined using one-way analysis of variance, followed by Tukey's (Fig. 3) or Kruskal–Wallis (Fig. 4) multiple comparisons test. Results with p < 0.05 were considered statistically significant.
Ethical consideration
All study protocols were approved by the institutional review board of the Kao Corporation (Study 1, 799-20160927, approved on 19 Oct 2016; Study 2, T208-190315, approved on 25 Apr 2019). These studies were performed in accordance with the Declaration of Helsinki.
Results
Construction of high-throughput evaluation system for salivary anti-IAV activity
To simultaneously evaluate the anti-IAV activity in many saliva samples, we constructed a new high-throughput evaluation system using neuraminidase (NA) derived from IAV as an index. Figure 1A shows the schematic of the measurement flow of salivary anti-IAV activity. The measurement consisted of the following steps: (1) mixing the virus and saliva, (2) infection of MDCK cells using the IAV solution and saliva mixture, (3) collecting the supernatant of the cells after 16–18 h of infection, and (4) measurement of sialidase activities of the supernatant for multiplicated virus quantification. The NA activity value when PBS was used instead of saliva was set to 100%, and the relative value was calculated from the NA activity value of saliva samples. The value obtained by subtracting the relative NA activity value from 100 was defined as the anti-IAV activity. A high correlation (ρ = 0.916; p < 0.001) was confirmed between the NA activity value in the supernatant of infected cells and the number of infected cells stained for NP protein when MDCK cells were infected with IAV mixed with various saliva samples (Fig. 1B).
Construction of high-throughput assay of salivary anti-IAV activities. (A) Schematic of the measurement of salivary anti-IAV activities. (B) Correlation between viral sialidase activity and infected cell count. The plot indicates significant moderate positive correlation. ρ Spearman's correlation coefficient. ***p < 0.001.
Individual differences in salivary anti-IAV activity
Using the established system, salivary anti-IAV activity against the PR8 IAV strain (H1N1) in 92 individuals was assessed. Anti-IAV activity varied widely between individuals, ranging from 2 to 97% (Fig. 2A). Furthermore, salivary anti-IAV activity against the Memphis IAV strain (H3N2) was significantly positively correlated with anti-IAV activity against the PR8 IAV strain (ρ = 0.638; p < 0.001) (Fig. 2B). We then performed a correlation analysis of salivary anti-IAV activity against the PR8 IAV strain and the amount of various salivary components measured using proteomic analysis and some kits to explore factors associated with individual differences in salivary anti-IAV activity (Fig. 2C). The results showed the highest positive correlation between salivary anti-IAV activity and BSA levels (ρ = 0.473; p < 0.001). In addition, the concentration of several proteins, such as AGTN/gp-340, ZG16B, SGP28, and C6orf58, significantly positively correlated with anti-IAV activity.
Individual differences of salivary anti-IAV activities. (A) Salivary anti-IAV activities against PR8 IAV strain (n = 92). Data are presented as mean ± SD from three independent experiments. A bar graph shows the activity in each subject. (B) Correlation between salivary anti-IAV activity against PR8 and Memphis IAV strains (n = 65). The plot shows significant moderate positive correlation. (C) The correlation between salivary anti-IAV activity against the PR8 IAV strain and amount of various salivary components (n = 72–85). A bar graph shows Spearman’s correlation coefficient (ρ) in each subject. *p < 0.05; **p < 0.01; ***p < 0.001.
Contribution of BSA to salivary anti-IAV activity
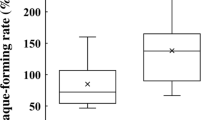
To clarify whether BSA contributes directly to the anti-IAV activity of saliva, we assessed saliva samples that were heat-treated or in which BSA was enzymatically removed. The amount of BSA in the saliva was significantly reduced by α(2-3,6)-sialidase treatment and was not altered by heat treatment (Fig. 3A). While the anti-IAV activity of untreated saliva samples averaged about 50%, the anti-IAV activity of heat-treated saliva samples decreased to an average of 25% (Fig. 3B). In contrast, the anti-IAV activity of sialidase-treated saliva samples decreased to less than 10% on average (Fig. 3B). To further elucidate the contribution of BSA to salivary anti-IAV activity, a hemagglutination inhibition (HI) test was performed. Similar to the anti-IAV activity, large individual differences were observed in HI titers in some saliva samples (Fig. 3C). In addition, individual HI titers highly positively correlated with anti-IAV activity (Fig. 3D). Although no effect of heat treatment on HI titer was observed, sialidase treatment significantly decreased the HI titer (Fig. 3E).
Contribution of protein-bound sialic acid to salivary anti-IAV activity. Effect of α(2-3,6)-sialidase or heat treatment on (A) the concentration of BSA and (B) salivary anti-IAV activity against the PR8 IAV strain. (C) Hemagglutination inhibition assay with saliva (n = 8, A–H). The bar graph shows the HI titers of each subject. This experiment was repeated three times with similar results. (D) Correlation between salivary anti-IAV activity against the PR8 IAV strain and the HI titer of saliva. The plot shows a moderately positive correlation. (E) The effect of α(2-3,6)-sialidase and heat treatment on the HAI titer of saliva. Data are presented as mean ± SD for each group. Average plateau values were compared using one-way analysis of variance and Tukey’s test. ρ Spearman's correlation coefficient. *p < 0.05; ***p < 0.001.
Age-related differences in salivary anti-IAV activity
We then compared salivary anti-IAV activity across a wide range of ages (5–79 years divided into three groups, young people (aged 5–19), middle adults (aged 20–59 years), and elderly people (aged 60–79 years), using the high-throughput evaluation system we constructed. As a result of comparing the gender differences within each group, no difference was observed in the mean value of saliva secretion rate, anti-IAV activity, and BSA level (data not shown). Therefore, subsequent analyses did not distinguish between gender. The mean value of saliva secretion rate decreased with increasing age and was lowest in elderly people (Fig. 4A). Moderate correlation was observed for between age and salivary anti-IAV activity in individuals (rs = 0.29, p < 0.001). The mean value of salivary anti-IAV activity was similar in middle adults and elderly people, and was significantly lower in young people (Fig. 4B). The mean value of BSA concentration in saliva was significantly lower in elderly people than middle adults (Fig. 4C). In each age group, the salivary anti-IAV activity significantly positively correlated with salivary BSA level (young; ρ = 0.366, p < 0.05, middle adults; ρ = 0.589, p-value < 0.001, elderly; ρ = 608, p-value < 0.001, total; ρ = 0.511, p-value < 0.001) (Fig. 4D).
Age-related differences in salivary anti-IAV activity. Comparison of (A) the saliva secretion rate (mL/min), (B) salivary anti-IAV activity against the PR8 IAV strain, (C) BSA concentration in saliva, and (D) correlation between salivary anti-IAV activity against the PR8 IAV strain and the concentration of BSA in young people (30 boys and 30 girls aged 5–19 years), middle adults (60 men and 60 women aged 20–59 years), and elderly people (30 men and 30 women aged 60–79 years). Data are presented as mean ± SD of each group. Average plateau values were compared using one-way analysis of variance and the Kruskal–Wallis test. *p < 0.05; ***p < 0.001.
Discussion
In this study, we constructed a high-throughput system to evaluate salivary anti-IAV activity. Using this system, we found that there are large individual differences in salivary anti-IAV activity, and that the amount of BSA in saliva is important for these individual differences.
In general, the main method of measuring the viral infection titer is to count the number of infected cells by staining with antibodies against the virus and clumps of cellular degeneration associated with viral infection. While these methods are suitable for measuring the virus titer, it is difficult to evaluate multiple samples because this approach requires a great deal of time and labor because of the need for operations, such as multi-step dilutions and infected cells or colony counting. In our constructed system, the neuraminidase activity derived from the propagated influenza virus in the culture medium was measured using a multi-plate reader, which eliminates the operations described above and is suitable for the analysis of multiple samples. A significant positive correlation was observed between salivary anti-IAV activity against the PR8 (H1N1) and Memphis (H3N2) strains (Fig. 2B), indicating that individual differences in salivary anti-IAV activity are similar across influenza virus strains. It is expected that this method can be used in the evaluation of many influenza virus strains in the future.
Using the constructed system, it was clarified that the individual variations in salivary anti-IAV activity most positively correlated with the amount of BSA (Fig. 2C). Moreover, the HI titers of saliva were significantly reduced by treatment with α(2-3,6)-sialidase (Fig. 3B). Some studies have reported that saliva contains soluble factors that can exert anti-influenza activity, such as sialic acid binding protein and lectins. Although glycolipids and glycopolysaccharides are also present in saliva, their concentrations are much lower than those of proteins20,21. Thus, their contribution as a source of sialic acid is considered to be low. It has been reported previously that several purified salivary proteins, such as AGTN/gp-340 and A2M, can inhibit the hemagglutination of erythrocytes by presenting a sialic acid ligand for the viral HA12,13,14,15. In addition, Qin et al. reported that saliva containing more α2-3/6-linked sialic acid residues has high binding properties to IAV22. These reports are consistent with our results, and indicate that the total amount of sialic acid possessed by these proteins determines the anti-IAV activity of saliva. In other words, the amount of BSA in the saliva is expected to be an indicator of frontline oral defenses against influenza viruses.
MUC5B has potent inhibitory activity against IAV. However, our data showed that salivary MUC5B levels weakly correlated with the anti-IAV activity of saliva. White et al. reported that the sialic acid of MUC5B is more easily cleaved than that of gp-340, the levels of which highly correlated with anti-IAV activity. Our results suggest that the sialic acid of MUC5B is cleaved by sialidase in the oral cavity23,24,25, and the anti-IAV activity of MUC5B in saliva may be reduced. In considering individual variations in the anti-IAV activity of saliva, it is necessary to consider oral sialidase activity. In this study, SGP28, C6orf58, and PIP also strongly correlated with the anti-IAV activity of saliva. To date, the biological functions of SGP28 and C6orf58 have not been clarified. PIP, on the other hand, is known as a water-soluble antimicrobial protein26. It binds to and agglutinates bacteria, the detailed mechanism of its antimicrobial properties is not fully understood. These molecules are known to have sugar chains27,28,29. Although the presence of sialic acid of these molecules is not clear, they may also contribute to the anti-IAV activity of saliva via sialic acid, which is less susceptible to cleavage by sialidase. It is known that sialic acids are structurally diverse and that O-acetylated sialic acids are resistant to bacterial sialidase30. In the future, it will be necessary to clarify the presence or absence of sialic acid in these molecules, including their structural features.
Interestingly, the anti-IAV activity of saliva was reduced following excessive sialidase treatment, but was not completely lost (Fig. 3A, B). Moreover, salivary anti-IAV activity also decreased following heating, which did not affect the concentration of sialic acid. Therefore, it is possible that sialic acid-independent factors are also involved in anti-IAV activity. One of the possible factors is the action of lectin-like proteins, such as ZG16B, which have shown a high correlation with anti-IAV activity31,32. Lectin (carbohydrate-binding proteins), including conglutinin, SP-D, MBL, and SAP, bind selectively to specific carbohydrate structures (mannose- over galactose-type sugars) located at the head of the influenza HA of susceptible strains, thereby blocking the ability of HA to bind to sialylated cell-surface receptors16,17. Furthermore, lectin-like proteins may also inhibit the fusion of the viral membrane with the host cell by binding to sialic acid on the surface of the host cell18. In our evaluation system, since host cells were exposed to a mixture of saliva and virus for 30 min, the action of lectin-like proteins in saliva that inhibit the contact between host cells and virus would also contribute to the anti-IAV activity of saliva. On the other hand, in our evaluation system, the activity of molecules that inhibit the later mechanisms of virus propagation, such as replication and release, is not reflected in the anti-IAV activity. For this reason, defensin and lactoferrin, which are known to inhibit replication of IAV12,33,34,35,36,37, showed only a weak correlation with anti-IAV activity in our study.
The current thinking regarding influenza infection morbidity is that the risk factors are related to age, higher rates of infection in school-aged children relative to adults, and lower rates in the elderly. Children spend a great deal of time in communities where daily contact with other people is extensive; for example, in schools, playgroups, and daycare centers, and it is assumed that close contact favors infection38,39. The results of evaluating differences in salivary properties by age (Fig. 4) showed that salivary volume, which has been reported as a predictive risk factor for influenza transmission8, was higher in the younger age group. On the other hand, the mean value of anti-IAV activity of saliva in the young group was significantly lower than that in the middle and elderly groups. These results suggest that low salivary anti-IAV activity may also be associated with a high rate of IAV transmission in school-aged children. In the present study, although the mean value of BSA concentration in saliva of young group was not significantly different from that of the middle and elderly groups, salivary anti-IAV activity was highly correlated with sialic acid in all age group, indicating that the determinants of anti-IAV activity were not as sensitive to age. Previous studies have shown that glycoproteins capable of binding IAV are lower in the saliva of children than in adults and the elderly, supporting our results22. Thus, qualitative control of saliva in young people may be effective in preventing IAV infection.
There are two major limitations to this study as follow: First, we have not been able to define the contribution of proteins such as SGP28, C6orf58, and PIP that are significantly correlated with anti-IAV activity. It is important to prepare these purified proteins from saliva and conduct experiments to determine the relationship between glycosylation levels of these proteins and anti-IAV activity. Second, we have not been able to examine the effect of salivary anti-IAV activity on respiratory illnesses. Retrospective study the relationship between salivary anti-IAV activity and history of respiratory disease and recent antibody prevalence against influenza viruses, or prospective intervention trials with techniques that provide control of salivary anti-IAV are needed to gain insight into the contribution of salivary anti-IAV activity.
Our study revealed that there are large individual differences in the anti-IAV activity of saliva, which mainly depends on the amount of BSA. BSA directly contributes to salivary anti-IAV, which may contribute to influenza morbidity in young people. In conclusion, the understanding of individual differences in salivary anti-IAV activity may provide new insights into creating effective methods to prevent influenza infection.
References
Stöhr, K. Influenza—WHO cares. Lancet Infect. Dis. 2, 517. https://doi.org/10.1016/s1473-3099(02)00366-3 (2002).
World Health Organization. Influenza (seasonal). http://www.who.int/mediacentre/factsheets/fs211/en/ (2014).
Molinari, N. A. et al. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 25(27), 5086–5096. https://doi.org/10.1016/j.vaccine.2007.03.046 (2007).
Kamali, A. & Holodniy, M. Influenza treatment and prophylaxis with neuraminidase inhibitors: A review. Infect. Drug Resist. 6, 187–198. https://doi.org/10.2147/IDR.S36601 (2013).
Sun, H. et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc. Natl. Acad. Sci. U.S.A. 117, 17204–17210. https://doi.org/10.1073/pnas.1921186117 (2020).
Bai, G. R. et al. Amantadine- and oseltamivir-resistant variants of influenza A viruses in Thailand. Biochem. Biophys. Res. Commun. 390, 897–901. https://doi.org/10.1016/j.bbrc.2009.10.071 (2009).
Sreebny, L. et al. Saliva: Its role in health and diseases. Int. Dent. J. 42, 291–304 (1992).
Iwabuchi, H. et al. Relationship between hyposalivation and acute respiratory infection in dental outpatients. Gerontology 58, 205–211. https://doi.org/10.1159/000333147 (2012).
Malamud, D. et al. Antiviral activities in human saliva. Adv. Dent. Res. 23, 34–37. https://doi.org/10.1177/0022034511399282 (2011).
Seltsam, J. H., Lanni, F. & Beard, J. W. Inhibitory effect of human saliva on hemagglutination by influenza virus A (PR8 strain) and the swine influenza virus. J. Immunol. 63, 261–271 (1949).
Roella, G. Investigations of some effects of human saliva on influenza virus. 2. Kinetics of haemagglutination inhibition. A comparison of unspecific inhibition by saliva and inhibition by immune serum. Acta Pathol. Microbiol. Scand. 64, 224–228. https://doi.org/10.1111/apm.1965.64.2.224 (1965).
White, M. R. et al. Multiple components contribute to ability of saliva to inhibit influenza viruses. Oral Microbiol. Immunol. 24, 18–24. https://doi.org/10.1111/j.1399-302X.2008.00468.x (2009).
Hartshorn, K. L. et al. Lung and salivary scavenger receptor glycoprotein-340 contribute to the host defense against influenza A viruses. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L1066–L1076. https://doi.org/10.1152/ajplung.00057.2003 (2003).
Hartshorn, K. L. et al. Salivary agglutinin and lung scavenger receptor cysteine-rich glycoprotein 340 have broad anti-influenza activities and interactions with surfactant protein D that vary according to donor source and sialylation. Biochem. J. 393, 545–553. https://doi.org/10.1042/BJ20050695 (2006).
Chen, C. H. et al. The essentiality of alpha-2-macroglobulin in human salivary innate immunity against new H1N1 swine origin influenza A virus. Proteomics 10, 2396–2401. https://doi.org/10.1002/pmic.200900775 (2010).
Hartshorn, K. L. et al. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Investig. 94, 311–319. https://doi.org/10.1172/JCI117323 (1994).
Hartshorn, K. L. et al. Role of viral hemagglutinin glycosylation in anti-influenza activities of recombinant surfactant protein D. Respir. Res. 9, 65. https://doi.org/10.1186/1465-9921-9-65 (2008).
Ng, W. C. et al. Soluble host defense lectins in innate immunity to influenza virus. J. Biomed. Biotechnol. 2012, 732191. https://doi.org/10.1155/2012/732191 (2008).
Limsuwat, N. et al. Susceptibility of human and avian influenza viruses to human and chicken saliva. J. Med. Virol. 86, 872–878. https://doi.org/10.1002/jmv.23751 (2014).
Slomiany, B. L. et al. Lipid composition of human parotid and submandibular saliva from caries-resistant and caries-susceptible adults. Arch. Oral Biol. 27, 803–808. https://doi.org/10.1016/0003-9969(82)90033-4 (1982).
Pogrel, M. A. et al. Hyaluronan (hyaluronic acid) in human saliva. Arch. Oral Biol. 41, 667–671. https://doi.org/10.1016/s0003-9969(96)00050-7 (1996).
Qin, Y. et al. Age- and sex-associated differences in the glycopatterns of human salivary glycoproteins and their roles against influenza A virus. J. Proteome Res. 12, 2742–2754. https://doi.org/10.1021/pr400096w (2013).
Lewis, A. L. & Lewis, W. G. Host sialoglycans and bacterial sialidases: A mucosal perspective. Cell Microbiol. 14, 1174–1182. https://doi.org/10.1111/j.1462-5822.2012.01807.x (2012).
Kamio, N. et al. Neuraminidase-producing oral mitis group streptococci potentially contribute to influenza viral infection and reduction in antiviral efficacy of zanamivir. Cell Mol. Life Sci. 72, 357–366. https://doi.org/10.1007/s00018-014-1669-1 (2015).
Gilbertson, B. et al. Mouse saliva inhibits transit of influenza virus to the lower respiratory tract by efficiently blocking influenza virus neuraminidase activity. J. Virol. 91, e0010145-17. https://doi.org/10.1128/JVI.00145-17 (2017).
Nistor, A. et al. Influence of mouse prolactin-inducible protein in saliva on the aggregation of oral bacteria. Oral Microbiol. Immunol. 24, 510–513. https://doi.org/10.1111/j.1399-302X.2009.00543.x (2009).
Udby, L. et al. An ELISA for SGP28/CRISP-3, a cysteine-rich secretory protein in human neutrophils, plasma, and exocrine secretions. J. Immunol. Methods 263, 43–55. https://doi.org/10.1016/s0022-1759(02)00033-9 (2002).
Ramachandran, P. et al. A comparison of N-linked glycoproteins in human whole saliva, parotid, submandibular, and sublingual glandular secretions identified using hydrazide chemistry and mass spectrometry. Clin. Proteomics 4, 80–104. https://doi.org/10.1007/s12014-008-9005-0 (2008).
Wiegandt, A., Behnken, H. N. & Meyer, B. Unusual N-type glycosylation of salivary prolactin-inducible protein (PIP): Multiple LewisY epitopes generate highly-fucosylated glycan structures. Glycoconj. J. 35, 323–332. https://doi.org/10.1007/s10719-018-9826-7 (2018).
Barnard, K. N. et al. Modified sialic acids on mucus and erythrocytes inhibit influenza A virus hemagglutinin and neuraminidase functions. J. Virol. 94, e01567-e1619. https://doi.org/10.1128/JVI.01567-19 (2020).
Perumal, N. et al. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci. Rep. 6, 29629. https://doi.org/10.1038/srep29629 (2016).
Saitou, M. et al. Functional specialization of human salivary glands and origins of proteins intrinsic to human saliva. Cell Rep. 33, 108402. https://doi.org/10.1016/j.celrep.2020.108402 (2020).
Hartshorn, K. L. et al. Innate defense against influenza A virus: Activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 176, 6962–6972. https://doi.org/10.4049/jimmunol.176.11.6962 (2006).
Salvatore, M. et al. Alpha-defensin inhibits influenza virus replication by cell-mediated mechanism(s). J. Infect. Dis. 196, 835–843. https://doi.org/10.1086/521027 (2007).
Park, M. S. et al. Towards the application of human defensins as antivirals. Biomol. Ther. (Seoul) 26, 242–254. https://doi.org/10.4062/biomolther.2017.172 (2018).
Ammendolia, M. G. et al. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 106, 12–19. https://doi.org/10.1179/2047773212Y.0000000004 (2012).
Wakabayashi, H. et al. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 20, 666–671. https://doi.org/10.1016/j.jiac.2014.08.003 (2014).
Riley, S. et al. Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med. 8, e1000442. https://doi.org/10.1371/journal.pmed.1000442 (2011).
Viboud, C. et al. Risk factors of influenza transmission in households. Br. J. Gen. Pract. 54, 684–689 (2004).
Acknowledgements
We would like to thank Editage (http://www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
T.M. and N.O. designed the project; K.K., C.S., and H.K. performed the research; K.K., C.S., H.K., T.M., Y.K., and T.S. analyzed the data; K.K., C.S., and T.M. wrote the paper. T.M., N.O., Y.K., and T.S. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Kaori Kobayashi, Chika Shono, Takuya Mori, Hidefumi Kitazawa, and Noriyasu Ota are employees of Kao Corporation. Kao Corporation had no involvement in preparing this article in terms of study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit the article for publication. Yuki Kurebayashi and Takashi Suzuki have no competing interests to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kobayashi, K., Shono, C., Mori, T. et al. Protein-bound sialic acid in saliva contributes directly to salivary anti-influenza virus activity. Sci Rep 12, 6636 (2022). https://doi.org/10.1038/s41598-022-10559-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10559-4
- Springer Nature Limited