Abstract
We have analyzed the histopathological, clinical, and genetic characteristics in hereditary breast and ovarian cancer patients of counselled families from 1996 up to today in the southwestern Finland population. In this study we analyzed the incidence of different BRCA1 and BRCA2 pathogenic variants (PV). 1211 families were evaluated, and the families were classified as 38 BRCA1 families, 48 BRCA2 families, 689 non-BRCA families and 436 other counselled families (criteria for genetic testing was not met). In those families, the study consisted of 44 BRCA1 breast and/or ovarian cancer patients, 58 BRCA2 cancer patients, 602 non-BRCA patients and 328 other counselled patients. Breast cancer mean onset was 4.6 years earlier in BRCA1 carriers compared to BRCA2 (p = 0.07, a trend) and ovarian cancer onset almost 11 years earlier in BRCA1 families (p < 0.05). In BRCA families the onset of ovarian cancer was later than 40 years, and BRCA2-origin breast cancer was seen as late as 78 years. The BRCA PV (9%) increases the risk for same patient having both ovarian and breast cancer with a twofold risk when compared to non-BRCA group (4%) (95% CI p < 0.05). Triple-negativity in BRCA1 (42%) carriers is approximately 2.6 times vs more common than in BRCA2 carriers (16%) (p < 0.05). The risk ratio for bilateral breast cancer is approximately four times when compared BRCA2 (17%) and other counselled patients’ group (4%) (p < 0.05). 27% southwestern BRCA2-families have a unique PV, and correspondingly 39% of BRCA1-families. The results of this analysis allow improved prediction of cancer risk in high-risk hereditary breast and ovarian families in southwestern Finland and improve long term follow-up programs. According to the result it could be justified to have the discussion about prophylactic salpingo-oophorectomy by the age of 40 years. The possibility of late breast cancer onset in BRCA2 carriers supports the lifelong follow-up in BRCA carriers. Cancer onset is similar between BRCA2 carries and non-BRCA high-risk families. This study evaluated mutation profile of BRCA in southwestern Finland. In this study genotype–phenotype correlation was not found
Similar content being viewed by others
Introduction
Breast cancer is the second most common cancer for females worldwide1. The risk for breast cancer is approximately 13% in Finland2 and approximately 5–10% of all breast cancers are inherited3. In Finland pathogenetic variants (PVs) that lead to a risk of 40% or higher for breast cancer are classified as high-risk variants4. Hereditary breast cancer susceptibility genes consist of high-risk variants and moderate-risk variants. It has been suggested that 25% of the hereditary breast cancer is due to BRCA1 or BRCA2 PV5. Published early studies in Finland 2000 and 2002 showed that BRCA PVs were associated with 20% of breast cancer families6,7, but lately the association has decreased as more patients are being tested due to widened gene test criteria, technological improvement in testing, and the refining of referral criteria and their easy discoverability online. In southwestern Finland the amount of BRCA PV in relation to all high-risk families so is 9.5% (unpublished observation). The ratio of BRCA PV in relation to all breast cancer patients varies geographically. In Finland proportion of BRCA breast cancer of all breast cancers is relatively low, the exact value is not known currently. In a Swedish study, the prevalence of BRCA1 and BRCA2 PVs was 1.8% of all unselected breast cancer patients8,9. Among breast cancer patients with cancer onset age under 40 years PV incidence has been shown to be higher than in other age groups: for example, in the United States Buys et al. observed BRCA1 or BRCA2 PV in about 8–14% of all young breast cancer patients10. In this study we investigated the onset of breast and ovarian cancer in different breast and ovarian cancer families according to family cancer risk type, result of BRCA test and type of BRCA PV. Additionally, we compared histopathologic characters in different risk groups.
The approximate risk of breast cancer is 65–79% with BRCA1 PV and 61–77% for BRCA2 PV11. The approximate risk of ovarian cancer is 40% for BRCA1 PV and 20% in BRCA2 PV11,12. Men with BRCA2 PV have approximately a 6% risk of breast cancer, for men with BRCA1 PV the risk is approximately 1%13. After 40 years of age BRCA2 PV causes up to 5 times higher prostate cancer risk compared to men in general population14. 40–60 year old BRCA1 carrier men’s cancer risk is twice that of men in general population14. Some genotype-fenotype correlation has been detected only in few PVs15. Currently, the knowledge about genotype–phenotype correlation is still not sufficient to use in individual risk assessment16. In our study we compared mutation profile to cancer onset.
Detecting families with BRCA PV is essential as it improves the cancer prognosis via follow-up and prophylactic surgery. Family’s females with BRCA1 or BRCA2 PV can participate in a breast screening. Most carriers have ovarian and fallopian tube removal that decreases ovarian cancer risk significantly and may halve the breast cancer risk17,18,19. It is also possible to organize gynecologic follow-up from 40 years onwards if patient does not want prophylactic bilateral salpingo-oophorectomy20. Skin-sparing mastectomy and breast reconstruction are also possible for patients with BRCA PV as mastectomy reduces breast cancer risk significantly21,22. For those BRCA PV patients who have had breast cancer it is possible to receive tamoxifen, to reduce risk of contralateral breast cancer, if prophylactic mastectomy is not done23. Usage of PARP inhibitors is possible in certain ovarian cancer patients including patients with BRCA PV24.
Currently gene testing is not done for all breast cancer patients as it has not been found cost-effective25, but there are studies investigating the cost-effectives of widespread BRCA screening8,26. BRCA1 and BRCA2 tumor analyses is done for all ovarian cancer patients.
Methods
Materials
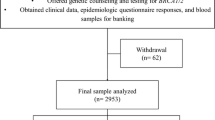
A retrospective cohort study was made of all families who had had genetic counseling at the Department of Clinical Genetics in Turku University Hospital because of hereditary breast cancer suspect. Counseling has been held between 1996 and 2019. The counseled patients were given referrals from southwestern area of Finland and this area is named as “The expert responsibility area (ERVA) of the Tyks Turku University Hospital”. This analysis compares the onset of breast and ovarian cancer in BRCA1, BRCA2, non-BRCA and other counselled families. Genetic testing has been done for all patients except those in other counselled group, as they did not meet the criteria for genetic testing. We also compare the onset of breast and ovarian cancer in relation to different BRCA genes and to different BRCA pathogenic variants.
Families in our cohort are categorized to families with high breast cancer risk using modified familial high-risk criteria (Table 1). In our department genetic testing has been done after doctor’s evaluation based on family tree, patients medical record and genetic testing criteria. Genetic testing criteria has changed during the years, and we have followed the guidelines presented in Table 2 when evaluating possible benefits of genetic testing. Genetic testing in the family is always started from the family member who has had cancer, as then it is most likely to find the family PV. DNA is isolated from white blood cells in normal venous blood sample. If the family member has died, it is possible to isolate the DNA from one’s healthy tissue sample with relative’s approval.
BRCA 1 and BRCA2 gene test analysis
Since 1996 genetic testing methods have developed significantly. The whole gene sequencing is necessary as the mutation can locate in any part of the gene. Since 2011 both BRCA genes have been checked with Sanger sequencing. In addition, genes were tested with MLPA-reaction to detect deletions and duplications.
In 2017, next-generation sequencing (NGS) were used in 17% of breast and ovarian cancer panel. By 2019 all screening studies were done by NGS gene panel to obtain a family diagnosis. With gene panels it is possible to analyze multiple breast and ovarian cancer-associated gene mutations at once and it is faster than Sanger sequencing. For analysis, NGS libraries were prepared using BRCA Mastr Plus Dx kit (Agilent) and sequenced with Nextseq 500 sequencer (Ilumina). Bioinformatics analysis was performed with Sophia DDM (Sophia Genetics). Large genomic copy number variation was analysed with SALSA MLPA P002 and P045 probe kits for BRCA1 and BRCA2, respectively. Fragment were analyzed with ABI 3500 xl Dx sequencer and GeneMarker software (Softgenetics).
Gene panel includes genes that are associated with increased breast cancer risk: BRCA1, BRCA2, TP53, PTEN, STK11, CDH1, PALB2, CHEK2, ATM, FANCM. Panel also includes genes that are associated especially with increased ovarian cancer risk (BRIP1, RAD51C, RAD51D) and genes associated with Lynch syndrome, which can increase the ovarian cancer risk (EPCAM, MLH1, MSH2, MSH6, PMS2)4,25,27,28,29,30. In this study we analyzed the incidence of BRCA1 and BRCA2 PV in cancer patients of counseled families.
Variant nomenclature and classification
For variant classification, ACMG guidelines were used and variants were described according to HGVS nomenclature29,30. Pathogenicity predictions were made with Align GVGD, SIFT, Mutation Taster, PolyPhen-2 and CADD tools and Enigma, BIC, Clinvar, HGMD and GnomAD databases. Genbank reference sequences NM_007294.3 and NM_000059.3 were used for variant nomenclature.
Statistical analysis
SAS Studio software version 3.8 (SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses. Sociodemographic and clinical variables were summarized using descriptive statistics, such as mean and standard deviation (SD) and frequencies and percentages. Dichotomous outcomes between different groups were reported using risk ratio (RR) with 95% confidence intervals (CI) and significance was analyzed using the Fischer’s exact test. Mean difference of cancer age was evaluated using Students’s T-Test, cancers with unknown age was discarded from the mean age test. All tests were two-sided and p-value less than 0.05 was considered to be statistically significant.
Ethics approval and consent to participate
This study is hospital quality research, which has been authorized by Turku University Hospital and has valid ID. The study was not an experimental study. In the study analyzed data was from patients who had previously been treated at the hospital. Consent was obtained from all subjects or their legal guardians during treatment. All methods were carried out in accordance with relevant guidelines and regulations. As no new samples in this study were required a separate ethics board permit was not required. This is as guided by the ethics committee at Turku Clinical Research Center.
The Turku Clinical Research Center provides services in the field of health scientific research for researchers of the University of Turku and the Turku special responsibility area it also hosts the ethics committee.
Results
1211 families were evaluated in southwestern Finland with clinical and family history that suggested hereditary breast and ovarian cancer. The families were classified as BRCA1 families, BRCA2 families, non-BRCA families, and other counselled families. The amount of cancer patients in these groups are shown in Table 3.
Table 4 shows the amount of breast and ovarian cancer cases in the families and their details. Note that if the patient had a bilateral breast cancer, it was calculated as two breast cancer cases.
Significance of breast cancer onset age was analyzed between groups by T-test. Table 5 shows significant or trending result of this analysis, non-significant results are not shown.
Significance of ovarian cancer onset age was analyzed between groups by T-test. Table 6 shows the significant or trending result of this analysis, non-significant results are not shown.
Triple-negativity was analyzed by calculating the risk ratio (RR) of triple-negative breast cancer patients between different groups with exact Fisher test. Significant and trending results are shown in Table 7, non-significant results are not shown.
Bilateral breast cancer was analyzed by calculating the risk ratio (RR) of bilateral breast cancer patients between different groups with exact Fisher test. Significant and trending results are shown in Table 8, non-significant results are not shown.
The risk ratio (RR) of a patient having ovarian and breast cancer (single or bilateral) was analyzed with exact Fisher test. Significant and trending results are shown in Table 9, non-significant results are not shown.
Breast and ovarian cancer onset age was also evaluated with age brackets to compare their distribution. Due the difference in N values distribution instead of absolute values were used. Figure 2 shown the cumulative breast cancer cases as a function to age and Fig. 3 for ovarian cancer cases correspondingly.
There are several BRCA PVs. If the same PV appears in several families, it is considered a founder PV. The list of founder PV is in Table 10. 15 BRCA1 families have a PV that does not appear in other families. 23 BRCA1 families have a common PV. 13 BRCA2 families have a unique PV. 35 BRCA2 families a common PV.
Table 11 shows the three PV found in southwestern Finland and that are very rare in other parts of Finland.
The variants have a slightly different cancer onset age. Figure 4 shows this for the most common variants in southwestern Finland. There are no clusters in the breast cancer onset. The germline variants of BRCA1 and BRCA2 identified in this study are shown in Supplementary Table 1 and Table 2.
Discussion
Onset of breast and ovarian cancer in BRCA1 and BRCA2 families
In this study breast cancer onset was 4–6 years earlier for BRCA1 patients compared to patients in all other groups. BRCA1 compared to BRCA2 result was only a trend, most likely due to the low number of breast cancer incidences. These observations are similar than reported in other studies7,15. Of interest is that in southwestern Finland breast cancer onset was similar between BRCA2 families and in non-BRCA families and other counselled families.
In this study ovarian cancer onset was 8–11 years earlier for BRCA1 patients compared to patients in BRCA2 and non-BRCA group. Compared to others group the difference was not significant most likely due to low number of incidences. These observations are similar than reported earlier15.
Recommendations for follow-up programs are updated regularly43. In this study in BRCA families the onset of ovarian cancer is later than 40 years and breast cancer later than 26 years. Therefore, magnetic resonance imaging (MRI) screening for breast cancer from the age of 25 years is supported by our results. In non-BRCA group a very early onset of breast and ovarian cancer of less than 30 years’ was seen. This result may reflect that early breast cancer onset age is affected by polygenetic factors44,45, which are not well known currently. In our study, the risk of breast cancer decreases significantly after 70 years of age in BRCA patients but is still higher than in average population. The observation of our study supports the lifelong follow-up in BRCA1 and BRCA2 carriers as is the current recommendations46. MRI is recommended for BRCA1 and BRCA2 carriers47. After 70 years of age MRI can be replaced with mammography.
Risk-reducing prophylactic bilateral salpingo-oophorectomy is recommended for BRCA patients shortly after 40 years if the patient is willing for the surgery48. In the study of Kuchenbaecker et al.11 the incidence of BRCA2 ovarian cancer is rising from the age of 50 years and BRCA1 ovarian cancer over 10 years earlier11. It is opposite to the results of our study where the ovarian cancer onset for both BRCA1 and BRCA2 patients was soon after 40 years. According to the result it could be justified to have the discussion about prophylactic ovarian removal with a gynecologist by the age of 40 years.
Other histopathologic and clinical features in BRCA1 and BRCA2
The amount of triple-negativity represents 10–20% of invasive breast cancers in general population49. In our study triple-negativity is seen in 38% of BRCA1 breast cancers. This is similar that has been seen in other studies50. Also, the ratio of triple-negativity between this study’s groups was in line with other studies50.
In all patients with breast cancer the cumulative incidence for contralateral breast cancer increases approximately 6% after 15 years23,51. Contralateral breast cancer risk is significantly higher in BRCA carries (about 39%)23. The 10-year risk of contralateral cancer is approximately 43% for BRCA1 carriers and 35% for BRCA2 carriers52. In our material in BRCA2 carriers bilateral breast cancer was more common compared to BRCA1 carriers, but this result was not significant.
This study also shows that the risk having both ovarian cancer and breast cancer is higher in BRCA1 than in non-BRCA group (trend). This finding is in line with earlier studies, that have concluded that having both breast cancer and ovarian cancer raise the suspicion of BRCA PV.
Type of pathogenic variants in southwestern Finland
More than 1800 pathogenic variants have been detected in both BRCA1 and BRCA2 genes16. This study is the first study, which investigates the mutation profile in southwestern Finland. So far there are 23 different PV types in BRCA1 and 18 in BRCA2 in counseled families. According to prior publications in the group of BRCA2 families same PV appears more often in many families than in the group of BRCA1 families7,9. We found that ten BRCA2 families (21%) share the same PV c.771_775delTCAAA, which is very common in Finland, and 73% of all BRCA2 families share a common PV. Of all BRCA1 families 61% share a common PV. This observation is different to earlier studies in which in Finland 80% of BRCA PVs are common7,9,33. Our observation suggests that in southwestern Finland families more often have a unique mutation than in other parts of Finland.
We observed that all BRCA2 PVs that were seen in more than a single families are also common in other part of Finland4,6,9,33. In Finland the large number of common PV in BRCA families is due to a strong founder effect. Finland is a geographically and culturally isolated country. A small population inhabited area that is nowadays known as Finland. The mutations of this population have enriched different Finnish regions over the years32,33,34. Spectrum of BRCA1 founder PV is wider than BRCA2 PV, where a small group of founder PVs are over presented in breast- and ovarian cancer families32. Due to the founder effect the most common founder PVs in Finland are not as common in Caucasian or European population, however there are some common PVs naturally37.
We also observed a common PV named 6-KB DUP EX13 (more specifically c.4186-1787_ 4358-1668dup6081), which is very rare in other part of Finland, but common in Sweden. To our best knowledge, this pathogenic variant has not been published in any other part of Finland. Common PV found in other parts of Finland, but that were not found in our study at Southwestern Finland were c.4327C > T, c.2684del2, c.5251C > T, c.1687C > T32,34,37. Large genomic alterations are uncommon in BRCA1 or BRCA2 gene in the Finnish population53.
Type of pathogenic variant in association to onset of breast and ovarian cancer
In our study there were clear differences in the age of onset between different common PVs. For example, all cases of breast cancer for c.3626delT patients were before the age of 40. This information could be used to further improve when counselling is provided and when surveillance is started. However, the sample size was too small make statistical analysis of these differences.
Multiple breast cancer cluster regions (BCCR) and ovarian cancer cluster regions (OCCR) have been observed in BRCA1 and BRCA2 and are associated with relatively elevated breast cancer risk and lower ovarian cancer risk or inversely15,54. In our study’s for 56% (10/18) of all patients with BRCA1 origin ovarian cancer the PV was located in the OCCR published in the study of Rebbeck15, whereas for BRCA2 origin ovarian cancer no PV were located in the ovarian cluster region15.
Genotype–phenotype correlation is a topic for a follow-up study with greater family and patient amounts.
Conclusion
In conclusion, more specific knowledge about different genetic prognostic factors allows us to evaluate the cancer risk and improve existing treatment guidelines. According to the result it could be justified to have the discussion about prophylactic salpingo-oophorectomy by the age of 40 years. The observation of our study supports the lifelong follow-up in BRCA1 and BRCA2 carriers as breast cancer can be diagnosed as late as approximately 80 years in BRCA2 carriers. Onset of breast and ovarian cancer is similar between BRCA2 carries and non-BRCA families. We observed that 39% of BRCA1 and 27% of BRCA2 family PVs were unique in Southwestern Finland. Genotype–phenotype correlation was not found in southwestern Finnish population in this study.
Data availability
The data and materials are stored anonymously at the IT system of the Department of Clinical Genetics, Turku University Hospital, Turku, Finland.
Abbreviations
- NGS:
-
Next generation sequencing
- BCCR:
-
Breast cancer cluster regions
- BRCA:
-
Breast cancer susceptibility gene
- DNA:
-
Deoxyribonucleic acid
- PARP:
-
Poly (adenosine diphosphate)-ribose polymerase
- TNBC:
-
Triple-negative breast cancer
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65(2), 87–108 (2015).
Finnish Cancer Registry. Cancer Statistics Application (Finnish Cancer Registry, 2020).
Nagy, R., Sweet, K. & Eng, C. Highly penetrant hereditary cancer syndromes. Oncogene 23(38), 6445–6470. https://doi.org/10.1038/sj.onc.1207714 (2004).
Wendt, C. & Margolin, S. Identifying breast cancer susceptibility genes—A review of the genetic background in familial breast cancer. Acta Oncol. 58(2), 135–146. https://doi.org/10.1080/0284186X.2018.1529428 (2019).
Melchor, L. & Benítez, J. The complex genetic landscape of familial breast cancer. Hum. Genet. 132(8), 845–863. https://doi.org/10.1007/s00439-013-1299-y (2013).
Vahteristo, P., Eerola, H., Tamminen, A., Blomqvist, C. & Nevanlinna, H. A probability model for predicting BRCA1 and BRCA2 mutations in breast and breast-ovarian cancer families. Br. J. Cancer 84(5), 704–708 (2001).
Eerola, H., Aittomäki, K. & Nevanlinna, H. Genetic susceptibility to breast cancer. Finnish Med. J. 46, 4695–4701 (2002).
Li, J. et al. Prevalence of BRCA1 and BRCA2 pathogenic variants in a large, unselected breast cancer cohort. Int. J. Cancer 144(5), 1195–1204. https://doi.org/10.1002/ijc.31841 (2019).
Syrjäkoski, K. et al. Population-based study of BRCA1 and BRCA2 mutations in 1035 unselected Finnish breast cancer patients. J. Natl. Cancer Inst. 92(18), 1529–1531. https://doi.org/10.1093/jnci/92.18.1529 (2000).
Buys, S. S. et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 123(10), 1721–1730. https://doi.org/10.1002/cncr.30498 (2017).
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23), 2402–2416. https://doi.org/10.1001/jama.2017.7112 (2017).
Chen, S. & Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 25(11), 1329–1333. https://doi.org/10.1200/JCO.2006.09.1066 (2007).
Tai, Y. C., Domchek, S., Parmigiani, G. & Chen, S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 99(23), 1811–1814. https://doi.org/10.1093/jnci/djm203 (2007).
Mersch, J. et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 121(2), 269–275. https://doi.org/10.1002/cncr.29041 (2015).
Rebbeck, T. R. et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313(13), 1347–1361. https://doi.org/10.1001/jama.2014.5985 (2015).
GeneReviews. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. https://www.ncbi.nlm.nih.gov/books/NBK1247/ (Accessed 20 June 2020).
Metcalfe, K. et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br. J. Cancer 121(1), 15–21. https://doi.org/10.1038/s41416-019-0446-1 (2019).
Tschernichovsky, R. & Goodman, A. Risk-reducing strategies for ovarian cancer in BRCA mutation carriers: A balancing act. Oncologist 22(4), 450–459. https://doi.org/10.1634/theoncologist.2016-0444 (2017).
Terry, M., Daly, M. & Phillips, K. Y. Risk-reducing oophorectomy and breast cancer risk across the spectrum of familial risk. J. Natl. Cancer Inst. 111(3), 331–334 (2019).
Auranen, A. Perinnöllisen syöpäalttiuden tunnistamisella on merkitystä myös gynekologisten syöpien ehkäisyssä. Duodecim 134, 1262–1264 (2018).
Kotsopoulos, J. BRCA mutations and breast cancer prevention. Cancers (Basel) 10(12), 524. https://doi.org/10.3390/cancers10120524 (2018).
Li, X. et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: A meta-analysis and systematic review. Clin. Cancer Res. 22(15), 3971–3981. https://doi.org/10.1158/1078-0432.CCR-15-1465 (2016).
Pierce, L. J. et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J. Clin. Oncol. 24(16), 2437–2443. https://doi.org/10.1200/JCO.2005.02.7888 (2006).
George, A., Kaye, S. & Banerjee, S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat. Rev. Clin. Oncol. 14(5), 284–296. https://doi.org/10.1038/nrclinonc.2016.191 (2017).
Robson, M. E. et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J. Clin. Oncol. 33(31), 3660–3667. https://doi.org/10.1200/JCO.2015.63.0996 (2015).
Manchanda, R., Sun, S. & Patel, S. Economic evaluation of population-based BRCA1/BRCA2 mutation testing across multiple countries and health. Cancer 12(7), 1929. https://doi.org/10.3390/cancers12071929 (2020).
Yang, X. et al. Cancer risks associated with germline PALB2 pathogenic variants: An international study of 524 families. J. Clin. Oncol. 38(7), 674–685. https://doi.org/10.1200/JCO.19.01907 (2020).
Kankuri-Tammilehto, M., Vihinen, P. & Schleutker, J. Heredity of cancer. Finnish Med. J. 14, 880–886 (2019).
Kiiski, J. I. et al. FANCM mutation c.5791C>T is a risk factor for triple-negative breast cancer in the Finnish population. Breast Cancer Res. Treat. 166(1), 217–226. https://doi.org/10.1007/s10549-017-4388-0 (2017).
Mavaddat, N., Antoniou, A. C., Easton, D. F. & Garcia-Closas, M. Genetic susceptibility to breast cancer. Mol. Oncol. 4(3), 174–191. https://doi.org/10.1016/j.molonc.2010.04.011 (2010).
Barkardottir, R. et al. Haplotype analysis in Icelandic and Finnish BRCA2 999del5 breast cancer families. Eur. J. Hum. Genet. 9(10), 773–779 (2001).
Vehmanen, P. et al. Low proportion of BRCA1 and BRCA2 mutations in Finnish breast cancer families: evidence for additional susceptibility genes. Hum. Mol. Genet. 6, 2309 (1997).
Huusko, P. et al. Evidence of founder mutations in Finnish BRCA1 and BRCA2 families. Am. J. Hum. Genet. 62, 1544 (1998).
Sarantaus, L. et al. Multiple founder effects and geographical clustering of BRCA1 and BRCA2 families in Finland. Eur. J. Hum. Genet. 8(10), 757–763 (2000).
Thomassen, M. et al. BRCA1 and BRCA2 mutations in Danish families with hereditary breast and/or ovarian cancer. Actancology 47, 772–777 (2008).
Moller, P. et al. Genetic epidemiology of BRCA mutations—Family history detects less than 50% of the mutation carriers. Eur. J. Cancer 43, 1713–1717 (2007).
Rebbeck, T. et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum. Mutat. 35, 593 (2018).
Li, J. et al. Cancer therapy and prevention open access prevalence of BRCA1 and BRCA2 pathogenic variants in a large, unselected breast cancer cohort. Int. J. Cancer 144(5), 1195–1204 (2019).
Kremeyer, B. et al. The BRCA1 exon 13 duplication in the Swedish population. Fam. Cancer 4, 191–194 (2005).
Tonin, P. et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat. Med. 2, 1179–1183 (1996).
Iyevleva, A. G. et al. Non-founder BRCA1 mutations in Russian breast cancer patients. Cancer Lett. 298, 258–263 (2010).
Ghadirian, P. et al. The contribution of founder mutations to earlyonset breast cancer in French-Canadian women. Clin. Genet. 76, 421–426 (2009).
Daly, M. B. et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J. Natl. Compr. Cancer Netw. 15(1), 9–20. https://doi.org/10.6004/jnccn.2017.0003 (2017).
Yang, X. et al. Evaluation of polygenic risk scores for ovarian cancer risk prediction in a prospective cohort study. J. Med. Genet. 55(8), 546–554. https://doi.org/10.1136/jmedgenet-2018-105313 (2018).
Mars, N. et al. The role of polygenic risk and susceptibility genes in breast cancer over the course of life. Nat. Commun. 11(1), 6383. https://doi.org/10.1038/s41467-020-19966-5 (2020).
Finnish Breast Cancer Group. Rintasyövän valtakunnallinen diagnostiikka- ja hoitosuositus 2019. (Accessed 20 June).
Phi, X. A. et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥ 50 years: Evidence from an individual patient data meta-analysis. J. Clin. Oncol. 33(4), 349–356. https://doi.org/10.1200/JCO.2014.56.6232 (2015).
Paluch-Shimon, S. et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 27(suppl 5), 103–110. https://doi.org/10.1093/annonc/mdw327 (2016).
Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 23(Suppl 6), 7–12. https://doi.org/10.1093/annonc/mds187 (2012).
Foulces, W. D., Smith, I. E. & Reis-Filho, J. S. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948. https://doi.org/10.1056/NEJMra1001389 (2010).
Schaapveld, M. et al. The impact of adjuvant therapy on contralateral breast cancer risk and the prognostic significance of contralateral breast cancer: A population based study in the Netherlands. Breast Cancer Res. Treat. 110(1), 189–197. https://doi.org/10.1007/s10549-007-9709-2 (2008).
Metcalfe, K. et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 22(12), 2328–2335. https://doi.org/10.1200/JCO.2004.04.033 (2004).
Laurila, E., Syrjäkoski, K., Holli, K., Kallioniemi, A. & Karhu, R. Search for large genomic alterations of the BRCA1 gene in a Finnish population. Cancer Genet. Cytogenet. 163(1), 57–61. https://doi.org/10.1016/j.cancergencyto.2005.05.014 (2005).
Singer, C. F. et al. Association between family history, mutation locations, and prevalence of BRCA1 or 2 mutations in ovarian cancer patients. Cancer Med. 8(4), 1875–1881. https://doi.org/10.1002/cam4.2000 (2019).
Acknowledgements
The authors would like to thank research assistant Juho Järvinen for collecting the data together with authors.
Funding
No specific funding has been provided for this study. VTR (Valtion tutkimusrahoitus yliopistotasoiseen terveyden tutkimukseen) funding was used for the salary of a research assistant. In accordance with the Health Care Act (1326/2010), state research funding (VTR) is intended for distribution to university-level health research projects. The Ministry of Social Affairs and Health pays the funding to the Research Committee for Special Responsibilities, also to “The expert responsibility area (ERVA) of the Tyks Turku University Hospital”. Minna Kankuri-Tammilehto has been given the funding for breast cancer genetic project. The funding was used for the salary of research assistant.
Author information
Authors and Affiliations
Contributions
T.P., main writer of the manuscript together with M.K.-T., analyzing the results with other authors. S.L., collecting the data, analyzing the results with other authors. T.J., analyzing the results with other authors, especially the results concerning ovarian cancer. R.A., analyzing the results with other authors, especially the results concerning breast cancer. P.P., expert of laboratory methods. M.K.-T., senior advisor, analyzing the results, responsible for statistical results, tables and images, main writer of the manuscript together with T.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pallonen, T.AS., Lempiäinen, S.M.M., Joutsiniemi, T.K. et al. Genetic, clinic and histopathologic characterization of BRCA-associated hereditary breast and ovarian cancer in southwestern Finland. Sci Rep 12, 6704 (2022). https://doi.org/10.1038/s41598-022-10519-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10519-y
- Springer Nature Limited