Abstract
The doubly labelled water (DLW) method is widely used to determine energy expenditure. In this work, we demonstrate the addition of the third stable isotope, 17O, to turn it into triply labelled water (TLW), using the three isotopes measurement of optical spectrometry. We performed TLW (2H, 18O and17O) measurements for the analysis of the CO2 production (rCO2) of mice on different diets for the first time. Triply highly enriched water was injected into mice, and the isotope enrichments of the distilled blood samples of one initial and two finals were measured by an off-axis integrated cavity output spectroscopy instrument. We evaluated the impact of different calculation protocols and the values of evaporative water loss fraction. We found that the dilution space and turnover rates of 17O and 18O were equal for the same mice group, and that values of rCO2 calculated based on 18O–2H, or on 17O–2H agreed very well. This increases the reliability and redundancy of the measurements and it lowers the uncertainty in the calculated rCO2 to 3% when taking the average of two DLW methods. However, the TLW method overestimated the rCO2 compared to the indirect calorimetry measurements that we also performed, much more for the mice on a high-fat diet than for low-fat. We hypothesize an extra loss or exchange mechanism with a high fractionation for 2H to explain this difference.
Similar content being viewed by others
Introduction
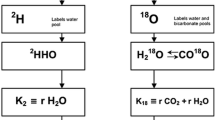
The doubly labelled water (DLW) method, first proposed by Lifson et al. in the middle of the last century, is a reliable, harmless and non-invasive method to determine the energy expenditure and body composition for humans and free-living animals1,2,3,4,5,6. In practice, a dose of known concentration water, highly enriched in the isotopes 2H and 18O, is introduced to the body, where the mixtures will quickly spread evenly through the body water pool and thus get diluted. The general principle of the method is based on the fact that hydrogen leaves the body through water turnover, while oxygen leaves both through water turnover and through respiratory CO2. The 2H and 18O abundances of body fluids are measured from the initial to the final time points, and then the isotope elimination rates can be determined. The difference between these two turnover rates is then proportional to the CO2 production (rCO2), which can be further converted to energy expenditure if the composition of the food intake is known1,4,7,8,9.
Although the basic theory is straightforward, several complications are involved when conducting the actual rCO2 calculation2,4,10,11,12,13,14. For example, we need to consider the oxygen isotope fractionation between CO2 and body water, as well as the fractionation between water and water vapour. These fractionation factors are well known from various laboratory experiments in the past4,8,15,16,17,18,19. However, certain aspects of the process are less well-known (and possibly variable), such as the level of (non-)equilibrium in the fractionation process during evaporative H2O loss, and the fraction of the water that leaves the body through evaporation. Different approaches for body water pool size calculations also matter for the final results13,20, although one can argue that the choice for the optimal way of calculation is clear from a principle point of view.
In the history of DLW, a series of calculation protocols have been used and studied. The comparison with other methods estimating energy expenditure methods, like the indirect calorimetry method, is highly valuable13,21,22,23.
In addition to the traditional DLW method, some researchers proposed or even used three isotopes instead of two (the combination of 2H, 3H and 18O or 2H, 17O and 18O), to trace isotope changes for quantifying the body water and CO2 fluxes10,16,24. The additional use of the third isotope reduces the analytical error, helps to check the data quality and in principle even gives the possibility to quantify the evaporative water loss fraction. However, 3H (tritium) is rare and radioactive, and therefore not attractive to complement 2H and 18O as a tracer. Addition of the other naturally occurring rare stable isotope of oxygen, 17O, was for a long time unattractive due to the complicated measurement methods when using Isotope Ratio Mass Spectrometry (IRMS), due to the mass overlap of the 12C17O16O and the 13C16O16O isotopologues. Avoiding this overlap could either be done by reduction of CO2 to O2 by fluorination25,26 or by direct water electrolysis27. In practice, however, 17O was never used due to these complicated measurements.
For a long time, IRMS has been the technique for DLW water analysis. As IRMS functions with pure gases, pre-treatment for the (water) samples is necessary, preceded by distillation if the body fluid is blood2,4,9,28. Optical spectroscopic measurement of water vapour has become a reliable alternative, initially thanks to the pioneering activities in our laboratory14,29,30. At present, there is commercial equipment available, which enables the measurement of the isotope ratios for the DLW method faster and easier, but with equivalent precision and accuracy compared with IRMS20,31,32,33,34. The advantage of optical spectrometry is that all water isotopologues can be measured simultaneously, so the analysis of 2H, 18O and 17O of water samples and biological fluids is possible in one sample measurement. This provides the possibility to add this third isotope, 17O, to DLW analysis, and turn it into Triply Labelled Water (TLW). Of course, then also water highly enriched in all three rare isotopes should be administered to the study subjects. At the moment, due to almost absent demand, water enriched in both 18O and 17O water is a non-standard product, but that might change when the use of TLW will become more widespread.
In this study, we make use of this possibility, and demonstrate, to our knowledge for the first time, complete TLW measurements for the analysis of the CO2 production of mice in different diet types. Triply highly enriched 2H, 17O and 18O water was injected into mice, and isotope enrichment of the distilled blood samples were measured by optical spectrometry, using available reference waters of 2H and 18O, and home-made 17O reference waters. We describe how we conduct the TLW method and give several calculation protocols. Then we analyze the advantage of the TLW method, the difference of calculation protocols, the deviation of CO2 production measured by TLW and indirect calorimetry, and the influence of different nutrition for mice. The last step, converting the produced CO2 to energy expenditure, is a mere multiplication by the energy equivalent value for the food. Since this study focusses on method evaluation, we refrain from this step and stick with the produced CO2.
Giving this study an additional goal, we chose to repeat a previous experiment by our group13, in which we compared the energy expenditure measured by DLW to Indirect Calorimetry (IC), for mice on low fat (LF) and High Fat (HF) diets. That experiment showed systematic differences between DLW and IC results, and by its repetition, now using TLW, we wanted to find out if this difference occurred again, and if so, if the addition of 17O would shed light on this difference.
Material and methods
Animals and housing
Twenty male C57BL6/J mice were individually housed on a 12:12 light–dark cycle with food and water ad libitum, and a controlled temperature (22 ± 1 °C) (more details in Ref.13). At the age of 27 weeks, ten of the mice were maintained on regular chow diet, the so-called low-fat diet (LF) group (17.5 kJ/g; fat content 13.5%; protein content 28%; carbohydrate content 58%). The other ten mice were changed to a high-fat sucrose diet (HF) (21.8 kJ/g; fat content 28%; protein content 19.5%; carbohydrate content 52.5%) eleven weeks prior to the TLW injection.
Preparation of the triply labelled water
We produced a highly enriched TLW mixture by mixing the 2H, 18O and 17O “mother” waters (around 8.0, 12.4 and 6.2 g, respectively, determined with 0.1 mg precision). The “mother” waters are purely 2H water ([2H] > 99.9%, Sigma-Aldrich, Netherlands), 18O water ([18O] ≈ 98%, Rotem industries, Rehovoth, Israel) and 17O water with high enrichment levels ([17O] ≈ 41%, [18O] ≈ 43%, Rotem industries, Rehovoth, Israel). This resulted in a mixture ([2H] = 29.7%, [18O] = 55.88%, [17O] = 8.55%). This is equivalent to enrichment factors of ≈ 1900, 280 and 225, respectively, so in our experiments we expect higher enrichments for δ2H than for δ18O and δ17O, whereas the latter two will be roughly equal. Given the measurement uncertainty of our Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) analyzer, we expect similar accuracies in the measurements for all three isotopes this way.
Using this mixture for the injection in the mice, we estimated that a 0.17 g injection would result in the initial samples (the most enriched ones) having δ2H and δ18O values close to the international enriched reference water IAEA-609 (δ2H = 16,036.4‰, δ18O = 1963.7‰35). Therefore, the suite of enriched water references IAEA-609,608 and 607 are suitable for δ2H and δ18O calibration of all mice blood samples. However, as these reference waters are not (or only mildly) enriched in 17O, we cannot use them for the calibration of our δ17O measurements, where we expect initial values of around 1700‰. Therefore, to calibrate TLW measurements, we made a range of four reference waters enriched in all three isotopes, by gravimetrically mixing the highly enriched TLW mixture ([2H] = 29.7%, [18O] = 55.88%, [17O] = 8.55%) with varying quantities of demineralized tap water (δ2H = − 42.49‰, δ18O = − 6.36‰, δ17O = − 3.39‰). Different amounts of the TLW mixture, from 0.25 to 0.6 g (~ 0.1 mg precision), were put into a 2 ml glass vial, and then immersed into a glass bottle which contains about 100 g demineralized water (~ 0.1 mg precision). These bottles were sealed after mixing and shaken periodically for several hours.
Table 1 shows the values of these four TLW-references along with their uncertainty. The δ2H and δ18O values were measured by OA-ICOS and calibrated using IAEA-609, 608 and 607. The error of δ2H is based on several measurement repetitions, but for δ18O, the measurement uncertainty is small (less than 1‰), so the uncertainty of IAEA waters are important for the δ18O error of these TLW-references35.
The IAEA waters are unfortunately only mildly enriched in 17O, so for the δ17O value determination of our four TLW reference waters, we conducted several dilution experiments to bring the resulting δ17O values of the diluted TLW reference waters within the range of the IAEA waters (with IAEA-609 having the maximum δ17O value of 126.6‰). By using the accurately determined dilution factor, we could in this way calibrate the δ17O of TLW reference waters using IAEA-609,608 and 607. All calculations of isotope abundances and δ-values were performed using a thoroughly validated Excel spreadsheet36. Based on the measurement uncertainties, the uncertainties in the values quoted for the IAEA waters, and the dilution uncertainties we attribute a conservative ± 1% relative uncertainty to our δ17O values (see Table 1). Besides the best estimates for the δ17O of each TLW reference water, we also could determine the abundances of the highly enriched TLW ([2H] = 29.7%, [18O] = 55.88%, [17O] = 8.55%). We separately determined that the 18O mother water has a 98.42% abundance, deviating somewhat from 98.2% provided by the manufacturer (but within their specification). The 17O mother water contains 34.47% [17O] and 42.65% [18O] (for [17O] deviating from the manufacturer's specification of 41.1% whereas [18O] with 43% agrees). The 2H mother water is virtually pure. The enriched reference waters and the highly enriched TLW mixtures were stored in thoroughly closed bottles inside a sealed container, filled with dry N2 gas at slight overpressure. This prevented the uptake of—and thus dilution by—atmospheric moisture.
Experimental design
For the experiments, each mouse was intraperitoneally injected with about 0.17 g (weighted to the nearest 0.1 mg) of the highly enriched TLW mixture. Before injection, we took 4 blood samples separately from 5 mice for background isotope analysis. After exactly 2 h after the TLW injection, the initial blood samples were taken. Then, the mice were transferred to the indirect calorimetry (IC) cages for two consecutive days. The indirect calorimetry (IC) was shortly interrupted at exactly (deviations less than 2 min) 24 and 48 h after the initial sample time. In this study, the background and TLW mixture blood were all sampled by tail snip (4 times per sample every time), and then flame-sealed into 25 µl glass capillaries until the micro-distillation process13. Mice body masses were measured by a balance (~ 0.1 g precision), and fat, lean weight and water content of all the mice were measured by a magnetic resonance imaging machine (EchoMRI-100, Echo Medical Systems, Houston, TX, US) just before injection37. All experimental procedures were approved and guided by the local Animal Experimentation Committee (DEC) of the University of Groningen.
Indirect calorimetry
In the IC cages (Homemade polypropylene cage, 43 × 30 × 21 cm), the housing and feeding conditions were not changed. The detailed description of the IC method in our lab is in Ref.13. The IC system measured the O2 and CO2 concentration difference of the dried inlet air (3 Å molecular sieve drying beads; Merck, Darmstadt, Germany) and dried outlet air going through the chambers. The flow rate of the inlet was set at 20 l/h, and only 6 l/h outlet air passed through the drying system and subsequently to the gas analyzers. The mass-flow controllers (Type 5850; Brooks Instrument, Veenendaal, The Netherlands) were calibrated before and after the trials (the variation < 1%). O2 was measured by a paramagnetic O2 analyzer (Xentra 4100, Servomex, Egham, UK), and CO2 by an infrared gas analyzer (Servomex 1440). The CO2 and O2 analyzers were calibrated daily with two certified gas standards (Linde Gas, The Netherlands), with values 19.5612% for O2 and 0.0006% for CO2, and 20.8743% for O2 and 0.5133% for CO2, respectively. The maximum overall error of the method is ≤ 2%. For validation purposes, the respiratory quotients (RQ = rCO2/rO2) and metabolic rates (MR) were also recorded and calculated13,38.
Analysis method of the TLW samples
The δ2H, δ17O and δ18O of mice blood samples and reference waters were measured by a commercial Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) Liquid Water Isotope Analyser (LWIA 912-0050, Los Gatos Research, San Jose, CA, USA). Before injection into the analyser, all the samples and references were prepared by a home-built micro-distillation system (detailed distillation procedures are described in Ref.39). In brief, a capillary is broken in an evacuated system, and the water is collected in a freeze finger immersed in liquid nitrogen. The system is then again evacuated, and the water is finally transferred, again using liquid nitrogen, into a small insert tube, which can be measured directly on the OA-ICOS analyser. The reference waters (the IAEA series and our TLW references) are treated identically, so also transferred from capillaries.
The distilled samples and references were introduced into the OA-ICOS instrument through an auto-injector (Pal, CTC Analytics, Zwingen, Switzerland), and there is a heated injector block to evaporate the liquid water. This vapour expands into a high-finesse optical cavity, and the δ2H, δ18O and δ17O values were calculated from fits to the relative transmission spectrum. The distilled IAEA-609,608 and 607 and our local TLW references are interleaved with samples during the measurement series for calibration and instrumental drift correction. Each reference and sample water was injected 12 times. Before each distillate reference, the same reference water, but without distillation, is also injected 12 times to check the micro-distillation quality and stability, and also to reduce memory effects. However, only the distilled references were used for calibration.
Raw data from the instrument were analysed using a bespoke data analysis program (written in R), through which memory effects and drifts were corrected, and calibration was performed (for details see40). Specifically, to correct for memory effects we do not ignore the first few injections of a sample, but instead use all of them and correct for the memory effects using a 2–3 pool exchange algorithm39. This is quite meaningful for TLW blood samples, which have a minimal sample size (less than 15 µl). Figure 1 shows a representative part of a measurement batch (containing “initial” samples), in which both the raw, and memory corrected values for several samples and reference waters are shown. The improvement in precision is remarkable: standard deviations of the 12 δ2H measurements of the samples around 6000‰, for example, reduce from 210 to 30‰ when measured just after natural (demineralized Groningen tap-) water (δ2H ≈ − 42‰).
The memory effect of δ2H observed in an initial samples’ measurement batch. The measured (raw) values are showed by the black circles and the open red circles indicate the values after memory effect correction by the 3 pools exchange algorithm. In addition to the memory, there is also some drift visible which is also corrected by the data analysis program.
For calibration, mostly a “multiple-point” quadratic fit is chosen, which is based on three or more of the reference waters. This is based on our experience that for these highly enriched samples and the large range in δ-values in each series (e.g.: δ18O from 736 to 1963‰ for the IAEA reference waters), the instrument’s output is not fully linear. This is probably due to imperfect line fitting, which also makes itself noticeable through relatively high values for the so-called “Narrow Band” spectroscopic interference41. This tool is meant to be an indicator for contamination, but as contamination does not occur in our samples (and certainly not in the pure reference waters), here it is the result of an imperfection in the spectroscopic fit of these triply labelled waters.
As illustrated in Ref.39, duplicate analysis of DLW samples is necessary and helpful, due to the dominant uncertainty contribution of the actual procedure of flame-sealing and micro-distillation. In this study, if the δ2H value of a duplicate analysis deviates more than 2% of its value from the first (or 1.5% for δ18O, 1.5% for δ17O), a third sample is analyzed. A third sample is also taken if the quality of a capillary is questionable (for example not tight or containing too much air). The average of the duplicate (or triplicate if an outlier cannot be identified) analyses, along with the standard error in the mean is taken as the final result. The OA-ICOS measurement uncertainty for individual samples is usually negligibly small compared to the spread between duplicate samples.
For the TLW method analysis, we use isotope abundances instead of the δ-values. First, the sample’s xδs values need to be converted into abundance ratios xRs (x = 2, 17 and 18), using the isotope abundance ratios for Vienna Standard Mean Ocean Water (VSMOW), which are 1.5576 × 10–4, 3.799 × 10–4, and 2.0052 × 10–3 for 2H, 17O and 18O, respectively42:
From these ratios, the absolute isotope concentrations xCs are computed, usually expressed in parts per million (ppm):
Calculations
After the injection of the enriched TLW mixtures into the mice, the enriched rare isotopes are gradually exchanged with the surroundings, and the turnover rate (k; h−1) describing the rare isotope concentration decrease can be expressed as:
C is the concentration of the isotope 2H, 18O or 17O. “i” means the initial, and in this study, the initial sample is the 2-h samples taken after injection. “b” is background (concentrations corresponding to δ2H = − 27.3‰, δ18O = − 4.85‰, δ17O = − 2.61‰, as established based on sampling five mice prior to the injection of TLW), and “f” is the the final sample (taken either 24 h or 48 h after the initial sample), therefore, time duration “t” is equal to 24 or 48 h.
The dilution space of the isotopes in the body, and thus the size of the body water pool, can be calculated using the measurement of the initial concentration by the so-called plateau method4:
where N (mol) represents the dilution space or body water pool for 2H (N2H), 18O (N18O) and 17O (N17O). Molinj is the number of the moles of the injection TLW (19.81 g/mol) and Cinj is the injected enrichment ([2H] = 29.7%, [18O] = 55.88%, [17O] = 8.55%). In this expression, the loss of enriched isotopes in the 2 h between the injection and the initial measurement is ignored. Alternatively, one can take this loss into account by extrapolating the turnover rate back to the injection time. This is called the intercept method4:
where Ci–ic is the concentration extrapolated back to the time of injection (0 h), and ti–tinj is equal to 2 h in our case.
Whereas the plateau method is expected to underestimate the body water pool slightly (as the loss of enriched isotopes during the first 2 h is ignored), the intercept method, on the other hand, possibly overestimates the body water pool, as the loss of enriched isotopes during the first two hours is probably less than later, since the enriched isotopes have not distributed themselves over the entire body water pool. Therefore, calculating and comparing both is a good practice.
It is generally observed that the body water pool as determined by 2H is slightly, but significantly, larger than that by 18O4,13,43. This is commonly attributed to the exchange of hydrogen (and thus 2H) with body tissues, which does not occur with oxygen. For this reason, we expect the body water pool determination using 17O to be identical to that with 18O.
The amount of total body water (TBW, g) for each individual animal is then simply:
M is the molar mass of water (18.02 g/mol). In terms of carbon dioxide production, in a simple expression ignoring the fractionation effects, the difference between 2H and 18O turnover is proportional to the rate of CO2 production (rCO2; mol/h):
Also here, several fractionation effects occur in the process. Therefore, this Eq. (10) is not suitable for an accurate calculation of rCO2. However, as the deviations are relatively small, this equation can be used for uncertainty propagation calculations. The full expression contains the following fractionation factors: the (partly kinetic, partly equilibrium) evaporation of water for 2H (f1) and 18O (f2,18O), and the CO2-H2O fractionation for 18O (f3,18O), which is assumed to be in equilibrium:
rG is the fraction of the water loss due to evaporation, as it happens in the lungs. By lack of a firm determination or estimate, most studies use a value of 0.5. The isotopic fractionation process leads to relatively lower abundances of the heavy isotopes in the vapour phase. All fractionation factors are shown in Table 2, and Eq. (11) is from Ref.4. If instead of on 18O and 2H turnover, rCO2 is calculated based on the 17O and 2H turnover, we arrive at the identical equation, but with the 17O decay rate, and two fractionation factors now for 17O:
where k17 is the turnover rate for 17O, and f2,17O and f3,17O are the fractionation factors for 17O fractionation in the water evaporation and the CO2-H2O equilibrium, respectively.
In Table 2, we list the fractionation factors obtained from literature, as well as the 'mixed' results by the equilibrium/kinetic as a ratio of 3:14, and the final (f2–f1)/2f3 calculation results. All the factors are equal to the values listed in Refs.4,16, expect the f3,17. Its value of 1.0202 is obtained based on the equation from17 at 37 °C, and ln(α17)/ln(α18) = 0.522944.
There are several classical equations to calculate the CO2 production, which differ in the selection of fractionation factor values, portion of fractionation water (rG) and body water pool models, and are also dependent on the research subjects (animals or humans)4,16,45,46,47. Equations (11) and (12) use a single pool model, they are reproduced as equationns 1-1 and 1-2 in Table 3. For 18O, equation 1-1, is similar to the expression in Ref.4 except the number of decimal places, and equation 1-2 is for 17O based on the same calculation principle. When we consider the two-pools model, which means that the effective body water pool is taken differently for 2H than for 18O (or 17O), the Coward 198546 and Speakman 199345 models are more logical and suitable for animal CO2 production calculation. The equations 2-1, 2-2, 3-1 and 3-2 in Table 3 are based on their model principle, separately from Coward 198546 and Speakman 199345. The fractionation factors (from Table 2) used for the equations in Table 3 are the same, irrespective of the model. The Rdil in 3-1 and 3-2 is the mean dilution space ratio N2H/NO (the dilution space calculated by 2H divided by the dilution space calculated by 17O or 18O) for different group members, so different for the low and high fat diet mice.
Ethics approval
All experimental procedures involving animals were approved and guided by the local Animal Experimentation Committee (DEC) of the University of Groningen.
Consent for publication
All authors whose names appear on the submission consent to this version of the manuscript to be published.
Results
Body composition
After 11 weeks on a high-fat diet, the high-fat diet mice gained more than 5 g of weight. On the basis of the body mass gain (> 10 g or < 10 g), 5 mice were assigned to be obesity-resistant (HF-OR), and 5 mice were in the obesity-prone (HF-OP) group. The body mass weighed just before injection were used as body weight, together with the fat mass, lean mass, and body water measurement by EchoMRI. From the 10 mice on low fat diet (LF), two had to be discarded from the data set due to blood sampling problems (only one successful capillary for the initial sampling, and large discrepancies between their calculated total body water by TLW and by EchoMRI).
Figure 2 illustrates the average values of the body-weight, lean content (g), fat percentage (fat/body-weight) and body water percentage (body-water/body-weight) of the three mice groups: LF, HF-OR and HF-OP (with 8, 5 and 5 individuals, respectively). When analyzing the individual differences in body content, we find that the fat percentage is positively correlated with the body-weight, and negatively correlated with the body-water percentage. When analyzing the group difference, Fig. 2 clearly shows that the HF-OP mice group, which is heaviest (the average 45 g), has the lowest water percentage (the average 50%) and the highest fat percentage (37%). The average weight difference between the LF and HF-OR groups is not large (30.6 g and 33.8 g, respectively), but the fat-% and water-% are quite different. The lean contents for LF and HF-OR groups are similar (nearly 24 g), and the average lean content of the HF-OP group is only 2.5 g higher than the other two groups.
Indirect calorimetry
After taking the initial blood samples, the 20 mice were put into the indirect calorimetry box and the actual rCO2, rO2 and RQ were measured over the two following days. The data around the interruptions (taking blood samples) were removed, and the IC data summary of the two days is listed in Table 4. Table 4 also shows the mean food intake (kJ/day) for the three categories. The individual variability is large (especially for the HF-fed mice), but on average the LF mice have taken in a higher caloric value of food than the HF ones.
All observed values are equal within the error for day 1 and day 2, except for rCO2 of the LF mice. The rCO2 of the LF and HF-OR groups are similar and their difference is within the error, but the CO2 production of the HF-OP group is about 8 ml/h more than that of the other two groups. The rO2 values differ significantly between the groups, and that of the HF-OP group is the highest.
The respiratory quotient (RQ) resembles the low-fat or high-fat food intake difference between the LF and the two HF groups. There is no difference between the HF-OR and HF-OP groups, and they are both lower than the RQ values of LF. As the Metabolic Rate values are directly computed from rCO2 and the RQ, they show the same trends. The MR value of HF-OP is highest (about 5 kJ/day more than HF-OR, 11 kJ/day more than LF).
Turnover rates k
The turnover rates for 2H (k2H), 18O (k18O) and 17O (k17O) were separately calculated from the logarithmic decline of the initial isotope abundance (2-h after injection) and the isotope abundance of two finals (24 h and 48 h after the initial sample taken). The average k2H, k18O, k17O for the three mice groups are shown in Fig. 3, whereas Table 5 gives the numerical values, and in addition the turnover rate ratios and differences. All are presented for the 24 h and the 48 h period. It is clear that the LF group has the highest turnover rates of the three groups, and the differences are highly significant between LF and HF groups. The turnover rates of the HF-OR group are slightly higher than the HF-OP group, but for k2H and k18O, the difference is not significant.
In Table 5, it is clear that the uncertainty in k-48 h is lower than that in k-24 h, this is because of the larger difference between the isotope values for the 48 h-finals and the initials. In Table 5 and Fig. 3, for k2H, we can see the difference between 24- and 48 h is significant, k2-48 h being higher than k2H-24 h for all three mice groups. For k18O and k17O, on the other hand, the differences between the turnover rates for 24 h and 48 h are small and not significant. The k17O and k18O agree with each other within the uncertainties for both of the two times. This is to be expected, as both the 17O and 18O label are subject to the same processes. The fact that they do agree within the uncertainty increases the confidence in the experimental results (both the animal handling side and the isotopic analysis of the blood samples). Therefore, it is possible to obtain the turnover of oxygen by taking the average of k17O and k18O, which lowers the uncertainty of kO (turnover for oxygen isotopes).
As shown in Table 5, the turnover rates ratios kO/k2H are typically 1.9 (LF) and 2.3 (HF) for 24 h finals, while for 48 h, they are a bit lower: 1.7 (LF), 2.0 (HF), fully caused by the increase of k2H. In the analysis of kO–k2H, which carries the rCO2 signal information, the kO–k2H values are obviously lower for 48 h than for 24 h because of the higher k2H-48 h values. On the other hand, the differences between the three groups for the same final are not significant in most of the cases, expect the k17O–k2H for HF-OR group at 48 h-final.
Total body water (TBW) and dilution space
The body dilution space (N) can be measured based on the plateau method (Eq. (6)) or on the intercept method (Eq. (7)), and the total body water is calculated using Eq. (9). In Fig. 4 we compare the body water percentage (water/mass) results calculated by TLW with those by EchoMRI. To illustrate the differences better, Fig. 4 shows the differences between the two. In terms of individual variation, the water percentage values from the calculation (TLW) and measurement (EchoMRI) are consistent with each other (not shown here). Moreover, the dilution space difference obtained by the intercept method based on the k-24 h or k-48 h is not significant (less than 0.1%), so we only illustrate the percentage difference of the plateau and 48 h-intercept method in Fig. 4.
It is clear in Fig. 4, and expected (see above), that the plateau method gives higher water percentages than the intercept method for both 2H, 18O, and 17O, and also that the water percentages based on 2H are the highest (nearly 2% higher than the EchoMRI values for the plateau method). On the other hand, the intercept method results for 18O and 17O give lower water percentages than that of EchoMRI. Still, given the combination of the indicated uncertainties for the TLW method (as indicated in Fig. 4) and the uncertainty of the EchoMRI method (estimated to be ≤ ± 2%), all differences shown in Fig. 4 are not significant. Furthermore, although the water percentage values for the three different mice groups are different (see Fig. 2), the differences between the TLW and EchoMRI methods do not seem to correlate with these percentages themselves.
The absolute total body water or dilution space (N) values calculated by three isotopes and two methods are listed in Table 6 for the three mice groups. As Fig. 4 already showed, the values of the plateau method are higher than that of the intercept method for each mice group irrespective of the isotope used. N2H is always higher than NO, and N17O matches N18O. The most important feature in Table 6 is that the dilution space of HF-OP group is significantly higher than that of LF and HF-OR, while the dilution spaces of HF-OR and LF are equal for most of the cases.
CO2 production
The average rCO2 of each LF, HF-OR and HF-OP group are calculated by three kinds of equations which are listed in Table 3. We took the rG = 0.5. The rCO2 results are shown in Fig. 5, and for each group, there are two kinds of doubly labelled water methods: left columns based on 18O and 2H, and right columns based on 17O and 2H. We also consider the different dilution space (N) calculation methods (plateau or intercept methods) and two different finals (24 h or 48 h). The grey horizontal line in the bottom is the average rCO2 value for 2 days from the indirect calorimetry method for the three mice groups. Therefore, in summary, we consider 4 factors for each mice group to calculate the rCO2: two finals (24 h or 48 h), two N calculation methods (plateau or intercept), three models (Table 34,45,46), and two isotope combinations (based on 2H–18O or 2H–17O). The relative uncertainties of the classical DLW (18O and 2H) methods in this study are 8.5% (24 h) and 5.1% (48 h), while for (17O, 2H) DLW they are 6.3% (24 h) and 4.0% (48 h). The relative uncertainty in the indirect calorimetry values is estimated to be ≤ ± 2%.
The average rCO2 (ml/h) of the three mice groups (LF, HF-OR and HF-OP) based on different calculation protocols. There are three models (Speakman single- or two-pools model, Coward two-pools model), two kinds of doubly labelled water method (18O–2H, or 17O–2H), two dilution space calculation methods (plateau or intercept), and two different finals (24 h or 48 h). The grey horizontal lines in the bottom are the average rCO2 values of two days from the indirect calorimetry method.
For all three mice groups, the 24 h rCO2 data are much higher than the 48 h data, irrespective of the calculation method, in other words, the differences between solid and hollow symbols in each column are the same. The differences are all caused by the k2H-48 h value being larger than the k2H-24 h one (see Fig. 3, Table 5). Of course, this difference could indicate a real different behavior, but the IC values for the two days do not show such a difference (Table 4). In terms of different oxygen isotopes for each mice group, the difference is random (from 0 to 8 ml/h), just within the largest errors no matter which model is used. For each of the oxygen isotope and models in each mice group, the rCO2 calculated by the intercept method (yellow area) is lower than that by the plateau method. However, the differences are within the uncertainties. Interestingly, the intercept points are more scattered than the plateau ones, caused by the extra influence the turnover rates k have when using the intercept method. As was stated before, one can expect the plateau method to give an overestimation of the water pool size, and the intercept an underestimation. This results in and over- and underestimation of the rCO2, respectively. The average of the two values would probably produce the best result, while their differences would give an estimate of the uncertainty.
Obviously, the most striking feature of Fig. 5 is the discrepancy between all the TLW results on the one hand, and the IC results on the other. The discrepancy is the smallest for the 48 h two-pools models, with the solid triangles (Coward 1985, 48 h-) having the lowest discrepancy with IC for each mice group. The lowest difference of TLW and IC is 8 ml/h (LF) and 16 and 15 ml/h (HF-OR and HF-OP) for these results (the equation 2-2 for 48 h). The TLW and IC results agree in the sense that both show the highest rCO2 for the HF-OP group. However, the average IC values for the HF-OR and LF groups are similar, while in the TLW methods, the rCO2 of HF-OR is higher than the rCO2 of the LF group (about 10 ml/h).
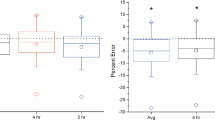
The discrepancy between TLW and IC needs an explanation. Figure 6a shows the individual rCO2 measured by the 2-1 and 2-2 (Coward 1985) models with the intercept method at 48 h finals, and the individual IC values of day two. It is very clear that rCO2 measured based on the 18O–2H and 17O–2H pairs are consistent, and their difference is within the uncertainty. For the LF and HF-OP groups, the rCO2 (18O) is slightly higher than r′CO2 (17O), but the difference is only about 4 ml/h and not significant. Figure 6b clearly illustrates the deviation of the TLW and IC methods. The rCO2 values of the TLW method are calculated by averaging the rCO2 (18O) and r′CO2 (17O), and the IC rCO2 values are the same as in Fig. 6a. The uncertainty in the difference (TLW minus IC) in Fig. 6b is around 3.5 ml/h (based on 3% relative error for TLW and 2% for IC). The average difference between the TLW and IC values for the LF mice is 7.2 ml/h, while the average distance between TLW and IC for HF groups is 18.1 ml/h, much larger than that of the LF group. The individual IC and TLW data show a similar pattern, which is a firm indication that the difference between TLW and IC is of a systematic nature. As the IC technique is straight forward and less assumption-prone, we suspect the deviation to be caused by isotopic effects not accounted for.
(a) Individual rCO2 values (ml/h) measured by 18O–2H or 17O–2H DLW using the Coward 1985 two-pools model by the intercept method at the 48 h finals. The blue points are the 18O-based results, with 5.1% uncertainty, and the orange points are for 17O with 4% uncertainty. The grey points are the individual indirect calorimetry vales for day two (2% uncertainty). (b) The difference between the rCO2 values calculated by the TLW and IC methods. The rCO2 values of the TLW method are the average the rCO2 (18O) and r′CO2 (17O) in (a). The uncertainty in the difference is around 3.5 ml/h. The values in the plot are the mean ± the SE, for LF, and HF (OR and OP taken together).
Discussion
The triply labelled water method
Because the measurement of δ17O has become simple, fast and accurate by the optical spectroscopic system, the classical Doubly Labelled Water (DLW) can easily be extended to Triply Labelled Water (TLW), and to our knowledge we demonstrated that here for the first time. The isotope abundance measurement uncertainties of 17O and 18O in the blood samples are similar, and the individual turnover rates of 17O and 18O are also expected to be equal no matter the subject treatment or the turnover time chosen, and our data confirmed this (Fig. 3). The same holds for the dilution space difference between N17O and N18O (Fig. 4). In terms of rCO2 calculated based on 18O, 2H, or on 17O, 2H, the values also match with each other for the same models (Fig. 5). These findings are consistent with our assumptions: although processes with 17O and 18O are governed by different fractionation factors, these differences can be accounted for, and do not cause a significant difference in the TLW method calculation. Moreover, using the TLW as extension to DLW, we can double-check the data quality of 18O based on the 17O data, and lower the measurement uncertainty of the CO2 production. In this study, we lower the calculated rCO2 uncertainty to 3% when taking the average of rCO2 (18O, 2H, 5%) and r′CO2 (17O, 2H, 4%).
Another use of the third isotope is to help quantify the evaporative water loss fraction, in other words, the TLW method enables us, at least in principle, to derive a direct estimate of the fractionated losses fraction (rG). This value of fractional evaporative water losses—rG—has been subject of discussion since many decades4,5,48,49. As the rCO2 (18O, 2H) should be equal to r′CO2 (17O, 2H), we can derive the individual rG values by equating the two Eqs. (11) and (12). However, the influence of the value of rG is limited: in this paper, we took rG = 0.5, a value that is also widely used in free-living mammals. If we would use rG = 0.25 as other researchers have done4,48, the rCO2 will increase by less than 2%, which is within the uncertainty band of our TLW average values (3%). Alternatively, one can say that in order to determine rG from the combination (18O, 2H) and (17O, 2H) to ± 0.1, one would need an uncertainty in rCO2 ≤ ± 1%, out of reach of the present measurement methods, as was already concluded in Ref.5.
At the moment, highly enriched 17O water is more expensive than pure 18O water, this is mainly because of less demand for it. However, 17O can now be easily detected by the optical systems such as the one we use. Also, one only needs a factor of 7 less 17O label to achieve the same enrichment factor as for 18O water, and this reduces the costs. Therefore, adding 17O to the classical DLW method is practically easy now, and it is also worthy to use TLW to check the method and improve the precision of the CO2 production. At the moment, certified reference waters (such as Ref.35) are not yet available for highly enriched 17O, so laboratories should make their own references by gravimetrical mixing. However, if demand increases, such reference waters will be made available, by international bodies such as IAEA, or commercial suppliers.
Calculation protocols
We considered three models for CO2 production, one is based on the single-pool model (1-1 and 1-2 in Table 3), another two series of equations are based on the two-pools model (Table 3). In addition, we used the best available values from the literature for the fractionation factors (including 17O). The two-pool equations based on Ref.46 take the individuals’ specificity more into account, while the equations based on Ref.45 use a group average for the dilution space ratio. Therefore, we consider the equations based on Ref.46 to be the best form a principal standpoint. Nonetheless, we compared two different group averaging methods for Rdil in equations 3-1 and 3-2, one is for the whole group of 18 mice regardless of the feeding methods, the other is separate averages for the three mice subgroups (as used for Fig. 5). Differences in rCO2 were less than 2%, so the group difference of Rdil is not essential. The results in Figs. 5 and 6 show that the rCO2 calculated by Ref.46 are indeed closer to the CO2 production obtained by indirect calorimetry, but there still is a significant discrepancy, much larger for the 24 h results than for the 48 h ones, and much larger for HF groups than LF groups: The TLW method leads to higher numbers for the rCO2.
We found a clear increase from k2H-24 h to k2H-48 h, but no significant change for k18O and k17O. This leads to increased values for rCO2 in the first 24 h compared to the second 24 h. One might speculate that this can be caused by the disturbance of the mice during the first day: we injected the labelled water, took the 2 h as well as the 24 h blood samples and put them in and out of the IC box at the first day, but we only took the 48 h samples on the second day. However, the IC results show no significant changes between the first and second day. Due to this low k2H-24 h, the TLW rCO2 results for the 24 h deviate much more form the IC results than the 48 h results (see Fig. 5). Still, also the 48 h results for rCO2 are high compared to the IC result, which fact one could alternatively formulate as: k2H-24 h is much too low, k2H-48 is still too low, but by less. If we consider the IC results as straight forward and trustworthy, this would lead to the speculation that the 2H label disappears form the body water at a lower rate than the water loss itself, so involving a process with very high fractionation. We discuss this possibility further below, in which discussion the extension of DLW to TLW appears useful.
Influence of the nutritional conditions
Previous work in our lab focused on different nutrition and body composition effects on rCO2 of mice by the DLW technique13. They also separated their mice in the same three groups, only their mice were younger. They also found that the rCO2 measured by DLW matches IC results much better for low-fat mice than for the high-fat feeding mice, so the high-fat diet is a relevant factor to explain the overestimation of DLW. Intuitively, one might expect that the high-fat fielding mice would consume a higher caloric value of food than the low-fat ones, but this appears to not be the case, rather the opposite (Table 4). However, the individual scatter is large, and does not correlate at all with the individual deviation between IC and TLW values for rCO2. Rather, the main difference we consider between our three groups is the turnover rates difference (kO–k2H), because the TLW-determined body water agrees well with the EchoMRI-determined one, implying that the dilution space for the water is correctly determined. Yet, the rCO2 determined by TLW is systematically higher than that with IC, even for the “most agreeing” calculation method (see Figs. 5, 6). This difference is the lowest, regardless of the individual difference, for the LF mice group, close to 8 ml/h, still significantly higher than the largest error of TLW (± 3%) and IC (± 2%). For the HF-OR and HF-OP mice groups, this difference is more than double that amount. The (too) high rCO2 values must be caused by too low k2H-values, and/or too high k17O and k18O ones. The number of possible explanations is limited, because the body water pool is correctly determined by TLW. The only thinkable way of getting too high 18O and 17O rates is assuming a strongly fractionating water loss process that preferably takes up 17O and 18O over 16O, and with the same fractionation factor for both. Such a process is next to impossible to imagine, as fractionation factors for 17O are typically half those for 18O. So, thanks to the extension of the experiments from DLW to TLW, we can now rule that out. For 2H on the other hand, we would need an extra water loss or uptake process that heavily discriminates against 2H, and such processes are thinkable, and in fact known: electrolysis of water, for example, manifests fractionations of − 600 to − 700‰ (so fractionation factors of 0.4 to 0.3), and also bacterial uptake is known to fractionate considerably (albeit not to the extent of electrolysis). A rough calculation shows that if a 10% extra loss/exchange effect would exist with a 2H fractionation factor of 0.4, this would lead to an overestimation of rCO2 by 10 ml/h. As the effect is considerably larger for the 24 h than for the 48 h results, the process might in fact be an exchange effect that reaches equilibrium at some point. If that is the case, the TLW-IC difference must gradually disappear. As the discrepancies are much larger for the HF than for the LF mice, body composition (fat content) and/or food digestion must play a profound role in this mechanism.
Identifying the cause of the discrepancy with IC is essential for the reliability of the DLW/TLW methods alike. Thanks to the addition of 17O, the mechanism must be some kind of fractionating 2H loss. This requires further study, and eventually inclusion of that mechanism into the equations of Table 3.
Conclusions
This study extends the traditional doubly labelled water technique to triply labelled water to estimate the CO2 production for mice held in different nutrition conditions.
The results for both combinations 2H–17O and 2H–18O agree well, and hence the calculated rCO2 uncertainty is lower and the values are more robust. However, we found that systematic deviations between the DLW (now TLW) method and indirect calorimetry still exist13. Thanks to the addition of 17O, we can now conclude that a process of extra water removal/uptake with a high degree of discrimination against 2H must be the cause of the too high rCO2 results. This uptake apparently is dependent on the food intake and/or the body composition. More detailed isotope analysis (such as gastric fluids50) can probably reveal this extra water loss/exchange channel.
Data availability
The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). All data described in the manuscript are in the database system of the Centre for Isotope Research, and are available upon request.
Code availability
The custom-made R data processing program described in the manuscript is available upon request.
References
Lifson, N., Gordon, G. B. & McClintock, R. Measurement of total carbon dioxide production by means of D2O18. J. Appl. Physiol. 7, 704–710. https://doi.org/10.1152/jappl.1955.7.6.704 (1955).
Westerterp, K. R. Doubly labelled water assessment of energy expenditure: Principle, practice, and promise. Eur. J. Appl. Physiol. 117, 1277–1285. https://doi.org/10.1007/s00421-017-3641-x (2017).
Black, A. E., Coward, W. A., Cole, T. J. & Prentice, A. M. Human energy expenditure in affluent societies: An analysis of 574 doubly-labelled water measurements. Eur. J. Clin. Nutr. 50, 72–92 (1996).
Speakman, J. R. Doubly Labelled Water: Theory and Practice (Chapman and Hall, 1997).
Visser, G. H., Boon, P. E. & Meijer, H. A. J. Validation of the doubly labeled water method in Japanese Quail Coturnix C. japonica chicks: Is there an effect of growth rate? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 170, 365–372. https://doi.org/10.1007/s003600000112 (2000).
Visser, G. H. & Schekkerman, H. Validation of the doubly labeled water method in growing precocial birds validation of the doubly labeled water method in growing precocial birds: The importance of assumptions concerning evaporative water loss. Physiol. Biochem. Zool. 72, 740–749. https://doi.org/10.1086/316713 (1999).
Westerterp, K. R. Exercise, energy expenditure and energy balance, as measured with doubly labelled water. Proc. Nutr. Soc. 77, 4–10. https://doi.org/10.1017/S0029665117001148 (2018).
Speakman, J. R. The history and theory of the doubly labeled water technique. Am. J. Clin. Nutr. 68, 932–938. https://doi.org/10.1093/ajcn/68.4.932S (1998).
Guidotti, S. et al. Total energy expenditure assessed by salivary doubly labelled water analysis and its relevance for short-term energy balance in humans. Rapid Commun. Mass Spectrom. 30, 143–150. https://doi.org/10.1002/rcm.7412 (2016).
Speakman, J. R. & Hambly, C. Using doubly-labelled water to measure free-living energy expenditure: Some old things to remember and some new things to consider. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 202, 3–9. https://doi.org/10.1016/j.cbpa.2016.03.017 (2016).
Butler, P. J., Green, J. A., Boyd, I. L. & Speakman, J. R. Measuring metabolic rate in the field: The pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168–183. https://doi.org/10.1111/j.0269-8463.2004.00821.x (2004).
Berman, E. S. F. et al. Inter-and intraindividual correlations of background abundances of 2H, 18O and 17O in human urine and implications for DLW measurements. Eur. J. Clin. Nutr. 69, 1091–1098. https://doi.org/10.1038/ejcn.2015.10 (2015).
Guidotti, S., Meijer, H. A. J. & van Dijk, G. Validity of the doubly labeled water method for estimating CO2 production in mice under different nutritional conditions. Am. J. Physiol. Endocrinol. Metab. 305, E317–E324. https://doi.org/10.1152/ajpendo.00192.2013 (2013).
Van Trigt, R. et al. Validation of the DLW method in Japanese quail at different water fluxes using laser and IRMS. J. Appl. Physiol. 93, 2147–2154. https://doi.org/10.1152/japplphysiol.01134.2001 (2002).
Dansgaard, W. Stable isotopes in precipitation. Tellus 16, 436–468. https://doi.org/10.3402/tellusa.v16i4.8993 (1964).
Haggarty, P., McGaw, B. A. & Franklin, M. F. Measurement of fractionated water loss and CO2production using triply labelled water. J. Theor. Biol. 134, 291–308. https://doi.org/10.1016/S0022-5193(88)80060-2 (1988).
Brenninkmeijer, C. A. M., Kraft, P. & Mook, W. G. Oxygen isotope fractionation between CO2 and H2O. Chem. Geol. 41, 181–190. https://doi.org/10.1016/S0009-2541(83)80015-1 (1983).
Majoube, M. Fractionnement en oxygène 18 et en deutérium entre l’eau et sa vapeur. J. Chim. Phys. 68, 1423–1436. https://doi.org/10.1051/jcp/1971681423 (1971).
Merlivat, L. Molecular diffusivities of H216O, HD16O, and H218O in gases. J. Chem. Phys. 69, 2864–2871. https://doi.org/10.1063/1.436884 (1978).
Berman, E. S. F. et al. Maximizing precision and accuracy of the doubly labeled water method via optimal sampling protocol, calculation choices, and incorporation of 17O measurements. Eur. J. Clin. Nutr. 74, 454–464. https://doi.org/10.1038/s41430-019-0492-z (2020).
Ravussin, E., Harper, I. T., Rising, R. & Bogardus, C. Energy expenditure by doubly labeled water: Validation in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab. https://doi.org/10.1152/ajpendo.1991.261.3.e402 (1991).
Schoeller, D. A. Insights into energy balance from doubly labeled water. Int. J. Obes. 32, S72–S75 (2008).
Hall, K. D. et al. Methodologic issues in doubly labeled water measurements of energy expenditure during very low-carbohydrate diets. BioRxiv 1, 21 (2018).
Kerstel, E. R. T. et al. Assessment of the amount of body water in the Red Knot (Calidris canutus): An evaluation of the principle of isotope dilution with 2H, 17O, and 18O as measured with laser spectrometry and isotope ratio mass spectrometry. Isotopes Environ. Health Stud. 42, 1–7. https://doi.org/10.1080/10256010500503197 (2006).
Barkan, E. & Luz, B. High precision measurements of 17O/16O and 18O/16O ratios in H2O. Rapid Commun. Mass Spectrom. 19, 3737–3742. https://doi.org/10.1002/rcm.2250 (2005).
Baker, L. et al. A technique for the determination of 18O/16O and 17O/16O isotopic ratios in water from small liquid and solid samples. Anal. Chem. 74, 1665–1673. https://doi.org/10.1021/ac010509s (2002).
Meijer, H. A. J. & Li, W. J. The use of electrolysis for accurate δ17O and δ18O isotope measurements in water. Isotopes Environ. Health Stud. 34, 349–369. https://doi.org/10.1080/10256019808234072 (1998).
De Groot, P. A. Handbook of Stable Isotope Analytical Techniques (Elsevier Inc, 2004).
Kerstel, E. R. T. et al. Simultaneous determination of the 2H/1H, 17O/16O, and 18O/16O isotope abundance ratios in water by means of laser spectrometry. Anal. Chem. 71, 5297–5303. https://doi.org/10.1021/ac990621e (1999).
Van Trigt, R., Kerstel, E. R. T., Visser, G. H. & Meijer, H. A. J. Stable isotope ratio measurements on highly enriched water samples by means of laser spectrometry. Anal. Chem. 73, 2445–2452. https://doi.org/10.1021/ac001428j (2001).
Melanson, E. L. et al. Validation of the doubly labeled water method using off-axis integrated cavity output spectroscopy and isotope ratio mass spectrometry. Am. J. Physiol. Endocrinol. Metab. 314, E124–E130. https://doi.org/10.1152/ajpendo.00241.2017 (2018).
Speakman, J. R. The role of technology in the past and future development of the doubly labelled water method. Isotopes Environ. Health Stud. 41, 335–343. https://doi.org/10.1080/10256010500384283 (2005).
Thorsen, T. et al. Doubly labeled water analysis using cavity ring-down spectroscopy. Rapid Commun. Mass Spectrom. 25, 3–8. https://doi.org/10.1002/rcm.4795 (2011).
Mitchell, G. W., Guglielmo, C. G. & Hobson, K. A. Measurement of whole-body CO2 production in birds using real-time laser-derived measurements of hydrogen (δ2H) and oxygen (δ18O) isotope concentrations in water vapor from breath. Physiol. Biochem. Zool. 88, 599–606. https://doi.org/10.1086/683013 (2015).
Faghihi, V. et al. A new high-quality set of singly (2H) and doubly (2H and 18O) stable isotope labeled reference waters for biomedical and other isotope-labeled research. Rapid Commun. Mass Spectrom. 29, 311–321. https://doi.org/10.1002/rcm.7108 (2015).
Faghihi, V., Meijer, H. A. J. & Gröning, M. A thoroughly validated spreadsheet for calculating isotopic abundances (2H, 17O, 18O) for mixtures of waters with different isotopic compositions. Rapid Commun. Mass Spectrom. 29, 1351–1356. https://doi.org/10.1002/rcm.7232 (2015).
Tinsley, F. C., Taicher, G. Z. & Heiman, M. L. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes. Res. 12, 150–160. https://doi.org/10.1038/oby.2004.20 (2004).
Weir, J. B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109, 1–9 (1949).
Guidotti, S. et al. Doubly Labelled Water analysis: Preparation, memory correction, calibration and quality assurance for δ2H and δ18O measurements over four orders of magnitudes. Rapid Commun. Mass Spectrom. 27, 1055–1066. https://doi.org/10.1002/rcm.6540 (2013).
Wang, X., Jansen, H. G., Duin, H. & Meijer, H. A. J. Measurement of δ18O and δ2H of water and ethanol in wine by off-axis integrated cavity output spectroscopy and isotope ratio mass spectrometry. Eur. Food Res. Technol. https://doi.org/10.1007/s00217-021-03758-2 (2021).
Brian Leen, J., Berman, E. S. F., Liebson, L. & Gupta, M. Spectral contaminant identifier for off-axis integrated cavity output spectroscopy measurements of liquid water isotopes. Rev. Sci. Instrum. https://doi.org/10.1063/1.4704843 (2012).
Gonfiantini, R. Advisory Group Meeting on Stable Isotope Reference Samples for Geochemical and Hydrochemical Investigations, Viena, 19–21 September 1983, report to the Director General (International Atomic Energy Agency, 1984).
Schoeller, D. A. et al. Energy expenditure by doubly labeled water: Validation in humans and proposed calculation. Am. J. Physiol. Regul. Integr. Comp. Physiol. https://doi.org/10.1152/ajpregu.1986.250.5.r823 (1986).
Barkan, E. & Luz, B. High-precision measurements of 17O/ 16O and 18O/16O ratios in CO 2. Rapid Commun. Mass Spectrom. 26, 2733–2738. https://doi.org/10.1002/rcm.6400 (2012).
Speakman, J. R. How should we calculate CO2 production in DLW studies of mammals. Funct. Ecol. 7, 746–750 (1993).
Coward, W. A. et al. Measurement of CO2 and water production rates in man using 2H, 18O labelled H2O: Comparisons between calorimeter and isotope values. Eur. Nutr. Rep. 5, 126–128 (1985).
Schoeller, D. A. Measurement of energy expenditure in free-living humans by using doubly labeled water. J. Nutr. 118, 1278–1289. https://doi.org/10.1093/jn/118.11.1278 (1988).
Nagy, K. A. CO2 production in animals: Analysis of potential errors in the doubly labeled water method. Am. J. Physiol. https://doi.org/10.1152/ajpregu.1980.238.5.r466 (1980).
Lifson, N. & McClintock, R. Theory of use of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 12, 46–74. https://doi.org/10.1016/0022-5193(66)90185-8 (1966).
Pal, M. et al. Exploring triple-isotopic signatures of water in human exhaled breath, gastric fluid, and drinking water using integrated cavity output spectroscopy. Anal. Chem. 92, 5717–5723. https://doi.org/10.1021/acs.analchem.9b04388 (2020).
Acknowledgements
Xing Wang gratefully acknowledges support by the Chinese Scholarship Council.
Funding
X.W. has received support by the Chinese Scholarship Council and the scholarship from the University of Groningen.
Author information
Authors and Affiliations
Contributions
H.A.J.M. initiated the research. All authors contributed to the study concept and design. Material preparation and all experiments on and handing of the mice were conducted by D.K. and G.v.D. Micro-distillation and isotopic measurement of the blood samples were done by X.W. Data collection and analysis were performed by X.W. and H.A.J.M. H.A.J.M. wrote the data processing code. The draft of the manuscript was written by X.W. All authors read, commented and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Kong, D., van Dijk, G. et al. First use of triply labelled water analysis for energy expenditure measurements in mice. Sci Rep 12, 6351 (2022). https://doi.org/10.1038/s41598-022-10377-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10377-8
- Springer Nature Limited