Abstract
This study evaluated dental caries detection ability between the Qraycam and Qraypen on the same dental caries lesions. A total of 178teeth from 61patients were imaged using Qraypen C®(QC) and Qraycam Pro®(QP) devices and evaluated using analysis software (QA2). Occlusal, secondary, and proximal dental caries were evaluated and scored according to International Caries Detection and Assessment System(ICDAS II) and X-ray criteria. Bland–Altman plots were used to compare quantitative light-induced fluorescence(QLF) parameters obtained from the different QLF devices. Sensitivity, specificity, and area under the receiver operating characteristic curve(AUROC) were calculated. The ΔFaver. of the QLF-parameters showed that the mean difference between the two different QLF devices was close to zero and that the ± 5 error value was included in the mean ± 1.96SD range for the detection of dental caries. The accuracies for diagnosing occlusal dental caries were 0.83–0.96 and 0.81–0.82 and the accuracies for diagnosing proximal dental caries were 0.52–0.62 and 0.52–0.71 for the QC and QP devices, respectively. In conclusion, the ΔFaver. obtained from the QP showed diagnostic value mainly for screening of demineralized teeth. For teeth selected through screening, the depth of the lesion must be precisely evaluated using additional QP and radiographic imaging.
Similar content being viewed by others
Introduction
Over the past two decades, there has been a paradigm shift in the early detection of carious lesions without cavities and nonsurgical treatment of such lesions before cavity formation1,2,3,4. These changes require the early detection and evaluation of lesions; thus, accurate diagnosis is essential5.
In general, visual inspection is the simplest and most commonly used method for detecting caries in the clinical setting; however, this technique is limited in that it can only detect decay directly on the tooth surface. Due to the anatomical structure of the lesion, such detection is possible only after the lesion has progressed significantly to the dentin6,7,8. Thus, visual inspection for detecting noncavitary lesions varies widely in both sensitivity (0.2–0.96) and specificity (0.50–1.00) and is inconsistent across examiners as a diagnostic tool9,10. Radiography, which has long been used as a traditional diagnostic method, is now considered unsuitable for reliable diagnosis owing to its low sensitivity (0.14–0.38) and high specificity (0.59–0.90) in the detection of early carious lesions9,10. Additionally, the American Dental Association (ADA) discourages the use of routine radiographic examinations for screening purposes due to the inherent risks of radiation exposure11. Currently, the options available for detecting dental caries beyond visible assessment are limited.
In this context, various adjunctive diagnostic techniques, such as quantitative light-induced fluorescence (QLF), optical fiber-transmitted illumination using visible light, DIAGNOdent using laser light, and electrical conductivity measurement using electrical current, have been investigated10,12. The QLF technique can quantitatively evaluate minute changes in teeth based on autofluorescence that occurs when irradiated with 405 nm visible blue light13,14. The loss of fluorescence detected in teeth was highly correlated with the loss of minerals within the lesion, which can be used to effectively detect and monitor minute changes in demineralization/remineralization in early carious lesions without cavities15,16. Red fluorescence is derived from porphyrin-induced metabolites produced by oral microorganisms and is emitted by dental caries, dental plaques, and calculi17,18. This technique showed an intraclass correlation coefficient (ICC) of 0.96 and intra- and inter-examiner agreements of 0.93 and 0.92, respectively. Therefore, in the diagnosis of early carious lesions, QLF can reduce the probability of a diagnostic mismatch if only visual inspection and radiographic examinations are employed10.
Various types of devices using the QLF technology have been developed. Among them, the Qraypen C® (QC, AIOBIO, Seoul, Republic of Korea), with a small field of view (FOV), was introduced in 2018. This device comprises blue (emits 405-nm peak wavelength) and white LEDs, an inductor filter, and a 1/3-inch progressive complementary metal–oxide–semiconductor (CMOS) image sensor (HD 720p). The Qraycam Pro® (QP, AIOBIO, Seoul, Republic of Korea) with a larger FOV, introduced in 2018, is a third-generation QLF device that comprises a set of LEDs (in the same configuration as QLF-D), an Inspektor glass filter (with the same filter as in the QLF-D device), and a CMOS image sensor (FHD 1080p).
These two QLF devices are used differently for different imaging purposes, owing to their different FOV. The QC provides a large FOV and is useful for screening the overall dental condition in the oral cavity. However, the image quality of the QC is relatively deteriorated because it is placed outside the oral cavity. In contrast, the QP device is placed in the oral cavity, making it suitable for individual teeth and proximal imaging, enabling detailed evaluation.
Therefore, the purpose of this study was to evaluate the clinical applicability of QC as a screening tool to detect dental caries through quantitative evaluation through fluorescence loss (ΔF) and red fluorescence (ΔR) parameters compared to the QP, which could be evaluated relatively precisely for the same dental carious lesion.
Results
This study analyzed 178 teeth. Table 1 shows the mean and standard deviation of the QLF parameters for teeth diagnosed with 130 occlusal dental caries, 27 secondary dental caries, and 21 proximal dental caries.
We performed a Bland–Altman plot analysis to confirm the concordance between the fluorescence parameters of the two different devices using the confidence interval as the deviation ± 5 value of the error range provided by the manufacturer. Consequently, the ΔFaver. of the QLF parameters showed a mean difference between the two different QLF devices of close to zero and a ± 5 error value within the mean ± 1.96 SD range. Moreover, the probability of the ΔFaver. was within the confidence interval of 87.2% (Fig. 1).
Evaluation of the cut-off values and validity of the QLF parameters for diagnosing occlusal dental caries revealed an accuracy of 0.83–0.96, and an area under the receiver operating characteristic curve (AUROC) of 0.92–0.99 for the QC and 0.81–82 and 0.87–0.94, respectively, for the QP. Both devices showed a high diagnostic accuracy for dental caries. In particular, all parameter values showed higher accuracy in the diagnostic threshold for discriminating the degree of caries requiring treatment (D2) than in the diagnostic threshold for discriminating early dental caries (D1) (Table 2, Fig. 2A).
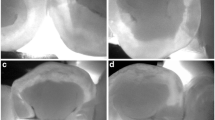
(A) Occlusal dental caries according to International Caries Detection and Assessment System (ICDAS) II criteria: (a–d) Score 1 (first visual change in the enamel) on the maxillary right second molar (#17). (e–h) Score 2 (distinct visual change in the enamel when viewed wet) on the maxillary right first premolar (#14). Score 3 (localized enamel breakdown) on the maxillary left second molar (#27). (a, e, i) Fluorescence image from the Qraypen C. (b, f, j) Fluorescence image from the Qraycam Pro. (c, g, k) Quantitative analysis of the Qraypen C image using QA2; (d, h, l) Quantitative analysis of the Qraycam Pro image using QA2. (B) Secondary dental caries according to the ICDAS II criteria: (a–d) Score 1 (first visual change in the enamel) on the maxillary left second molar (#27). (e–h) Score 2 (distinct visual change in the enamel when viewed wet) on maxillary left second molar (#27). (a, e) Fluorescence image from the Qraypen C. (b, f) Fluorescence image from the Qraycam Pro. (c, g) Quantitative analysis of the Qraypen C image using QA2. (d, h) Quantitative analysis of the Qraycam Pro image using QA2. (C) Proximal dental caries according to X-ray criteria: (a–d) Score 1 (radiolucency visible in the enamel) on the maxillary left first premolar (#24, arrow). (e–h) Score 2 (radiolucency restricted to the outer third of the dentin) on the maxillary left second premolar (#25, arrow). Score 3 (radiolucency extending to the middle third of the dentin) on the maxillary right first molar (#16, arrow). (a, e, i) Fluorescence image from the Qraypen C. (b, f, j) Bitewing radiograph. (c, g, k) Quantitative analysis of the Qraypen C image using QA2. (d, h, l) Quantitative analysis of the Qraycam Pro image using QA2.
Evaluation of the cut-off values and validity of the QLF parameters for diagnosing secondary dental caries showed an accuracy of 0.75 and AUROC of 0.90 for the QC and 0.89 and 0.82, respectively, for the QP (Table 3, Fig. 2B).
Evaluation of the cut-off values and validity of the QLF parameters for diagnosing proximal dental caries revealed an accuracy of 0.52–0.62, and an AUROC of 0.60–0.67 for the QC and 0.52–0.71 and 0.56–0.64, respectively, for the QP (Table 4, Fig. 2C).
Discussion
The comparisons of the two QLF devices in this study showed significant agreement between ΔFaver. for the four QLF parameters used to detect dental caries. Previous studies have also demonstrated the usefulness of the QLF parameter ΔF for detecting early occlusal carious lesions19. As mentioned previously, the degree of fluorescence loss detected in the tooth was highly correlated with mineral loss within the lesion, which enables lesion diagnosis by effectively detecting and monitoring minute changes in demineralization/remineralization in early carious lesions without cavities15,16. However, one outstanding question is the clinical usefulness of diagnosing early dental caries by evaluating only enamel demineralization. Another study argued that long-existing white spot lesions did not show any more demineralization effects and should be considered when diagnosing early dental caries. Because long-standing white spot lesions appear to be stable, it may be important for clinicians to carefully determine whether the lesion is active or arrested20. In this context, the results showing a higher degree of agreement between the two devices for dental caries (D2), which are considered to require treatment in this study, were meaningful.
Only the QLF parameter ΔFaver. showed significant agreement among the four QLF parameters analyzed in the present study, although the same sites were examined in both QC and QP images. This difference was attributed to the size (pixels) of the object photographed by the two devices. In general, the demineralization area of the occlusal surface is shown as a red or yellow area on QC images taken from a close distance, with finely divided pixels. In the QP image taken from a long distance, the pixels were displayed as black instead of a red or yellow area because the pixels could not be distinguished and, instead, were displayed together. Accordingly, our results demonstrated that QC images showing red/yellow regions had large ΔFmax values, while the QP images did not show the corresponding value and concomitantly showed low-intensity values.
Previous studies using the QLF technique mainly focused on red fluorescence for mature and pathogenic plaques21,22. However, Lee et al. first reported, through an in vitro experiment, that the red fluorescence observed in mature pathogenic plaques is also present in occlusal carious lesions23. Demineralized early enamel carious lesions contain fewer minerals and higher amounts of organic matter and water than healthy enamel 24,25. This can lead to the formation of porous pathways that connect the inside and outside of the lesion. These results indicated that the QLF technique can be used to evaluate the activity and extent of microorganisms inside carious enamel lesions, suggesting that the ΔR value can be a useful parameter for evaluating such lesions. In particular, when diagnosing secondary caries using the QP, the ΔR value may be a more reliable parameter than the ΔF value. In the case of secondary caries, it is more meaningful to evaluate the activity of mature microorganisms deposited around the restoration than the degree of enamel demineralization when diagnosing early caries. However, this study did not determine the inter-device agreement or diagnostic accuracy for ΔR values for the detection of dental caries.
We observed a low correlation in proximal caries diagnosis between the two QLF devices. In general, diagnosing proximal caries is challenging due to the anatomy of the lesions, as visual detection of these caries is limited because 75% of proximal lesions are in contact sites, with the rest beneath them15,26. Proximal lesions are detected only when dentin is affected or the marginal ridges become cavitated27. Thus, the number of proximal caries may be underestimated when using only visual examination. Although attempts have been made to diagnose proximal caries using the QLF technique, this method is also limited due to the anatomical location of the lesion. These sites produce less fluorescence because the lesion blocks the excitation light from the device as well as the backscattered fluorescence from the dentine. This results in reduced fluorescence, making it difficult to detect fluorescence in proximal dental caries15. The present study evaluated proximal caries by capturing fluorescence images of the occlusal surface. This indirect evaluation, as in previous evaluations of proximal caries26,27, was limited in its ability to confirm the relationship between the lesion depth and fluorescence parameters. However, another study showed that QLF technology can be used as a screening tool to detect proximal dental caries at the dentin level before radiographic examination28,29.
The in vivo repeatability and reproducibility of the QLF are excellent for the quantification of dental surface caries and this device has been applied in several clinical studies30. However, standardized conditions should be used to obtain images of consistent quality. In this study, the QC and QP images were obtained using the same process by a single clinician with more than 3 years of QLF imaging experience. Plaques and external stains that could fluoresce and complicate the analysis of QLF images were also removed before the examination. In addition, the QLF images were acquired in the same room, and the image conditions were kept consistent by reducing the ambient lighting as much as possible. Using these standardized conditions, the image acquisition process likely had a minimal impact on the results of this study.
A limitation of this study was the absence of a gold standard method such as histological analysis. Previous studies used histological analysis to calculate the precise lesion depth to reconfirm the validity of QLF parameters and provide clear evidence for the diagnosis of early dental caries31,32,33; However, the current study used the International Caries Detection and Assessment System (ICDAS-II) to validate carious lesions, biasing the key aspect of validation. The correlation between ICDAS and histology decreased from 0.782 (when SM was used for histological validation) to 0.511 (when microradiography with a contrast solution was used for histological validation)34. Dental plaque and stains remaining on the tooth surface even after thorough cleaning before QLF imaging may have affected the results of this study. However, in vitro experiments are limited because they do not accurately reflect the actual clinical setting. This study was meaningful in that it considered the actual clinical process. Another limitation was that the reliability of the experimental results may be reduced because of the small number of proximal or secondary caries samples that were analyzed.
In conclusion, the mean value of F (ΔFaver.) obtained from the QC device showed diagnostic value mainly for the screening demineralized teeth. For teeth selected through screening, the depth of the lesion must be precisely evaluated using the QP device and radiographic imaging.
Methods
Sample selection
This study was approved by the institutional review board of Kyung Hee University Dental Hospital (IRB No. KH-DT21019) and followed the tenets of the Declaration of Helsinki. Clinical data were collected from patients who visited Kyung Hee Dental Healthcare Center between April 2019 and August 2021. A total of 141 participants provided informed consent to participate and were initially enrolled in the study. After excluding 80 patients with no occlusal or proximal dental caries on QP examination, this study included 235 teeth from 61 patients. Among them, 166 teeth had occlusal caries, 29 had suspected proximal caries, and 40 had secondary caries. A total of 45 teeth were excluded following clinical examination for cavitated teeth, enamel hypoplasia, dens evaginatus, cracked teeth, and teeth with erosion, which could have affected the study results. Finally, this study included 178 teeth from 61 patients (Fig. 3). Among the 61 participants (35.6 ± 15.1 years), 29 were men (32.1 ± 12.0 years) and 32 were women (38.8 ± 17.0 years).
Acquisition of QLF images
White-light and fluorescence images were captured by a trained examiner using the QP and QC devices in a dark room to maintain image quality. The teeth were brushed with a toothbrush and gauze before QLF imaging to remove dental plaque or debris that could affect the results. The QLF images were captured with a QP (FOV, field of view (W × H × D, mm): 155 × 124 × 103, resolution: 1920 × 1080) using the “occlusal” imaging mode. This device was equipped with a metal tube and plastic shield to block external light and prevent interference with the fluorescence image. Using an intraoral photo mirror, the QP was placed vertically on the maxillary or mandibular occlusal surface containing the tooth to be analyzed and a mirror image was taken (Fig. 4A). The QC (field of view [FOV]: 5–45, resolution: 1280 × 720) was placed perpendicular to the occlusal surface of the corresponding tooth, and images were taken directly (Fig. 4B).
(A) White-light and fluorescent images of the occlusal surface of the maxilla or mandible captured using a Qraycam Pro® (QP, AIOBIO, Seoul, Republic of Korea). (a, b) A trained examiner using a Quantitative light-induced fluorescence (QLF) system in a dark room to maintain the image quality. This device is equipped with a metal tube that blocks external light to prevent contamination of the fluorescent image. (c) White-light image (d) Fluorescent image. (B) (a, b) QLF images of the occlusal surface of the examined tooth captured using a Qraypen C® (QC, AIOBIO, Seoul, Republic of Korea). (c) White-light image (d) Fluorescent image. (C) QA2 version 1.25 (Inspektor Research system BV, Amsterdam, The Netherlands). QA2 provides the QLF parameters (ΔFmax, ΔFaver., ΔRmax, ΔRaver.) of the area of interest (AOI); (a, b) Quantitative analyses of QP and QC images, respectively.
Bitewing radiography
After the QLF images were obtained, bitewing radiographs were obtained only for the 29 teeth suspected of having proximal caries. Bitewing images were obtained using a digital sensor (Kodak RVG, Carestream Dental, NY, USA) and an X-ray unit (Asahi Roentgen Industry, Kyoto, Japan) at exposures of 60 kV and 7 mA, with an average exposure length of 0.63 s.
Scoring of white QLF and bitewing images
The QLF and bitewing images were evaluated by an oral maxillofacial radiologist with > 10 years of experience. Occlusal and secondary dental caries were graded according to the ICDAS II criteria35, while proximal caries were graded according to X-ray criteria36. For lesions that were difficult to distinguish, a lower grade was assigned and the criteria were applied conservatively (Table 5).
Quantitative analysis of QLF images
Owing to the difference in fluorescence image size between the two devices, patches were drawn around the same area to be analyzed in each image and set as the region of interest (ROI). The fluorescence intensity of the ROI was quantified using two fluorescence parameters. The parameters ΔF and ΔR were measured as percentages using QA2 analysis software, version 1.25 (Inspektor Research Systems BV, Amsterdam, The Netherlands) (Fig. 4C). The ΔF value indicated the percentage decrease in fluorescence in the carious area relative to that of the normal enamel, which reflected changes in the mineral composition of the enamel. ΔR represented the increase in red fluorescence intensity due to porphyrins generated by bacterial metabolism as a percentage relative to that of normal enamel, which reflected the activity of microorganisms in the oral cavity.
Statistical analysis
To evaluate inter-observer reproducibility, 20 cases were randomly selected, and another oral and maxillofacial radiologist with > 10 years of experience analyzed the QLF and bitewing images. To evaluate intra-observer reproducibility, 20 randomly selected cases were further analyzed after 2 weeks. The resultant intraclass correlation coefficient (ICC, 0.84–0.91; P < 0.001; kappa value, 0.85) and interobserver coefficient (ICC, 0.75–0.85; P < 0.001; kappa value, 0.80) indicated high reliability. Bland–Altman plots were used to compare the fluorescence parameters obtained from two different QLF devices. Receiver operating characteristic curves were plotted between the two devices to verify the validity of the fluorescence parameters for the diagnosis of dental caries. The sensitivity, specificity, and AUROC were calculated at two critical points (D1 and D2): one to differentiate early caries (score, 1 vs. 2 or 3) and the other to differentiate severe caries requiring treatment (score, 1 or 2 vs. 3). The significance cut-off for all statistical tests was set at α = 0.05, using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA).
References
Takahashi, N. & Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 90, 294–303. https://doi.org/10.1177/0022034510379602 (2011).
Pitts, N. B. & Stamm, J. W. International consensus workshop on caries clinical trials (ICW-CCT)—final consensus statements: Agreeing where the evidence leads. J. Dent. Res. 83, 125–128. https://doi.org/10.1177/154405910408301s27 (2004).
Classifications, C. & Strategies, P. American academy on pediatric dentistry, American academy of pediatrics. Policy on early childhood caries (ECC). Pediatr Dent. 38, 52–54 (2016).
Pitts, N. B. Are we ready to move from operative to non-operative/preventive treatment of dental caries in clinical practice?. Caries Res. 38, 294–304. https://doi.org/10.1159/000077769 (2004).
Frencken, J. E. et al. Minimal intervention dentistry for managing dental caries–a review: Report of a FDI task group. Int. Dent. J. 62, 223–243. https://doi.org/10.1111/idj.12007 (2012).
Schwendicke, F., Tzschoppe, M. & Paris, S. Radiographic caries detection: A systematic review and meta-analysis. J. Dent. 43, 924–933. https://doi.org/10.1016/j.jdent.2015.02.009 (2015).
Dove, S. B. Radiographic diagnosis of dental caries. J. Dent. Educ. 65, 985–990 (2001).
Madiraju, G. Additional diagnostic value of digital bitewing radiographs in detecting proximal caries in primary molars. Compend. Contin. Educ. Dent. 32, E115-119 (2011).
Gomez, J., Tellez, M., Pretty, I. A., Ellwood, R. P. & Ismail, A. I. Non-cavitated carious lesions detection methods: A systematic review. Commun. Dent. Oral Epidemiol. 41, 54–66. https://doi.org/10.1111/cdoe.12021 (2013).
Pretty, I. A. Caries detection and diagnosis: Novel technologies. J. Dent. 34, 727–739. https://doi.org/10.1016/j.jdent.2006.06.001 (2006).
American Dental Association Council on Scientific Affairs. The use of dental radiographs: Update and recommendations. J. Am. Dent. Assoc. 137, 1304–1312, https://doi.org/10.14219/jada.archive.2006.0393 (2006).
Pretty, I. A. & Ekstrand, K. R. Detection and monitoring of early caries lesions: A review. Eur. Arch. Paediatr. Dent. 17, 13–25. https://doi.org/10.1007/s40368-015-0208-6 (2016).
Hope, C. K., de Josselin, J. E., Field, M. R., Valappil, S. P. & Higham, S. M. Photobleaching of red fluorescence in oral biofilms. J. Periodontal Res. 46, 228–234. https://doi.org/10.1111/j.1600-0765.2010.01334.x (2011).
Amaechi, B. T. & Higham, S. M. Quantitative light-induced fluorescence: A potential tool for general dental assessment. J. Biomed. Opt. 7, 7–13. https://doi.org/10.1117/1.1427044 (2002).
van der Veen, M. H. & de Josselin, J. E. Application of quantitative light-induced fluorescence for assessing early caries lesions. Monogr. Oral Sci. 17, 144–162. https://doi.org/10.1159/000061639 (2000).
Kim, H. E., Kwon, H. K. & Kim, B. I. Recovery percentage of remineralization according to severity of early caries. Am. J. Dent. 26, 132–136 (2013).
Kim, Y. S., Lee, E. S., Kwon, H. K. & Kim, B. I. Monitoring the maturation process of a dental microcosm biofilm using the quantitative light-induced fluorescence-digital (QLF-D). J. Dent. 42, 691–696. https://doi.org/10.1016/j.jdent.2014.03.006 (2014).
Han, S. Y., Kim, B. R., Ko, H. Y., Kwon, H. K. & Kim, B. I. Assessing the use of quantitative light-induced fluorescence-digital as a clinical plaque assessment. Photodiagnosis Photodyn. Ther. 13, 34–39. https://doi.org/10.1016/j.pdpdt.2015.12.002 (2016).
Gmür, R. et al. In vitro quantitative light-induced fluorescence to measure changes in enamel mineralization. Clin. Oral Investig. 10, 187–195. https://doi.org/10.1007/s00784-006-0058-z (2006).
Zantne, C., Martus, P. & Kielbassa, A. M. Clinical monitoring of the effect of fluorides on long-existing white spot lesions. Acta Odontol. Scand. 64, 115–122. https://doi.org/10.1080/00016350500443297 (2006).
Kim, Y. S., Lee, E. S., Kwon, H. K. & Kim, B. I. Monitoring the maturation process of a dental microcosm biofilm using the Quantitative Light-induced Fluorescence- Digital (QLF-D). J. Dent. 42, 691–696. https://doi.org/10.1016/j.jdent.2014.03.006 (2014).
Lee, E. S., de Josselin, J. E., Jung, H. I. & Kim, B. I. Red fluorescence of dental biofilm as an indicator for assessing the efficacy of antimicrobials. J. Biomed. Opt. 23, 1–6. https://doi.org/10.1117/1.JBO.23.1.015003 (2018).
Lee, H. S. et al. Caries detection and quantification around stained pits and fissures in occlusal tooth surfaces with fluorescence. J. Biomed. Opt. 23, 1–7. https://doi.org/10.1117/1.JBO.23.9.091402 (2018).
de Barbosa, S. F., Dias, S. J. & Sampaio, V. S. Natural enamel caries: A comparative histological study on biochemical volumes. Caries Res. 47, 183–192. https://doi.org/10.1159/000345378 (2013).
van der Veen, M. H., Thomas, R. Z., Huysmans, M. C. & de Soet, J. J. Red autofluorescence of dental plaque Bacteria. Caries Res. 40, 542–545. https://doi.org/10.1159/000095655 (2006).
de Oliveira, K. M. H. et al. Dental flossing and proximal caries in the primary dentition: A systematic review. Oral Health Prev. Dent. 15, 427–434. https://doi.org/10.3290/j.ohpd.a38780 (2017).
Geibel, M. A. et al. Radiographic diagnosis of proximal caries-influence of experience and gender of the dental staff. Clin. Oral Investig. 21, 2761–2770. https://doi.org/10.1007/s00784-017-2078-2 (2017).
Kim, E. S. et al. A new screening method to detect proximal dental caries using fluorescence imaging. Photodiagnosis Photodyn. Ther. 20, 257–262. https://doi.org/10.1016/j.pdpdt.2017.10.009 (2007).
Oh, S. H. et al. Detection of dental caries and cracks with quantitative light-induced fluorescence in comparison to radiographic and visual examination: A retrospective case study. Sensors (Basel). 21, 1741. https://doi.org/10.3390/s21051741 (2021).
Verdonschot, E. H. & Angmar-Mansson, B. Advanced methods of caries diagnosis and quantification. In Dental caries 1st edn (eds Fejerskov, O. & Kidd, E.) 134 (Blackwell, 2003).
Jung, E. H. et al. Development of a fluorescence-image scoring system for assessing noncavitated occlusal caries. Photodiagnosis Photodyn. Ther. 21, 36–42. https://doi.org/10.1016/j.pdpdt.2017.10.027 (2018).
Park, S. W. et al. Comparison of fluorescence parameters between three generations of QLF devices for detecting enamel caries in vitro and on smooth surfaces. Photodiagnosis Photodyn. Ther. 25, 142–147. https://doi.org/10.1016/j.pdpdt.2018.11.019 (2019).
Ko, H. Y., Kang, S. M., Kim, H. E., Kwon, H. K. & Kim, B. I. Validation of quantitative light-induced fluorescence-digital (QLF-D) for the detection of approximal caries in vitro. J. Dent. 43, 568–575. https://doi.org/10.1016/j.jdent.2015.02.010 (2015).
Campos, S. A. G., Vieira, M. L. O. & de Sousa, F. B. Correlation between ICDAS and histology: Differences between stereomicroscopy and microradiography with contrast solution as histological techniques. PLoS ONE 25, 1–12. https://doi.org/10.1371/journal.pone.0183432 (2017).
Gugnani, N., Pandit, I. K., Srivastava, N., Gupta, M. & Sharma, M. International caries detection and assessment system (ICDAS): A new concept. Int. J. Clin. Pediatr. Dent. 4, 93–100. https://doi.org/10.5005/jp-journals-10005-1089 (2011).
Ekstrand, K. R., Ricketts, D. N. & Kidd, E. A. Reproducibility and accuracy of three methods for assessment of demineralization depth of the occlusal surface: An in vitro examination. Caries Res. 31, 224–231. https://doi.org/10.1159/000262404 (1997).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oh, S.H., Choi, JY. & Kim, SH. Evaluation of dental caries detection with quantitative light-induced fluorescence in comparison to different field of view devices. Sci Rep 12, 6139 (2022). https://doi.org/10.1038/s41598-022-10126-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10126-x
- Springer Nature Limited