Abstract
The function of uric acid (UA) in the genesis and evolution of chronic kidney disease (CKD) has motivated numerous studies, but the results remain inconclusive. We sought to conduct a systematic review and meta-analysis of cohort studies aiming to analyze the association of UA levels with the incidence and progression of CKD. Pubmed/Medline, Lilacs/Bireme and Web of Science were searched to identify eligible studies, following the PRISMA protocol. Data were presented for CKD incidence and progression separately. For the meta-analysis, studies with data stratified by subgroups according to serum UA levels were selected. The inverse variance-weighted random effects model was used to generate a combined effect estimate. Meta-regressions were performed to identify the causes of heterogeneity. The Newcastle–Ottawa Scale was used to assess the risk of bias. The publication bias was tested by funnel plot and Egger’s test. Eighteen CKD incidence studies (n = 398,663) and six CKD progression studies (n = 13,575) were included. An inverse relationship was observed between UA levels and protection from CKD incidence and progression. Lower UA levels were protective for the risk of CKD incidence (RR 0.65 [95% CI 0.56–0.75]) and progression (RR 0.55 [95% CI 0.44–0.68]). UA seems to be implicated both in the genesis of CKD and its evolution.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a global public health problem affecting more than 697 million people1. The prevalence of CKD has been increasing worldwide, with an annual increase of 8 to 16%, which is higher than the general population growth2,3.
In recent decades, the function of uric acid (UA) in the genesis and evolution of CKD has motivated numerous studies, but the results remain inconclusive due to the complex and bidirectional interaction between the change in UA levels and renal function, which hinders the isolation of the causal effect of UA in the progression of CKD4,5,6. The coexistence of risk factors, such as hypertension and chronic inflammation7,8,9 and pathophysiological peculiarities of CKD make it complex to define the role of UA.
UA is the final product of purine catabolism from exogenous sources, through diet, and endogenous, by cell degradation10. The remnant of circulating UA accounts for more than half of the antioxidant potential of human blood11. However, when it is inside the cells, it exhibits a pro-oxidant behavior, stimulating the production of free radicals and pro-inflammatory cytokines, reducing the bioavailability of vasodilator substances and increasing vasoconstrictor substances such as angiotensin, which lead to oxidative stress, chronic inflammation and endothelial dysfunction, the tripod of the pathogenesis and progression of CKD12,13.
The growing interest in UA considerably increased the number of publications14, and some prospective observational studies15,16,17 and retrospective studies18,19,20 showed an association between UA and incident or prevalent CKD, while other studies found no association21,22, evidencing the controversial role of UA in the incidence and progression of CKD. Furthermore, to the best of our knowledge, no review has been performed to assess the association between different UA levels (subgroups of UA levels) and the incidence and progression, contemplating all stages of CKD. Therefore, we performed a systematic review and meta-analysis of cohort studies to analyze the association of serum UA levels with the incidence and progression of CKD.
Methods
Protocol and register
Systematic review study conducted according to protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO), under identification number CRD42020142073, respecting the recommendations of the Cochrane Collaboration, reference for the preparation and publication of systematic reviews and meta-analyses. The results were presented according to recommendations proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)23.
Search strategy
The identification and selection of the studies occurred from December 2019 to January 2020, completely independently by two researchers. The health databases consulted were: US National Library of Medicine National Institutes of Health (PubMed)/Medical Literature Analysis and Retrieval System Online (Medline), Latin American and Caribbean Center on Health Sciences Information (Lilacs) and Web of Science, main health science databases. The descriptors used in the search are indexed in the Medical Subject Headings (MeSH), which are: “Hyperuricaemia”, “Uric Acid”, “Renal Insufficiency, Chronic”, “Kidney Diseases”, “Kidney Failure, Chronic”, “Chronic Kidney Disease”. The following combinations were used: “Hyperuricaemia” or “Uric Acid” and “Renal Insufficiency, Chronic”; “Hyperuricaemia” or “Uric Acid” and “Kidney Diseases”; “Hyperuricaemia” or “Uric Acid” and “Kidney Failure, Chronic”; “Hyperuricaemia” or “Uric Acid” and “Chronic Kidney Disease”. In order to contemplate the entire scientific production, the publication period was not delimited.
Selection of studies
After searching the databases, duplicate studies were excluded. Next, a refining was carried out to select the studies related to the theme addressed through the reading of titles and abstracts.
Studies were included if they met the following criteria: longitudinal cohort studies that evaluated the role of UA in the progression and/or incidence of CKD and had a follow-up of at least one year.
To reduce any confounding variables that may affect the association between CKD and UA, we excluded: studies with animals, children, adolescents, pregnant women and kidney transplant recipients, cross-sectional or case–control studies (to reduce the reverse causation, bias from pre-existing CKD on UA levels), studies addressing acute kidney disease and/or specific types of kidney disease (contrast-induced nephropathy or persistent post-treatment, renal failure after percutaneous coronary intervention, immunoglobulin A nephropathy, autosomal dominant polycystic kidney disease, lupus nephritis), studies on drug effectiveness, studies whose outcome was mortality and studies that assessed risk factors in general for progression of CKD (obesity dyslipidemia, diabetes, hypertension, etc.), whose focus was not to evaluate the role of uric acid.
As they were different measures, the outcomes progression and incidence of CKD were presented separately. Incidence of CKD was defined as individuals who were free of CKD at baseline (glomerular filtration rate (GFR) ≥ 60 mL/min/1.73 m2) but experienced a decline in GFR to less than 60 mL/min/1.73 m2 during follow-up. Progression was defined as decline in GFR and/or end-stage renal failure, requiring renal replacement therapy.
Data extraction
The following data were extracted from the selected studies: author’s name, date of publication (year), study design (follow up time), characteristics of the studied population, GFR estimation equation, exposure variable, main outcome, adjustment for possible confounding factors in multivariate analyses and evidence quality analysis.
Evidence quality analysis
The Newcastle–Ottawa Scale (NOS) was used to assess the risk of bias for cohort studies. We used three items to assess study quality: (1) selection of participants (including four domains); (2) comparability of study results (including one domain); and (3) outcome (including two domains)24. Each domain had a rating of “yes,” “no”, or “unclear.” If there was adequate data against a domain in the included study and met the criteria, it was classified as low risk of bias (yes). Conversely, a domain was classified as high risk of bias if adequate information was not available (no) or not enough data was available to make an assessment (unclear). “Yes” was scored as “1”, and “no” or “unclear” was scored as “0.” Scores were tallied up to calculate the final cumulative score. A study was considered high quality if the cumulative score was ≥ 4, and low quality if < 425.
Statistical analyses
For the quantitative analysis, studies with data stratified by groups according to serum UA levels (quartiles or quintiles) were selected. To evaluate the gradient, the groups with the lowest levels of UA were compared to the other groups (For quartiles: Q1 vs. Q2; Q1 vs. Q3 and Q1 vs. Q4. For quintiles: Q1 vs. Q2; Q1 vs. Q3; Q1 vs. Q4; Q1 vs. Q5).
For incidence studies, the absolute number of incident and non-incident cases in each group was used to calculate the relative risk (RR), with a 95% confidence interval and p values. For progression studies, the absolute number of cases that progressed and did not progress in each group was used to calculate the RR, with a 95% confidence interval and p values.
The effect estimates rate ratios were combined in the meta-analysis to calculate the overall risk estimate. A random-effects model was used for analysis to account for the variation of real effects across studies. Heterogeneity was quantified using the I2 statistic, which describes the proportion of total variation in the study estimates due to heterogeneity26. The degree of heterogeneity was assessed from the I2 statistic using the following thresholds for interpretation: (1) 0% to 30%: marginal heterogeneity; (2) 30% to 50%: moderate heterogeneity; (3) 50% to 75%: substantial heterogeneity; and (4) 75% to 100%: represents considerable heterogeneity26. The estimation of each study and the standard error (SE) generated a combined estimate, graphically represented by the forest plot.
Meta-regressions (univariate and multivariate) were performed to identify the causes of heterogeneity. The following variables were investigated: sex (difference in percentage of men between comparison groups), serum UA level (mean difference between comparison groups), age (mean difference between comparison groups) and sample size (mean difference between comparison groups). Initially, a univariate analysis was performed and all variables that values of p ≤ 0.200 were included in the final multivariate model. Variables with p values < 0.05 in the multivariate analysis were considered significant.
The publication bias was tested by funnel plot and Egger test. All analyses were performed using the ‘meta’ package in STATA version 14.
Results
Selection of studies
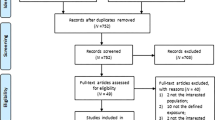
We identified 6889 publications at the electronic databases. After exclusion by duplicity, 4134 studies remained. In the paired selection through the screening by the titles and abstracts, 4084 more articles that did not fit our scope were eliminated. Among the 50 studies selected for full reading, we excluded 26 that did not meet the eligibility criteria. Finally, 24 studies were included and stratified into two groups: 186,15,16,18,20,27,28,29,30,31,32,33,34,35,36,37,38,39 related to the incidence of CKD in the general population and six5,17,21,40,41,42 on CKD progression. For the quantitative analysis, nine incidence studies6,16,28,30,32,34,35,37,39 and three progression studies5,17,21 were included. Figure 1 represents the flowchart of identification and selection of studies for systematic review and meta-analysis.
Characteristics of the studies
Tables 1 and 2 summarizes the characteristics of the 24 selected articles. All studies were longitudinal observational cohort studies, 18 prospective and six retrospective. Of the total, 14 studies were of Asian origin and 10 were conducted in Western countries between 2008 and 2019. In all, 412,238 participants were identified, 398,663 in incidence studies and 13,575 in progression studies. Regarding the confounding factors used in the adjustments of the analyzed studies, there was a diversity of variables: hypertension and/or blood pressure values; diabetes and/or related tests; dyslipidemia and/or lipidogram; use of medications (antihypertensive, diuretics, UA-lowering and lipid-lowering); proteinuria or albumin-creatinine ratio and GFR. All studies had a cumulative quality score of ≥ four and were thus considered high quality (Tables 1, 2, Supplementary table).
Synthesis of the results
UA levels and CKD incidence
An inverse relationship was observed between UA levels and protection for the CKD incidence (Fig. 2). Lower UA levels were protective for the risk of CKD incidence (RR 0.65 [95% CI 0.56–0.75]). With increasing quartiles or quintiles, protection for the CKD incidence decreased: Q1 versus Q2 (RR 0.88 [95% CI 0.78–0.99]), Q1 versus Q3 (RR 0.64 [95% CI 0.50–0.83]), Q1 versus Q4 (RR 0.51 [95% CI 0.37–0.71]), Q1 versus Q5 (RR 0.44 [95% CI 0.41–0.47]). In other words, the risk of CKD incidence increased with increasing levels of UA, and overall, the risk for incidence was 1.54.
The meta-analysis showed considerable heterogeneity by the I2 statistic (I2 = 97.7%). Regarding meta-regression (Table 3), in the univariate analysis, the age difference between the groups was the only variable with a p value < 0.200, therefore, the multivariate analysis was not necessary. We conclude that increasing the age difference between the groups increased the risk ratio between UA and the incidence of CKD (Coeff β 1.19 [95% CI 1.12–1.26]).
Funnel plot analysis qualitatively showed an asymmetric shape (Fig. 3), indicating the possibility of publication bias for the association between UA levels and CKD incidence. However, the Egger test showed no indication of publication bias (p = 0.249).
UA levels and CKD progression
An inverse relationship was observed between UA levels and protection from CKD progression (Fig. 4). Lower UA levels were protective for the risk of CKD progression (RR 0.55 [95% CI = 0.44–0.68]). With increasing quartiles or quintiles, protection for CKD progression decreased: Q1 versus Q2 (RR 0.74 [95% CI = 0.56–0.98]), Q1 versus Q3 (RR 0.53 [95% CI = 0.33–0.84]), Q1 versus Q4 (RR 0.42 [95% CI = 0.26–0.67]). In other words, the risk of CKD progression increased with increasing UA levels, and overall, the risk for progression was 1.81.
The meta-analysis showed considerable heterogeneity by the I2 statistic (I2 = 96.4%). Regarding meta-regression (Table 4), in the multivariate analysis no variable was significantly associated with the risk relationship between UA and CKD progression (Table 4).
Funnel plot analysis and Egger's test were not performed for CKD progression because few studies were selected.
Discussion
The present study found a gradient relationship between UA levels and CKD, with lower UA levels were protective for the risk of CKD incidence and progression. In addition, increasing the age difference between groups (with different levels of UA) increased the risk between UA and incidence of CKD.
UA levels are an independent predictor of the development of CKD and its progression. Some studies have shown that the prevalence of hyperuricaemia gradually increases with the decrease in renal function, being 10 times higher in stages 3–5 than in stage 143. This relationship highlights a potential benefit of UA screening in different phases of CKD44.
A retrospective cohort involving 13,133 healthy adults (without hypertension, diabetes, obesity or CKD) showed that increasing UA levels doubled the risk of incident CKD45. Similarly, a Japanese retrospective cohort with 5,507 adults followed-up for an average period of 4.6 years found a positive association of hyperuricaemia (≥ 7 mg/dl) with the incidence of CKD (adjusted HR = 1.58 [95% CI = 1.21–2.07]), but with no effect on its progression (HR = 1.08 [95% CI = 0.73–1.59])46.
Jalal47 made a critical review of observational and experimental studies on the potential effect of UA reduction therapy on the prevention of the incidence and progression of CKD and concluded that UA participates in inflammation and evolution of CKD. The authors acknowledge, however, that such conclusions are controversial because they are based on small studies and without placebo group.
Thus, two recently published double-blind, placebo-controlled, multicenter randomized trial48,49 concluded the use of allopurinol was not beneficial to prevent the progression of pre-existing kidney disease, with no statistical difference between using or not using the UA-lowering agents. On the other hand, a recent systematic review and meta-analysis of 11 randomized clinical trials, including 4,277 participants with CKD, suggested that UA-lowering therapy preserves GFR so that xanthine oxidoreductase inhibitors could improve renal outcomes50. This finding may indicate a different role for UA in the pathophysiology of CKD in addition to a nitrogen slag.
The definition of the role of UA is still complex, especially due to the coexistence of risk factors such as hypertension, chronic inflammation and pathophysiological peculiarities of CKD. Recent studies have shown that soluble UA exhibits a behavioral duality acting as pro-oxidant within the cell and antioxidant in the extracellular environment10,51,52.
In relation to the pro-oxidant effect, the UA stimulates the generation of reactive oxygen species culminating in oxidative stress and mitochondrial damage, endothelial dysfunction with activation of the renal-angiotensin-aldosterone system, reduction of nitric oxide bioavailability and afferent arteriolopathy53,54,55. Furthermore, UA induces the activation of dependent or independent pathways of NLRP3 inflamassome with release of pro-inflammatory cytokines (IL1β and IL18), necro-inflammation and interstitial fibrosis56. Such pathophysiological mechanisms may justify the role of UA in the incidence and progression of CKD.
There were several strengths to this systematic review: 1. it is one of the first studies to gather evidence from observational studies in the search for an association of UA levels, not only with the incidence of CKD, but also with its progression to terminal stages, thus contemplating all stages of CKD; 2. the literature search included several large databases with the search criteria designed to identify as many relevant articles as possible; 3. a proportion of the study selection, data extraction and quality assessment were conducted in duplicate by separate reviewers to reduce reporting bias; 4. all studies were classified as high quality and encourages future researches related to the use of UA reduction therapies for preventing CKD in at-risk populations.
There are some limitations to our study that need to be taken into consideration. First, the studies presented heterogeneous sample sizes, which was minimized with the use of appropriate statistical tests in the meta-analysis. Secondly, different definitions were observed for the final outcome (GFR < 60, decline in GFR, rapid decline of GFR) and for the independent variable (UA levels). Subgroup analysis based on the UA levels (quartiles or quintiles) was conducted to assess whether the presentation of exposure contributed to differences in study results. This analysis showed similar results regardless of the method of presenting the exposure. Thirdly, different forms of GFR estimation (MDRD, CKD-EPI, Cockcroft-Gault, Japanese equation, equation based on insulin clearance, iohexol technique) were adopted. Finally, there was some heterogeneity among risk estimates from the included studies, possibly due to some bias at the study level. However, the variables included in the meta-regression were not sufficient to explain the heterogeneity between studies. Other important variables that interfere in the UA metabolism could not be included, as they were not present in all studies, such as gout, basal GFR, diet components, use of UA-lowering agents.
In conclusion, the present study provides evidence that lower UA levels were protective for the risk of CKD incidence and progression. Increasing the age difference between the groups increased the risk between UA and the incidence of CKD.
References
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Jha, V. et al. Chronic kidney disease: Global dimension and perspectives. The Lancet 382, 260–272 (2013).
De Nicola, L. & Minutolo, R. Worldwide growing epidemic of CKD: Fact or fiction?. Kidney Int. 90, 482–484 (2016).
Bellomo, G. Uric acid and chronic kidney disease: A time to act?. World J. Nephrol. 2, 17–25 (2013).
Tsai, C. W. et al. Uric acid predicts adverse outcomes in chronic kidney disease: A novel insight from trajectory analyses. Nephrol. Dial. Transpl. 33, 231–241 (2018).
Chou, Y. C. et al. Elevated uric acid level as a significant predictor of chronic kidney disease: A cohort study with repeated measurements. J. Nephrol. 28, 457–462 (2015).
Shi, Y. Caught red-handed: Uric acid is an agent of inflammation. J. Clin. Investig. 120, 1809–1811 (2010).
Madero, M. et al. Uric acid and long-term outcomes in CKD. Am. J. Kidney Dis. 53, 796–803 (2009).
Mazzali, M. et al. Uric acid and hypertension: Cause or effect?. Curr. Rheumatol. Rep. 12, 108–117 (2010).
Kang, D. H. & Ha, S. K. Uric acid puzzle: Dual role as anti-oxidantand pro-oxidant. Electrolyte and Blood Pressure 12, 1–6 (2014).
Barata, R., Cardoso, F. & Pereira, T. Hyperuricemia in chronic kidney disease: A role yet to be explained. Port. J. Nephrol. Hypertens. 34, 30–35 (2020).
Gyurászová, M., Gurecká, R., Bábíčková, J. & Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2020/5478708 (2020).
Johnson, R. J. et al. Uric acid and chronic kidney disease: Which is chasing which?. Nephrol. Dial. Transpl. 28, 2221–2228 (2013).
Rudan, D., Polašek, O., Kolčić, I. & Rudan, I. Uric acid: The past decade. Croat. Med. J. 51, 1–6 (2010).
Obermayr, R. P. et al. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 19, 2407–2413 (2008).
Weiner, D. E. et al. Uric acid and incident kidney disease in the community. J. Am. Soc. Nephrol. 19, 1204–1211 (2008).
Hsieh, Y.-P. et al. The role of uric acid in chronic kidney disease patients. Nephrology 22, 441–448 (2017).
Kuo, C. F. et al. Hyperuricaemia and accelerated reduction in renal function. Scand. J. Rheumatol. 40, 116–121 (2011).
Kawashima, M., Wada, K., Ohta, H., Terawaki, H. & Aizawa, Y. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: A long-term retrospective cohort study. BMC Nephrol. 12, 31 (2011).
Wang, S. et al. Uric acid and incident chronic kidney disease in a large health check-up population in Taiwan. Nephrology 16, 767–776 (2011).
Liu, W. C. et al. Association of Hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin. J. Am. Soc. Nephrol. 7, 541–548 (2012).
Yü, T. F. & Berger, L. Impaired renal function in gout. Its association with hypertensive vascular disease and intrinsic renal disease. Am. J. Med. 72, 95–100 (1982).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6, e1000097 (2009).
Wells, G. et al. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10th October 2021.
Shajahan, S., Amin, J., Phillips, J. K. & Hildreth, C. M. Relationship between sex and cardiovascular mortality in chronic kidney disease: A systematic review and meta-analysis. PLoS One 16, e0254554 (2021).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Storhaug, H. M. et al. Uric acid is associated with microalbuminuria and decreased glomerular filtration rate in the general population during 7 and 13 years of follow-up: The Tromsø Study. BMC Nephrol. 16, 210 (2015).
Takae, K. et al. Serum uric acid as a risk factor for chronic kidney disease in a Japanese community—the Hisayama study. Circ. J. 80, 1857–1862 (2016).
Zhang, L., Wang, F., Wang, X., Liu, L. & Wang, H. The association between plasma uric acid and renal function decline in a Chinese population-based cohort. Nephrol. Dial. Transpl. 27, 1836–1839 (2012).
Mwasongwe, S. E. et al. Relation of uric acid level to rapid kidney function decline and development of kidney disease: The Jackson Heart Study. J. Clin. Hypertens. 20, 775–783 (2018).
Ben-Dov, I. Z. & Kark, J. D. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: The Jerusalem Lipid Research Clinic cohort study. Nephrol. Dial. Transpl. 26, 2558–2566 (2011).
Mok, Y. et al. Serum uric acid and chronic kidney disease: The Severance cohort study. Nephrol. Dial. Transpl. 27, 1831–1835 (2012).
Bellomo, G. et al. Association of uric acid with change in kidney function in healthy normotensive individuals. Am. J. Kidney Dis. 56, 264–272 (2010).
Ye, M. et al. The association between time-mean serum uric acid levels and the incidence of chronic kidney disease in the general population: A retrospective study. BMC Nephrol. 19, 190 (2018).
Kuwabara, M. et al. Elevated serum uric acid level predicts rapid decline in kidney function. Am. J. Nephrol. 45, 330–337 (2017).
Sonoda, H., Takase, H., Dohi, Y. & Kimura, G. Uric acid levels predict future development of chronic kidney disease. Am. J. Nephrol. 33, 352–357 (2011).
Cao, X., Wu, L. & Chen, Z. The association between elevated serum uric acid level and an increased risk of renal function decline in a health checkup cohort in China. Int. Urol. Nephrol. 50, 517–525 (2018).
Chini, L. S. N., Assis, L. I. S. & Lugon, J. R. Relationship between uric acid levels and risk of chronic kidney disease in a retrospective cohort of Brazilian workers. Braz. J. Med. Biol. Res. 50, e6048 (2017).
Kamei, K. et al. A slight increase within the normal range of serum uric acid and the decline in renal function: Associations in a community-based population. Nephrol. Dial. Transpl. 29, 2286–2292 (2014).
Nacak, H., Van Diepen, M., De Goeij, M. C. M., Rotmans, J. I. & Dekker, F. W. Uric acid: Association with rate of renal function decline and time until start of dialysis in incident pre-dialysis patients. BMC Nephrol. 15, 91 (2014).
Nacak, H. et al. Uric acid is not associated with decline in renal function or time to renal replacement therapy initiation in a referred cohort of patients with Stage III, IV and V chronic kidney disease. Nephrol. Dial. Transpl. 30, 2039–2045 (2015).
Sturm, G., Kollerits, B., Neyer, U., Ritz, E. & Kronenberg, F. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp. Gerontol. 43, 347–352 (2008).
Russo, E. et al. Association of uric acid with kidney function and albuminuria: The Uric Acid Right for heArt Health (URRAH) Project. J. Nephrol. https://doi.org/10.1007/s40620-021-00985-4 (2021).
Akhter, S. & Rizwan, A. S. M. Study of serum uric acid in different stages of chronic kidney disease. J. Adv. Med. Med. Res. 33, 70–79 (2021).
Son, Y.-B. et al. The effect of baseline serum uric acid on chronic kidney disease in normotensive, normoglycemic, and non-obese individuals: A health checkup cohort study. PLoS One 16, e0244106 (2021).
Tada, K. et al. Investigation the effect of serum uric acid on new-onset and progression of Chronic Kidney Disease(CKD) in Japanese general population: IKI Epidemiological Study of Artherosclerosis and Chronic Kidney Disease (ISSA-CKD) retrospective phase. Nephrol. Dial. Transpl. 35, gfaa142.P0778 (2020).
Jalal, D. I., Chonchol, M., Chen, W. & Targher, G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 61, 134–146 (2013).
Doria, A. et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 382, 2493–2503 (2020).
Badve, S. V. et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 382, 2504–2513 (2020).
Maruyama, Y. et al. Effect of uric acid-lowering therapy on renal function in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Replace. Ther. 71(7), 1–11 (2021).
Sautin, Y. Y. & Johnson, R. J. Uric acid: The oxidant-antioxidant paradox. In Nucleosides, Nucleotides and Nucleic Acids Vol. 27, 608–619 (NIH Public Access, 2008).
Yip, K., Cohen, R. E. & Pillinger, M. H. Asymptomatic hyperuricemia: Is it really asymptomatic?. Curr. Opin. Rheumatol. 32, 71–79 (2020).
Paravicini, T. M. & Touyz, R. M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 31, S170–S180 (2008).
Gliozzi, M., Malara, N., Muscoli, S. & Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 213, 23–27 (2016).
Joosten, L. A. B., Crişan, T. O., Bjornstad, P. & Johnson, R. J. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat. Rev. Rheumatol. 16, 75–86 (2020).
Mulay, S. R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 96, 58–66 (2019).
Author information
Authors and Affiliations
Contributions
D.L.N.G. and L.S.S. participated in the design of the study, the analysis and interpretation of data, the drafting the article and approved the final manuscript. T.R.M. participated in the analysis and interpretation of data, the drafting the article and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonçalves, D.L.N., Moreira, T.R. & da Silva, L.S. A systematic review and meta-analysis of the association between uric acid levels and chronic kidney disease. Sci Rep 12, 6251 (2022). https://doi.org/10.1038/s41598-022-10118-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10118-x
- Springer Nature Limited