Abstract
Patients with congenital heart disease who have a variety of cardiac/extracardiac problems are at high risk for deterioration. This study aimed to determine the effectiveness of post-intensive care unit (ICU) follow-up by a rapid response team (RRT) after congenital heart surgery. This before-and-after study was conducted at an urban regional tertiary hospital. We enrolled 572 consecutive patients who underwent congenital heart surgery and were transferred alive from the paediatric ICU (PICU) between April 2015 and March 2020. Post-ICU follow-up for 48 h was started in April 2018. The primary and secondary endpoints were unplanned ICU readmission and clinical outcomes at ICU readmission, respectively. Overall, 346 and 226 patients were analysed pre- and post-intervention, respectively. Patient demographics were similar between groups, but in the post-intervention group, patients tended to have had more complicated surgery. Unplanned ICU readmission rates within 30 days were similar between groups. Regarding the demographics and outcomes at ICU readmission, patients in the post-intervention group had lower predicted mortality rates (1.7% vs 5.3%, P = 0.001), required less ventilator days (median, 0.5 days [interquartile range (IQR) 0–1] vs median, 3 days [IQR 0.5–4], P = 0.02), and had a shorter ICU stay (median, 3 days [IQR 2–4] vs median, 6 days [IQR 3–9], P = 0.03), but there was no significant between-group difference in ICU mortality. Post-ICU follow-up by a RRT after congenital heart surgery did not decrease unplanned ICU readmission but improved several outcomes at ICU readmission.
Similar content being viewed by others
Introduction
Congenital heart disease (CHD) presents with a wide range of physiologic/anatomic circulatory abnormalities. The majority of patients with CHD continue to have various cardiac/extracardiac problems that need ongoing care and management1. Given their vulnerability, they have higher morbidity and mortality risks than the general population2, 3 and can become seriously ill from only the slightest perturbations, such as infection or injury.
The rapid response system (RRS) is one strategy to improve the prognosis of patients with sudden deterioration in the hospital. The RRS is a medical safety system that detects early signs of most serious conditions, such as respiratory and/or cardiac arrest, and prevents such situations. A team of specially trained professionals, such as a medical emergency team and rapid response team (RRT), needs to be organized to immediately respond to the needs of patients when their condition worsens. Various factors can trigger the RRS, such as abnormalities in the patient’s vital signs, concerns of medical personnel/patient’s family, or the follow-up of high-risk patients4. Recently, a systematic review of adult patients has reported that follow-ups by a specialized team after intensive care unit (ICU) transfer reduced unplanned ICU readmissions5. Meanwhile, it has been reported that deteriorating patients with CHD who required unscheduled ICU readmission had a poor outcome6, 7. Taken together, we hypothesize that post-ICU transfer follow-ups for patients with CHD would decrease unplanned ICU readmissions and improve their outcomes, but there are no previous reports to show this.
Therefore, we conducted a single-centre before-and-after study to examine whether post-ICU follow-ups by a specialized RRT reduce unscheduled ICU readmissions in patients after CHD surgery and reduce the severity of the illness and the prognosis of patients who require readmission.
Methods
Study design and setting
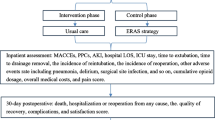
A before-and-after study was conducted at an urban regional tertiary hospital in Osaka, Japan; the 3-year before-implementation period (April 2015 to March 2018) was compared to the 2-year after-implementation period (April 2018 to March 2020) (here, we refer to the implementation of post-ICU follow-up by the RRT). This hospital has a multidisciplinary paediatric intensive care unit (PICU) with 12 beds; all patients, regardless of age, were admitted to the PICU after CHD surgery. Approximately 100 to 150 congenital heart surgeries are performed in this hospital annually. The protocol for this study was approved by the ethics committee of Osaka City General Hospital (reference number: 1805021) as being in compliance with the 1975 Declaration of Helsinki and Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, which waived the need to obtain informed consent from patients because only non-personally identifiable data from electronic patient records were used and no personal information will be disclosed.
Medical safety system for in-hospital deterioration
Our hospital has adopted a two-tiered RRS to detect in-hospital deterioration; the “Code Blue Team” deals with serious deteriorating events that require immediate resuscitation, including cardiac and/or pulmonary arrest, whereas the RRS is intended to prevent such critical events. The RRS was initially started for only adult patients in 2012 and was expanded to cover all ages in 2017. The type of response team is the so-called “RRT”, led mainly by nurses, consisting of nurses and physicians of the ICU, critical care centre, or operating room. The RRT is activated when the calling criteria are met; the nurse-in-charge first evaluates the deteriorating patient and, after consulting with the physician in charge of the RRT, decides on the necessary treatment plan. Furthermore, the actual therapeutic intervention and the need for ICU admission will be decided after consultation with the attending physician team and the ICU physician. However, using the calling criteria alone, the RRS for patients treated by highly specialized departments, such as paediatric cardiac surgery and cardiology, was almost never activated. In 2017, there were only 21 annual activations based on the calling criteria for all paediatric patients. Furthermore, there were no calls in patients whose primary department was paediatric cardiac surgery. Therefore, in addition to the usual activation of the RRT described above, we initiated the RRT follow-up after ICU transfer for patients with CHD with the highest risk of in-hospital deterioration in April 2018. The patients were periodically examined for at least 48 h after ICU transfer; visits at least twice a day, additional visits if condition deteriorates. The decision for readmission to the ICU was made through a consensus of the RRT staff, intensivist, and attending physician. During the study period, no major medical management policy changes were implemented in our hospital.
Selection of participants
We included consecutive patients who underwent congenital heart surgery, who were admitted to the PICU postoperatively, and who were discharged alive from the PICU between April 2015 and March 2020 and those for whom relevant data pertaining to the post-ICU transfer period of 30 days were available. Patients who met the selection criteria were stratified into the pre- and post-intervention groups.
Data collection
Several types of data were collected from electronic patient records at initial ICU admission. Demographic data included those on age (months), sex (male/female), weight (kg), single ventricle anatomy (Yes/No), diagnosis of chromosomal abnormalities (Yes/No), a chronic illness other than CHD (Yes/No), surgical history of tracheostomy (Yes/No), surgical history of gastrostomy (Yes/No), and home oxygen therapy (Yes/No). Operative data included those pertaining to Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) score (6 levels: 1–6; the higher the level, the greater the surgical complexity and the higher the risk of postoperative mortality), operative time (min), cardiopulmonary bypass (CPB) time (min), aortic cross-clamping time (min), water balance (mL/kg), and volume of blood products administered (mL/kg). ICU management data included those on the requirement for intensive care treatment (nitric oxide inhalation [Yes/No], extracorporeal membrane oxygenation [Yes/No]), ventilator days, length of ICU stay (days), and the requirement for new tracheostomy (Yes/No).
In addition, data at ICU readmission were collected on organ-specific classification (circulatory [e.g., acute heart failure]/respiratory [e.g., asthma attack]/infection [e.g., mediastinitis]/neurological [e.g., convulsive seizure]/cardiopulmonary arrest), presence of surgical complications (Yes/No), and surgical complication classification (cardiac tamponade/mediastinitis/phrenic nerve paralysis/intracranial haemorrhage).
Outcome measures
The primary evaluation item was unplanned ICU readmission within 30 days after ICU discharge. The secondary evaluation items were the severity at ICU readmission: predicted mortality (%, calculated from the Paediatric Index of Mortality 3 [PIM3]), the requirement for each intensive treatment (intubation/mechanical ventilation, nitric oxide inhalation, extracorporeal membrane oxygenation), and clinical outcomes at ICU readmission (ventilator days, length of ICU stay [days], and ICU mortality). As a secondary evaluation item, Unrecognized Situation Awareness Failure Events (UNSAFE) transfers (Yes/No) was also adopted; UNSAFE transfer is one of the leading indicators for the effectiveness of paediatric RRS. In children, cardiac arrest and death are extremely infrequent, making it difficult to show the effect of interventions, so UNSAFE was devised as a more frequently occurring indicator, defined as ward to ICU transfers after which patients are intubated, placed on vasopressors, or receive three or more fluid boluses in the first one hour after transfer8.
We used PIM3 to calculate the predicted mortality rate because it is a representative score for calculating predicted mortality in the paediatric intensive care field and is also used in the Japanese multicentre registry, in which our institution participates.
Statistical analyses
Descriptive statistics were used to summarize the characteristics of the patients. Continuous variables are expressed as medians with interquartile ranges (IQRs), whereas categorical variables are expressed as frequencies and percentages. Between-group differences in these types of variables were compared using the Wilcoxon rank-sum test and Fisher’s exact test, respectively. Multivariate logistic regression analyses for confounders were performed to examine the independent effects of the intervention on the primary outcome and variables significantly different between the two groups, and the results are presented as odds ratios (ORs) and their associated 95% confidence intervals (CIs). Variables that were significantly different between the two groups were selected as the confounders. All P values were two-sided, and a value < 0.05 was considered statistically significant. Statistical analyses were performed using the R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org).
Results
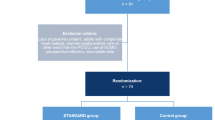
Overall, 572 patients were admitted to the PICU after congenital heart surgery and discharged alive from there during the study period, were included in the final analysis. We divided them into two groups: 346 patients were in the pre-intervention group, and 226 patients were in the post-intervention group (Fig. 1).
The patient demographics, operative variables, and ICU management variables are shown in Table 1. The patient demographics were similar between the two groups. The median age of the patients in both groups was approximately 12 months; the percentage of single-ventricular anatomy was approximately 10%. Few patients had a surgical history of tracheostomy or gastrostomy. Regarding operative variables, compared to the pre-intervention group, the post-intervention group had fewer cases with RACHS-1 score 1 and more with RACHS-1 score 3. Higher risk procedures tended to be performed more frequently in the post-intervention group than in the pre-intervention group. Longer operative and CPB time and larger volumes of blood products in the operation room were required for the post-intervention group than for the pre-intervention group. Regarding ICU management, more ventilator and PICU days were required in the post-intervention group than in the pre-intervention group.
Primary outcome
Unplanned ICU readmissions within 30 days from ICU discharge were similar between the pre- and post-intervention groups (4.3% vs 6.2%, P = 0.33). The logistic regression analyses showed that, compared to the pre-intervention group, the crude and adjusted ORs for unplanned ICU readmission within 30 days among the post-intervention group were 1.5 (95% CI 0.7–3.1) and 1.2 (95% CI 0.5–2.7), respectively. Variables adjusted for in the logistic regression analyses were age (months), RACHS-1 score four or more (Yes/No), operative time (min), volume of administered blood products (mL/kg), and length of PICU stay (days).
Reasons for paediatric intensive care unit readmission in both groups
Regarding the organ-specific classification of the ICU readmission cause, the post-intervention group tended to have more circulatory disorders (50.0% vs 13.3%, P = 0.05) and fewer respiratory disorders (21.4% vs 53.3%, P = 0.12) and cardiopulmonary arrests (0% vs 6.7%, P > 0.99). Moreover, surgical complications tended to be more common in the post-intervention group than in the pre-intervention group (85.7% vs 60.0%, P = 0.21); among them, cardiac tamponade was the most common (Table 2).
Secondary outcomes
Patients in the post-intervention group had a significantly lower predicted mortality rate (1.7% vs 5.3%, P = 0.001), lower UNSAFE transfer (86.7% vs 42.9%, P = 0.02) and required fewer ventilator days (median, 0.5 days [IQR 0.5, 4] vs median, 3 days [IQR 0, 1], P = 0.02) and a shorter length of ICU stay (median, 3 days [IQR 2, 4] vs median, 6 days [IQR 3, 9], P = 0.03) than those in the pre-intervention group. The ICU mortality rate was lower in the post-intervention group than in the pre-intervention group, although the difference was not statistically significant (0% vs 6.7%, P > 0.99). Furthermore, there was no significant difference in the treatments required between the two groups. All these results are shown in Table 3.
Discussion
Our study demonstrates that 48-h follow-ups after ICU discharge by the RRT for postoperative patients with CHD did not reduce the unplanned ICU readmission rate within 30 days, but it decreased the predicted mortality, UNSAFE transfer rate, ventilator days, and length of ICU stay at ICU readmission.
This is the first report to show the effect of RRT on post-ICU follow-up after congenital heart surgery. In the context of the rapidly increasing number of patients with CHD, appropriate treatment of in-hospital sudden deteriorations in patients with CHD is an urgent issue. However, cardiac surgery and cardiology, which are highly specialized departments that mainly treat patients with CHD, tend to avoid intervention by third parties, including RRS. Therefore, active activation of the RRS by the staff of these departments is rare, making it difficult to make the RRS function effectively9. In contrary, if the follow-up after ICU discharge is predetermined by the RRT, the RRS can function effectively regardless of the wishes of this department. Several positive effects of the intervention in this study will lead to improvement in the quality of medical care for patients with CHD in-hospital emergencies in the future.
In contrast to our study, post-ICU follow-ups reduced ICU readmissions in a systematic review of adults5. There are some possible reasons for this. First, the RACHS-1 score, length of PICU stay, and duration of ventilation in the post-intervention group were higher than in the pre-intervention group, suggesting that they were at higher risk of deterioration and ICU readmission. Therefore, it may have been difficult to show improvement by this intervention. Second, it is possible that the patient evaluation by the RRT, a third party other than the attending physician, may have had prompted ICU admission before the patient became seriously ill. In fact, there was a decrease in the illness severity among patients who required unplanned ICU readmission after the intervention in the current study, suggesting overcoming resistance of the primary teams. Third, surgical complications as a reason for ICU readmission, especially cardiac tamponade, tended to be more common in the post-intervention group than in the pre-intervention group. Since our treatment policy is that postoperative cardiac tamponade is always treated in the operating room with tamponade release and drainage, following which the patients must be admitted to the ICU, RRT interventions cannot reduce ICU readmissions. Therefore, the high incidence of cardiac tamponade was considered a reason for the lack of decline in the ICU readmission rate. A previous study of paediatric patients who were readmitted to the cardiac care unit found that the percentage of patients with increased pericardial effusion was 6.4%, which was lower than that in our post-intervention group (35.7%). The cause of the high incidence of cardiac tamponade in this study is unknown; no major changes in the perioperative treatment strategy, including anticoagulation management, existed before or after the intervention. The rate of respiratory disorders as the reason for ICU readmission decreased in the post-intervention group, suggesting that the post-ICU follow-ups might have prevented the progression of respiratory disorders.
The intervention reduced the severity of the illness and improved some outcomes of unscheduled PICU readmissions. The reason for this, as mentioned above, could be the fact that the third-party’s assessment could have prompted an ICU admission at an earlier stage, before the condition became severe. A single-centre before-and-after study of adults found that RRT activities, including follow-up after ICU discharge, reduced the Acute Physiology And Chronic Health Evaluation II score, the need for mechanical ventilation, and CPA in the ICU at ICU readmission10, similar to our results.
Study limitation
Several limitations of this study should be acknowledged. First, this was a single-centre study, and the results may not be generalizable to all centres because medical management varies between institutions. Since our hospital is a relatively small facility with 100–150 paediatric cardiac surgeries per year, it may be expected that a larger number of CHD patients will have more in-hospital sudden changes at a facility with a larger number of surgeries, and thus more benefit may be expected from RRT follow-up. Alternatively, large facilities with comprehensive care for CHD patients may have fewer in-hospital sudden changes to begin with. Differences depending on the size of the facility will be an important issue for further study. Second, the before-and-after study design is inherently unable to exclude potential bias (e.g. selection/assignment, history, Hawthorne, and reporting/publication bias)11. One report has pointed out the dangers of examining the effectiveness of paediatric RRS interventions using only a before-and-after study design12. The third limitation is the differences in the severity of illness (e.g. operative risk and PICU days) between the two groups. No major changes in treatment strategy or medical staffing were made during the study period, and the exact cause was unknown. Although we adjusted for these differences as confounding, we cannot deny the possibility that unmeasured and unknown confounding may have distorted the results. Fourth, the possibility of a measurement bias cannot be denied: the primary endpoints and the criteria for PICU readmission were not standardized.
A multifaceted approach is needed to improve the quality of care for the rapidly increasing number of patients with CHD. It is recommended to establish a sufficient number of specialized CHD centres, to establish a safe and smooth transport system13,14,15,16. Moreover, this post-ICU follow-up may be one of the effective countermeasures against in-hospital sudden deteriorations in patients with CHD. Further prospective, randomized, multi-centre studies are needed to demonstrate the real effects of the intervention.
In conclusion, this single-centre before-and-after study showed that post-ICU follow-ups of patients who underwent congenital heart surgery did not decrease their unplanned ICU readmission but improved the severity of the illness and several outcomes at ICU readmission. This may be one effective method to improve the prognosis of in-hospital deteriorating patients after congenital heart surgery.
Data availability
The authors confirm that the data supporting the findings of this study are available within the supplementary materials.
References
Warnes, C. A. et al. ACC/AHA. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology (American Heart Assoc. Task Force on Practice Guidelines) (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 52, e143-263 (2008).
Verheugt, C. L. et al. Mortality in adult congenital heart disease. Eur. Heart J. 31, 1220–1229. https://doi.org/10.1093/eurheartj/ehq032 (2010).
Greutmann, M. et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit. Heart Dis. 10, 117–127. https://doi.org/10.1111/chd.12201 (2015).
DeVita, M. A. et al. Findings of the first consensus conference on medical emergency teams. Crit. Care Med. 34, 2463–2478. https://doi.org/10.1097/01.CCM.0000235743.38172.6E (2006).
Niven, D. J., Bastos, J. F. & Stelfox, H. T. Critical care transition programs and the risk of readmission or death after discharge from an ICU: A systematic review and meta-analysis. Crit. Care Med. 42, 179–187. https://doi.org/10.1097/CCM.0b013e3182a272c0 (2014).
Bastero-Miñón, P., Russell, J. L. & Humpl, T. Frequency, characteristics, and outcomes of pediatric patients readmitted to the cardiac critical care unit. Intensive Care Med. 38, 1352–1357. https://doi.org/10.1007/s00134-012-2592-2 (2012).
Brunetti, M. A. et al. Unplanned readmission to the pediatric cardiac intensive care unit: Prevalence, outcomes, and risk factors. World J. Pediatr. Congenit. Heart Surg. 6, 597–603. https://doi.org/10.1177/2150135115594854 (2015).
Brady, P. W. et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics 131, e298–e308. https://doi.org/10.1542/peds.2012-1364 (2013).
Haga, T. et al. The prevalence and characteristics of rapid response systems in hospitals with pediatric intensive care units in Japan and barriers to their use. Int. J. Qual. Health Care 32, 325–331. https://doi.org/10.1093/intqhc/mzaa040 (2020).
Al-Qahtani, S. et al. Impact of an intensivist-led multidisciplinary extended rapid response team on hospital-wide cardiopulmonary arrests and mortality. Crit. Care Med. 41, 506–517. https://doi.org/10.1097/CCM.0b013e318271440b (2013).
Ho, A. M. H. et al. Bias in before–after studies: Narrative overview for anesthesiologists. Anesth. Analg. 126, 1755–1762. https://doi.org/10.1213/ANE.0000000000002705 (2018).
Joffe, A. R., Anton, N. R. & Burkholder, S. C. Reduction in hospital mortality over time in a hospital without a pediatric medical emergency team: Limitations of before-and-after study designs. Arch. Pediatr. Adolesc. Med. 165, 419–423. https://doi.org/10.1001/archpediatrics.2011.47 (2011).
Deanfield, J. et al. Management of grown up congenital heart disease. Eur. Heart J. 24, 1035–1084. https://doi.org/10.1016/s0195-668x(03)00131-3 (2003).
Landzberg, M. J. et al. Task force 4: Organization of delivery systems for adults with congenital heart diseases. J. Am. Coll. Cardiol. 37, 1187–1193. https://doi.org/10.1016/s0735-1097(01)01275-x (2001).
Therrien, J. et al. CCS Consensus Conference 2001 update: Recommendations for the management of adults with congenital heart disease. Part I. Can. J. Cardiol. 17, 940–959 (2001).
Mylotte, D. et al. Specialized adult congenital heart disease care: The impact of policy on mortality. Circulation 129, 1804–1812. https://doi.org/10.1161/CIRCULATIONAHA.113.005817 (2014).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
T.H., T.S., T.K., and T.M. conceptualized and designed the study. J.M., N.Y., and S.M. acquired the data. T.H. analysed and interpreted the data and drafted the manuscript. T.S., T.K., T.M., J.M., N.Y., and S.M. revised the manuscript critically for important intellectual content. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haga, T., Sakaguchi, T., Kazuta, T. et al. Effect of post-ICU follow-up by a rapid response team after congenital heart surgery. Sci Rep 12, 5633 (2022). https://doi.org/10.1038/s41598-022-09683-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09683-y
- Springer Nature Limited