Abstract
Particles in exhaled air (PEx) are generated when collapsed small airways reopen during breathing. PEx can be noninvasively collected by particle impaction, allowing the analysis of undiluted epithelial lining fluid (ELF). We used the endotoxin (LPS) challenge model to proof the concept that PEx can be used to monitor inflammatory changes in the lung. In this pilot study PEx were collected from ten healthy nonsmoking subjects using the PExA® instrument twice before and twice after a segmental LPS challenge (5, 21 h). Following a 4-week washout period, PEx were collected during the week before and 5 h after a whole lung LPS inhalation challenge. PEx biomarkers were compared to blood, bronchoalveolar lavage (BAL) following segmental challenge and induced sputum (ISP) following inhalation challenge. A clear LPS-induced inflammatory response was detectable in BAL fluid, ISP and blood. Albumin and surfactant–protein D were detectable in all PEx samples. While most baseline cytokines were close to or below the detection limit, the median (IQR) IL-6 and IL-8 concentrations in PEx increased significantly after segmental (0.04 (0.03; 0.06) fg/ng PEx; 0.10 (0.08; 0.17) fg/ng PEx) and inhalation LPS challenge (0.19 (0.15; 0.23) fg/ng PEx; 0.32 (0.23; 0.42) fg/ng PEx). Using a highly sensitive analysis platform, we were able to detect a cytokine response in PEx during the early phase of LPS-induced inflammation. This will broaden the spectrum of applications for this noninvasive method to monitor inflammatory processes in the lung, including its use in clinical trials for respiratory drug development.
Trial registration: The study has been registered on 07.02.2017 at Clinicaltrials.gov (NCT03044327).
Similar content being viewed by others
Introduction
Noninvasive monitoring of airway inflammation as a surrogate for disease activity is important both in basic respiratory research and in clinical airway research, e.g., to assess the efficacy of novel drugs. In this study, we used the endotoxin (LPS) challenge model1,2,3 to test the performance of exhaled breath particle analysis, a novel noninvasive tool to collect undiluted epithelial lining fluid from the peripheral airways4,5,6.
It is widely accepted that reopening of collapsed small airways during normal breathing is the dominant mechanism for PEx generation in the peripheral lung4,6,7. PEx can be collected by exhalation through cooled sampling systems as exhaled breath condensate or undiluted by impaction using, e.g., the PExA device6. The latter includes a particle counter to determine the number and size spectrum of the exhaled particles8. It is of utmost importance to compute the number and mass of PEx that are actually collected because despite using standardized breathing maneuvers, particle emission shows very large interindividual differences9, which could also contribute to increased variability in cross-sectional EBC studies and the insufficient reproducibility discussed in the last ERS Taskforce report10. We therefore used BAL, ISP and serum as reference for the PEx analysis.
LPS challenge of single lung lobes by bronchoscopic instillation or of the whole lung by controlled inhalation are well-established methods to induce a transient and well-controlled inflammatory response in the lungs of healthy subjects. It has a clear advantage for assessing the clinical value of novel inflammation-monitoring tools compared to using patients with chronic airway inflammation because it is not affected by potentially varying states of inflammation in patients. Broad experience has been gained in using this challenge model as an experimental research tool and for profiling the pharmacodynamic efficacy of investigational new drugs in early stages of clinical development1,2.
In this study, we included ten healthy volunteer subjects who underwent both segmental and inhalation challenges in two experimental blocks separated by a 4-week washout period. The inflammatory response was assessed by BAL after segmental challenge2 and by induced sputum after inhalation challenge3. Blood samples were also collected at the respective time points. This complex study was designed to further characterize the LPS challenge model for future applications and to test novel noninvasive tools to monitor airway inflammation. These included specific gas-enhanced MRI11, the analysis of volatile organic compounds12 and specific cell analysis by flow cytometry and Chipcytometry13. Here, we present proof-of-concept data for monitoring cytokines in PEx following LPS challenge.
Methods
Study design
The study is outlined in Fig. 1. Volunteers underwent a segmental LPS challenge and, following a four-week washout period, a whole lung inhalation challenge. Exhaled breath particles (PEx) were sampled during a screening visit (V1), a first baseline visit (V2), 5 h following the segmental LPS challenge (V3), 21 h after segmental LPS challenge (V4), at a second baseline visit (V5) and 5 h following the inhalation LPS challenge (V6). Blood samples were collected in the morning of V3 before the segmental LPS challenge, 6 h and 21 h after segmental challenge, and on V5 and 6 h after inhalation challenge on V6.
Study design. The study was designed to test the ability of novel noninvasive methods to detect airway inflammation. Here we focus on exhaled breath particles (PEx). BAL bronchoalveolar lavage, V visit (index pre: refers to samples taken before a challenge, post: refers to samples taken after a challenge), NaCl isotonic saline solution, LPS endotoxin, w week, h hours. PEx samples were collected on V3 in the afternoon 5 h after the segmental challenge. On V4 PEx were sampled prior to the BAL procedure 21 h after the LPS challenge. On visit 5 and 6 PEx were collected before the sputum induction. On V6 this was done 5 h following the inhalation LPS challenge.
The segmental and inhalation challenges were performed as previously reported1,2,3,13. The procedures of bronchoalveolar lavage and sputum induction have also recently been published13 and are described in detail in Additional file 1.
Subjects
Ten healthy, nonsmoking subjects (7 male and 3 females, smoking history < 1 pack-year, mean (SD) age 38 ± 10 years) with normal lung function (FEV1: 101 ± 14% pred.) were included. The study was conducted in accordance with local laws, regulations and GCP guidelines (CPMP/ICH/135/95), and the protocol was approved by the ethics committee of the Hannover Medical School and registered on 07.02.2017 at Clinicaltrials.gov (NCT03044327). Every subject provided written informed consent after being fully informed about the study.
PEx sampling
Breath samples were collected using a commercially available device for particle sampling (Particles in Exhaled Air [PExA], Gothenburg, Sweden. For a schematic graph of the device please refer to14). We aimed to sample 240 ng at each collection but limited the collection time to a maximum time of 30 min (Table S1, see Additional file 1). Estimations of the PEx mass are based on the data of the optical particle counter (Grimm Aerosol, Ainring, Germany), which is included in the PExA instrument. The number of PEx in 8 size ranges between 0.4 and 4.6 μm were determined. With this information and assuming spherical particles with a unit density of 1000 kg m−3, the mass of the collected particles can be estimated4. Participants were asked to perform repetitive breathing maneuvers, as described by Bake et al.4, to increase PEx emissions. PEx were collected on Teflon membranes (0.45 µm PTFE Membranes 25 mm, Merck FHLC02500, Darmstadt, Germany).
PEx analysis
Half of each collection filter was extracted using an adapted method based on a recommended procedure by PExA. In short, 200 µL of RIPA buffer, including a protease inhibitor cocktail (Merck, Darmstadt, Germany), was added to the membrane in a 1.7 mL tube and incubated at 30 °C for 30 min under constant rapid agitation. The fluid was transferred to an empty 1.5 mL low binding tube (Eppendorf, Hamburg, Germany) and directly used for analysis.
The Meso Scale Discovery (MSD) platform was used for analysis: S-Plex Human IL-6 Kit with a lower limit of detection (LLOD) of 1.1 fg/mL, V-Plex Human IL-8 with an LLOD of 0.04 pg/mL, combined R-Plex for MPO and PSP-D (SP-D) with LLODs of 11 pg/mL and 37 pg/mL, R-Plex Human albumin with an LLOD of 155 pg/mL.
Data analysis
Due to the exploratory character of this pilot study, a power calculation was not performed. Data are presented as median and IQR. Nonnormally distributed data were log-transformed prior to analysis. Parametric methods were used for comparison. For potential LPS effects, we included the presegmental challenge and the two postsegmental challenge time points in the ANOVA. The pre- and postinhalation time points were analyzed separately. Significance level was set to 0.05.
Ethics approval and consent to participate
The study was conducted in accordance with local laws, regulations and GCP guidelines (CPMP/ICH/135/95), and the protocol was approved by the ethics committee of the Hannover Medical School (Approval No. 7193). Every subject provided written informed consent after being fully informed about the study.
Results
LPS induced inflammatory response
Tables 1 and 2 summarizes the LPS-induced inflammatory response in BAL fluid, induced sputum, and blood. The segmental LPS challenge induced a massive influx of neutrophils and monocytes into the challenged segment. Tables 1 and 2 shows the cellular data obtained by standard microscopy. A more detailed analysis including flow cytometry and Chipcytometry has recently been published13. LPS also caused an increase in the total cell count as well as in the BAL fluid concentration of albumin, IL-6, IL8, and MPO of each subject. The whole lung LPS challenge resulted in a comparable inflammatory response with an increase in the percentage of neutrophils and monocytes and the concentrations of IL-6, IL8 and MPO in the sputum of all subjects. A pronounced inflammatory response was also observed in blood both 6 and 21 h after the segmental challenge and 6 h after the whole lung challenge.
PEx characterization and reproducibility between baseline visits
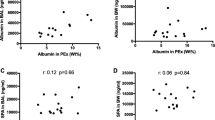
PEx were collected at screening and at two baseline visits prior to the respective LPS challenge (V1, V2, V5). We compared the baseline data of visit 2 and visit 5 to assess the reproducibility of PEx markers because subjects were unfamiliar with PEx sampling during their first visit. The numbers of PEx/L breaths and the PEx mass/L breaths showed a good correlation (r = 0.89, p = 0.001; r = 0.79, p = 0.006) between these visits, which were approximately five weeks apart. The total numbers of PEx/single breaths and the PEx mass/single breaths showed a similar result (r = 0.88, p = 0.001; r = 0.83, p = 0.003). The particle size distribution, expressed as the percentage of particles in each of the 8 size bins of the PExA particle counter, showed significant correlations (data not shown). Finally, we also detected good reproducibility for the concentration of SP-D in PEx between these baseline visits (r = 0.80, p = 0.01).
PEx inflammatory response
PEx were collected twice after segmental and once following whole lung LPS challenge (Fig. 1). Five hours after the segmental challenge, we observed an increase in particle emission, and the PEx concentration and mass concentration were significantly higher at this time point than before (p = 0.0008) and 21 h after (p = 0.03) segmental challenge. The particle size distribution was not significantly affected. Only a trend (p = 0.08) for higher numbers of smaller particles (0.55–0.70 µm) was observed, paralleled by lower numbers in bins 4–8 (0.92–4.55 µm). No significant increase in PEx concentration or PEx mass was observed after the inhalation challenge, and no shift in PEx number distribution was observed.
Despite very low concentrations of sample in the eluate from the collection membranes, we were able to detect SP-D and albumin in all samples using the MSD assay platform. Most MPO samples were below the detection limit of the available assay. This was also true for IL-6 and IL-8 with respect to most screening and baseline samples. However, a clear increase in the concentrations of IL-6 and IL-8 following segmental LPS challenge was detected. The response was even more pronounced after the inhalation challenge (Tables 1 and 2, Fig. 2).
Discussion
In this study, we were able to demonstrate that it is possible to detect an LPS-induced inflammatory response in exhaled breath particles of healthy volunteer subjects. Using this controlled experimental approach and with the availability of novel sensitive analysis assays, we were able to detect an increase in IL-6 and IL-8 in very small amounts of undiluted ELF. Our data prove the concept that PEx analysis can be applied as a noninvasive monitoring tool in respiratory medicine.
LPS challenge
In this study, we clearly showed the inflammatory response to both segmental and whole lung LPS challenge. As shown in previous studies, there was a clear increase in neutrophils and monocytes, which were detectable both in BAL fluid after segmental and in sputum after inhalation LPS challenge. The inflammatory response 24 h after segmental LPS challenge was also comparable with our previous study with respect to the five investigated biomarkers2.
In the segmental challenge, 200–400 ng LPS (depending on the subject’s weight) was applied to one lobe of the lung, and 2 µg was inhaled during the whole lung challenge. It is important to keep in mind that despite the massive inflammatory response in the one lobe, PEx are emitted and collected from the whole lung. Perhaps due to this, we observed a more pronounced increase in IL-6 and IL-8 following the inhalation challenge, which affects the entire lung and not only one lung segment. Interestingly, we observed a similar systemic response with respect to the increase in blood neutrophils following these different LPS challenges.
PEx analysis
We aimed to collect 240 ng PEx at each time point. As shown in Table S1 (see Additional file 1), this target was missed several times at visit 1 (V1), when subjects were still unfamiliar with the procedure. In one subject, this target was missed repeatedly, which resulted in 2 missing values and overall the lowest detected concentrations of albumin and SP-D. A total of 120 ng was used for the analysis of proteins and extracted according to a protocol recommended by PExA AB. The strong correlation of SP-D concentrations between the baseline visits indicates that the extraction process was efficient and apparently resulted in a reproducible yield.
The analysis of PEx collected at the baseline visits showed that physical aerosol parameters such as particle number emissions and the particle size distribution are reproducible within individuals even over a period of more than 4 weeks. This has been demonstrated in more detail before5,9. The particle number emission increased five hours after the segmental LPS challenge, potentially related to the developing edema in the challenged segment.
Due to the limited amount of available PEx, we selected five biomarkers for our analysis. We chose IL-6 and IL-8, as these were known from previous studies2,15, to show a clear detectable increase in BAL fluid. Importantly, high-sensitive assays were available for both of these cytokines. In addition, we selected MPO as a further marker for neutrophilic inflammation2,15. Albumin was included as a control because it is abundant in ELF and was previously shown to be detectable in PEx4. SP-D is part of the pulmonary surfactant system and a dominant constituent of ELF but has previously been shown to not respond significantly to LPS challenge2. We did not analyse amylase in PEx due to the limited amount of material available and because it has been shown previously that PEx do not contain amalyse16. An extensive discussion on the origin of PEx and why a contamination of PEx with larger aerosols from the upper airways is extremely unlikely has been published by Ljungkvist et al.14.
To the best of our knowledge, this is the first time that the inflammatory cytokines IL-6 and IL-8 were detected in PEx. The increase is compatible with cytokine levels obtained from the analysis of BAL fluid, induced sputum and serum. As we did not have high and low responders to the LPS challenge in our study and with the need to limit the number of subjects to 10 in this complex study design, we were not able to find significant correlations between the LPS induced changes in BAL and PEx.
Based on the theoretical origin and generation of PEx, it can be assumed that PEx represent undiluted ELF6. Bronchoalveolar lavage also collects ELF from the peripheral lung. Based on Rennard et al.17, it can be estimated that with a standard lavage procedure, the volume of ELF recovered from a healthy human is approximately 1.0 ml in 100 ml recovered BAL fluid. Based on this, we calculated and compared the approximate concentrations in PEx and BAL. With the numerous assumptions required, with data from different studies and subjects, and with biomarkers being analyzed with different assays, this can only be a rather crude comparison. Nevertheless, as shown in Table S2 (see Additional file 1), the IL-6, IL-8 and SP-D data show a plausibly similar order of magnitude. The estimated concentrations of IL-6 and IL8 appeared to be lower in PEx at the relevant time point of 5 h after segmental LPS challenge. This would be expected, as PEx are sampled from the whole lung, which leads to dilution with ELF from unaffected lung areas. In contrast, the IL-6 and IL-8 response in BAL fluid originates from the challenged lobe only with no dilution from unaffected lung areas. Interestingly, the estimated SP-D concentration is quite comparable between PEx and BAL. As SP-D is not significantly affected by LPS, the concentration in the challenged lobe is not much different from that in the rest of the lung; therefore, comparable results would make sense. For albumin, the estimated concentrations are on the same order of magnitude but much more variable and sometimes higher in PEx than in BAL, which is difficult to explain with our current knowledge about PEx.
Conclusion
In summary, this study has proven the concept that exhaled particles can be used for monitoring inflammatory changes in the lung, making PEx analysis an attractive noninvasive method for experimental lung research. With more sensitive biochemical analysis methods becoming available, the spectrum of potential analytes is constantly increasing. In addition, novel filter material and improved filter processing tools that were not available at the time of sample processing in our study are likely to further increase the yield of PEx for future applications. Due to the cost of the instrument, however, exhaled breath particle analysis will remain limited for use in general practice, but we do see a need for the noninvasive analysis of peripheral airway inflammation in clinical trials, especially for repeated evaluations where BAL analysis cannot be performed.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to data protection reasons but are available from the corresponding author on request.
Abbreviations
- BAL:
-
Bronchoalveolar lavage
- ELF:
-
Epithelial lining fluid
- FEV1 :
-
Forced expiratory volume in 1 s
- LPS:
-
Lipopolysaccharide, bacterial endotoxin
- IL6:
-
Interleukin-6
- IL8:
-
Interleukin-8
- IQR:
-
Interquartile range
- ISP:
-
Induced sputum
- LLOD:
-
Lower limit of detection
- MPO:
-
Myeloperoxidase
- MRI:
-
Magnetic resonance imaging
- MSD:
-
Meso scale discovery
- PEx:
-
Particles in exhaled air
- RIPA buffer:
-
Radioimmunoprecipitation assay buffer
- SD:
-
Standard deviation
- SP-D:
-
Surfactant protein D
- V:
-
Study visit
References
Hohlfeld, J. M. et al. Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: A randomized placebo-controlled trial. Pulm. Pharmacol. Ther. 21, 616–623. https://doi.org/10.1016/j.pupt.2008.02.002 (2008).
Holz, O. et al. Inter- and intrasubject variability of the inflammatory response to segmental endotoxin challenge in healthy volunteers. Pulm. Pharmacol. Ther. 35, 50–59. https://doi.org/10.1016/j.pupt.2015.10.011 (2015).
Janssen, O. et al. Low-dose endotoxin inhalation in healthy volunteers—A challenge model for early clinical drug development. BMC Pulm. Med. 13, 19. https://doi.org/10.1186/1471-2466-13-19 (2013).
Bake, B., Larsson, P., Ljungkvist, G., Ljungström, E. & Olin, A.-C. Exhaled particles and small airways. Respir. Res. 20, 8. https://doi.org/10.1186/s12931-019-0970-9 (2019).
Schwarz, K., Biller, H., Windt, H., Koch, W. & Hohlfeld, J. M. Characterization of exhaled particles from the healthy human lung—A systematic analysis in relation to pulmonary function variables. J. Aerosol Med. Pulm. Drug Deliv. 23, 371–379. https://doi.org/10.1089/jamp.2009.0809 (2010).
Beauchamp, J., Davis, C. & Pleil, J. Breathborne Biomarkers and the Human Volatilome 2nd edn. (Elsevier, 2020).
Morawska, L. et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 40, 256–269. https://doi.org/10.1016/j.jaerosci.2008.11.002 (2009).
Almstrand, A.-C. et al. Airway monitoring by collection and mass spectrometric analysis of exhaled particles. Anal. Chem. 81, 662–668. https://doi.org/10.1021/ac802055k (2009).
Schwarz, K., Biller, H., Windt, H., Koch, W. & Hohlfeld, J. M. Characterization of exhaled particles from the human lungs in airway obstruction. J. Aerosol Med. Pulm. Drug Deliv. 28, 52–58. https://doi.org/10.1089/jamp.2013.1104 (2015).
Horváth, I. et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. https://doi.org/10.1183/13993003.00965-2016 (2017).
Kern, A. L. et al. Noninvasive monitoring of the response of human lungs to low-dose lipopolysaccharide inhalation challenge using MRI: A feasibility study. J. Magn. Reson. Imaging. 51, 1669–1676. https://doi.org/10.1002/jmri.27000 (2020).
Holz, O. Breath Analysis Following Experimental Endotoxin Challenge in Healthy Volunteers (Breath Biopsy Conference, 2021).
Carstensen, S., Holz, O., Hohlfeld, J. M. & Müller, M. Quantitative analysis of endotoxin-induced inflammation in human lung cells by chipcytometry. Cytometry A https://doi.org/10.1002/cyto.a.24352 (2021).
Ljungkvist, G. et al. Two techniques to sample non-volatiles in breath-exemplified by methadone. J. Breath Res. 12, 16011. https://doi.org/10.1088/1752-7163/aa8b25 (2017).
O’Grady, N. P. et al. Local inflammatory responses following bronchial endotoxin instillation in humans. Am. J. Respir. Crit. Care Med. 163, 1591–1598. https://doi.org/10.1164/ajrccm.163.7.2009111 (2001).
Bredberg, A. et al. Exhaled endogenous particles contain lung proteins. Clin. Chem. 58, 431–440. https://doi.org/10.1373/clinchem.2011.169235 (2012).
Rennard, S. I. et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 1986(60), 532–538. https://doi.org/10.1152/jappl.1986.60.2.532 (1985).
Acknowledgements
Dr. A Suffredini, NIH Clinical Center, kindly provided Clinical Center Reference Endotoxin. The study was supported by Dr. J. Östling, Manager, Research and Biochemical Analysis at PExA as a consultant for the analysis of PEx samples. We also like to thank the Team of the Fraunhofer ITEM Clinical Airway Research for conducting the study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the German Center for Lung Research (DZL).
Author information
Authors and Affiliations
Contributions
J.M.H., O.H., M.M. designed the study. M.M. and S.C. were responsible for the biomarker measurements, and S.C. acquired and analyzed the cell data. A.C.O. provided the PExA instrument and was a consultant for PEx sampling and analysis. O.H. performed the data analysis and, together with J.M.H., outlined the manuscript. S.C., M.M., and A.C.O. participated in the manuscript preparation and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
OH, MM, SC and JMH have no competing interest. ACO is a chairholder of PExA AB.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holz, O., Müller, M., Carstensen, S. et al. Inflammatory cytokines can be monitored in exhaled breath particles following segmental and inhalation endotoxin challenge in healthy volunteers. Sci Rep 12, 5620 (2022). https://doi.org/10.1038/s41598-022-09399-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09399-z
- Springer Nature Limited
This article is cited by
-
Exhaled breath particles as a novel tool to study lipid composition of epithelial lining fluid from the distal lung
BMC Pulmonary Medicine (2023)