Abstract
Ubiquitous microthromboses in the pulmonary vasculature play a crucial role in the pathogenesis of COVID-19 associated acute respiratory distress syndrome (ARDS). Excess of Willebrand factor (vWf) with intravascular multimer formation was identified as a key driver of this finding. Plasma exchange (PLEX) might be a therapeutic option to restore the disbalance between vWf and ADAMTS13. We report the effects of PLEX on vWf, ADAMTS13, inflammatory cytokines and parameters of ventilation. We investigated 25 patients, who were on mechanical ventilation for COVID-19 pneumonia with ARDS at two German university hospitals. All patients received PLEX as an ultima ratio measure for refractory ARDS. VWf antigen (vWf:Ag), ADAMTS13 activity, a cytokine panel mirroring the inflammatory situation and clinical parameters were assessed before and after three to six PLEX therapies with fresh frozen plasma. Before the PLEX sequence there was an excessive release of vWf:Ag (425.4 ± 167.5%) and mildly reduced ADAMTS13 activity (49.7 ± 23.3%). After the PLEX series, there was a significant increase of ADAMTS13 activity to 62.4 ± 17.7% (p = 0.029) and a significant decrease of vWf:Ag to 336.1 ± 138.2% (p = 0.041) resulting in a 63% improvement of the ADAMT13/vWf:Ag ratio from 14.5 ± 10.0 to 23.7 ± 14.6, p = 0.024. Comparison of parameters before and after individual PLEX sessions (n = 35) revealed a mean reduction of vWf from 387.8 ± 165.1 to 213.2 ± 62.3% (p = 0.001) and an increase of ADAMTS13 activity from 60.4 ± 20.1 to 70.5 ± 14.0% (p = 0.001). Parallelly, monocyte chemotactic protein-1 and interleukin-18 decreased significantly (p = 0.034 each). Along the PLEX sequence lactate dehydrogenase (p = 0.001), C-reactive protein (p = 0.001), and positive end expiratory pressure (p = 0.01) significantly decreased accompanied by an improvement of Horovitz index (p = 0.001). PLEX restores the disbalance between ADAMTS13 and vWf:Ag, a driver of immunothrombosis. Moreover, it reduces the inflammatory state and is associated with a benefit of ventilation parameters. These findings render a further rationale to regard PLEX as a therapeutic option in severe COVID-19.
Similar content being viewed by others
Introduction
Immunothrombosis is a key feature of Coronavirus disease 2019 (COVID-19) affecting both macro- and microvasculature1. Regarding the macrovasculature, COVID-19 patients suffer from arterial and venous thrombembolic events including stroke, limb ischemia, myocardial infarction, deep vein thromboses, and pulmonary embolism2,3,4. Autopsy studies identified pulmonary microvascular thromboses as a major reason for ventilation-perfusion imbalance leading to acute respiratory distress syndrome (ARDS)5,6.
A disbalance of von Willebrand factor (vWf) and ADAMTS13 was identified as a main driver of immunothrombosis in COVID-197,8. COVID-19 is associated with ubiquitous endothelial damage leading to excessive release of vWf. ADAMTS13 is a protease that usually cleaves the large string-like molecules of vWf and is necessary to avoid accumulation of vWf multimers in the blood stream. In COVID-19, however, the excessive release of vWf exceeds the capacity of the protease leading to vWf multimers, that act as a matrix for platelet aggregation and thereby cause microvascular thromboses9. This mechanism resembles the situation in thrombotic thrombocytopenic purpura (TTP), in which a deficiency of ADAMTS13 causes microangiopathic thromboses. In COVID-19 the ADAMTS13/vWf ratio decreases with the severity of the disease and is a strong and independent predictor of mortality9.
The aggregation of platelets at vWf multimers cannot be inhibited by plasmatic anticoagulation, which might explain, why autopsy findings revealed extensive microthromboses in the lungs of COVID-19 patients despite prior plasmatic anticoagulation. Therefore, there is an urgent clinical need for novel preventive and therapeutic strategies to address immunothrombosis in COVID-19. In analogy to TTP, plasma exchange (PLEX) may be considered as a therapeutic option in this context, since it removes vWf and restores ADAMTS13. There are some first preliminary reports on PLEX in COVID-19 intended as a rescue therapy to eliminate proinflammatory cytokines with promising results10,11,12,13,14. These studies did not focus, however, on immunothrombosis.
In the present work we present experiences of two German university hospitals, that made use of PLEX as an ultima ratio therapy in COVID-19 associated ARDS. VWf, ADAMTS13, a cytokine panel, and parameters of mechanical ventilation (positive endexpiratory pressure [PEEP] and Horovitz index (partial pressure of oxygen [pO2]/fraction of inspired oxygen [FiO2]) were assessed before and after three to six sessions of PLEX.
Results
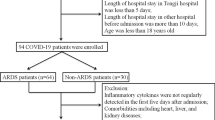
We enrolled 25 patients with SARS-CoV-2 infection and severe COVID-19 pneumonia with invasive and non-invasive mechanically ventilated ARDS, who underwent three to six sessions of PLEX with human plasma (median n = 5, overall n = 112). Mean age of the COVID-19 population was 67.0 ± 11.9 years. Gender distribution was homogeneous with n = 11 being female (44%). Characteristics of patients are summarized in Table 1. Eight (32%) had severe and 14 (56%) moderate ARDS. 14 (56%) patients died from COVID-19. Mean time of hospitalization was 40.8 ± 24.3 days. 21 patients (84%) were treated with dexamethasone, remdesivir was initiated in 10 (40%) cases (Table 1). Dexamethasone was permanently discontinued before the enrolment in our study in 16% of our patients due to a prior superinfection.
Evaluation of parameters before and after the complete PLEX sequence
ADAMTS13 activity was 49.7 ± 23.3% before the PLEX sequence and increased towards 62.4 ± 17.7% (p = 0.029). Parallelly, vWf:Ag decreased from 425.4 ± 167.5 to 336.1 ± 138.2% (p = 0.041). The ADAMTS13/vWf:Ag ratio improved significantly by 63.4% from 14.5 ± 10.0 to 23.7 ± 14.6 (p = 0.024) after completion of the PLEX sequence (Fig. 1, Suppl. Fig 1).
ADAMTS13 and von Willebrand factor (vWf) hemostasis and ventilation parameters before and after a sequence of three to six PLEX sessions. (A) ADAMTS13, (B) PEEP, (C) vWf:Ag, (D) Pinsp, and (E) ADAMTS13/vWf:Ag, and (F) the Horovitz index. Data are analysed by a paired two-tailed t-test. ***p = 0.001, **p < 0.01 and *p < 0.05 were regarded significant. PEEP positive endexpiratory pressure, Pinsp positive inspiratory pressure, vWf:Ag von Willebrand factor antigen.
As illustrated in Fig. 1, PEEP was significantly reduced by 26.5% after the PLEX series (11.7 ± 3.2 vs. 8.6 ± 4.7 mmHg, p = 0.009), while Pinsp tended to decrease (22.9 ± 4.6 vs. 18.1 ± 10.7 mmHg, p = 0.056). The Horovitz index ameliorated significantly by 41.0% from 135.3 ± 54.4 mmHg to 190.8 ± 95.4 mmHg (p = 0.001). We could not observe any significant differences in the need for vasopressors (p = 0.091).
After completion of the PLEX series, lactate dehydrogenase was reduced by 48.3% (608.5 ± 284.7 vs. 314.8 ± 94.6 IU/l, p = 0.001). CRP decreased by 75.2% after the PLEX sequence (16.9 ± 10.8 vs. 4.2 ± 4.7 mg/dl, p = 0.001). Table 2 presents the coagulation parameters of the study population before and after PLEX.
Evaluation of parameters before and after the individual PLEX sessions
Measurements of vWf:Ag and ADAMTS13 were conducted successfully right before and after 35 PLEX sessions (11 patients), as presented in Fig. 2. A PLEX session was associated with a mean reduction by 45.0% of vWf:Ag concentrations, from 387.8 ± 165.1 to 213.2 ± 62.3% (p = 0.001), while ADAMTS13 activity significantly increased by 16.7%, from 60.4 ± 20.1 to 70.5 ± 14.0%, p = 0.001). The ADAMTS13/vWf:Ag ratio was substantially lower before PLEX treatment (19.6 ± 12.5) and showed a significant increase to 36.3 ± 14.1 (p = 0.001; Fig. 2, Table 2). PLEX was associated with significant reductions of two cytokines of the panel described above: monocyte chemotactic protein-1 and interleukin-18 (p = 0.034 each; Fig. 3).
Individual mean concentrations of (A) monocyte chemotactic protein-1 (MCP-1) and (B) interleukin-18 (IL-18) before and after a singular PLEX session, each point representing one patient. Data before and after treatment are analysed by paired t-test. ***p = 0.001, **p < 0.01 and *p < 0.05 were regarded significant.
Discussion
The present retrospective analysis shows, that PLEX is able to reduce the excess of vWf, to increase ADAMTS13 activity, to readjust the ADAMTS13/vWf:Ag ratio and thereby to reduce the risk of immunothrombosis in COVID-19. These effects were accompanied by a reduction of proinflammatory cytokines and an improvement in parameters of mechanical ventilation. The restoration of vWf and ADAMTS13 hemostasis constitutes a novel rationale to regard PLEX as a therapeutic option in SARS-CoV-2 induced ARDS beyond the reduction of the inflammatory milieu. In both centers, PLEX was used as an ultima ratio therapy. Future studies will have to define the optimal timepoint for initiation of PLEX. Randomized trials are underway.
In our previous study, we identified TTP-like vWf multimer patterns as a driver of immunothrombosis in COVID-199. ADAMTS13 is unable to sufficiently abolish the excessive vWf multimer formation. The endothelium plays a crucial role in hemostasis, regulating not only oxidative stress, and permeability but complement activation and pro- and anticoagulant factors as well. Severe COVID-19 is associated with ubiquitous endotheliitis, triggered by the primary SARS-CoV 2 infection and secondarily via a microvascular inflammatory process15,16. Moreover, an in vitro study demonstrated that SARS-CoV-2 spike protein can activate the alternative complement pathway5,17. The disbalance between ADAMTS13 and vWf represents a further crucial pathomechanism of COVID-19 associated immunothrombosis.
PLEX constitutes a central therapeutic measure in the management of TTP. The goal is not only to remove ADAMTS13 inhibitors, but also to restore the patients’ serum with a sufficient amount of ADAMTS13. In COVID-19, one major effect is the removal of vWf. Noteworthy, however, there is first evidence on a generation of ADAMTS-13 autoantibodies as well18. Thus, PLEX restores vWf and ADAMTS13 hemostasis by means of both removal of vWf, delivery of ADAMTS13, and—potentially—elimination of autoantibodies to ADAMTS-13. These mechanisms likely reduce SARS-CoV-2 associated microthrombosis and improve organ perfusion.
With regard to individuals refusing vaccination and those with vaccination breakthrough, the ongoing pandemic constitutes an urgent need for either new therapeutic approaches or those that are already approved for other indications. In the latter context, PLEX was used in order to attenuate circulating cytokines and inflammatory mediators in critically ill patients with COVID-19 as described by small case series with promising results14,19. In these reports PLEX was associated with higher extubation rates, and lower 14 days and 28 days all-cause mortality20. With regard to the restoration of the physiological vWf/ADAMTS13 balance, our analysis identifies an additional novel explanation for these clinical benefits. The excess of vWf was immediately dampened even by a single session of PLEX. Within the next days, vWf tended to increase again, thus making a sequence of sessions with intervals of 1 or 2 days reasonable. Accordingly, vWf was lower and ADAMTS13 higher at the end of the sequence compared to baseline values. Our cytokine analysis confirms the antiinflammatory potential of PLEX as demonstrated in previous studies14,21. PLEX reduced MCP-1 and IL-18 concentrations in the individual sessions. A decrease of thrombocytes cannot clearly be explained. However, platelet consumption is regularly seen on patients undergoing extracorporeal blood treatment and/or treated on the intensive care unit22. Due to the amelioration of the thrombotic microangiopathy parameters along the PLEX sequence, this reduction in platelet count cannot be explained through a relative ADAMTS13 deficiency.
Respiratory and ventilatory insufficiency in SARS-CoV-2 associated ARDS results from two major causes: First, alveolar inflammation and second, microthromboses in the pulmonary circulation. Whereas current treatment strategies like dexamethasone or tocilizumab are intended to address excessive inflammation, there is no specific therapeutic option to reduce immunothrombosis so far. The use of plasmatic anticoagulants in therapeutic doses resulted in unacceptable rates of bleeding events and is not recommended for COVID-19 necessitating intensive care medicine as seen in trials like REMAP-CAP, ATTACC and ACTIV-423. PLEX might be a helpful therapeutic adjunct in this context. The extensive microvascular thromboses in the lungs of deceased COVID-19 patients—despite prior low dose or therapeutic anticoagulation—illustrate the need for new therapies to regenerate diffusion capacity and oxygenation. In line with this hypothesis the ventilatory situation was improved in our population as reflected by PEEP and Horovitz index.
In the present study, PLEX served as a rescue therapy. Different kinds of immunomodulatory drug based therapies (e.g. JAK-inhibition) showed favourable effects, but still none of them are seen as an evidence-based ultima ratio therapy for COVID-19. Furthermore, inhaled nitric oxide could provide immediate help and a delay in respiratory deterioration in COVID-19-induced moderate to severe ARDS. However the data appears conflicting, and reports only show a temporary amelioration24,25. Lung transplantation constitutes an ultimate rescue therapy of COVID-19 induced lung injury. Obviously, only a few carefully selected patients will be suitable for such therapy. In a letter to the Editor, the authors report a similar survival rate compared to lung transplant recipients, suffering from a different underlying pulmonary disease26. The long term outcome, especially with regard to an ongoing pandemic and immunosuppression, remains elusive, but lung transplantation for COVID-19 will not be suitable on a large scale.
The present analysis is limited by its sample size and its retrospective character. PLEX was used in Bochum as an ultima ratio therapy, when there were no other evidence-based ultima ratio therapies available. Due to the continuously evolving data on the management of COVID-19, e.g. concerning anticoagulation and the use of immunosuppressants like tocilizumab, it was not possible to define a sufficiently matching control group. Data about the course of the investigated parameters before and after the individual PLEX sessions, however, allow a precise determination of the PLEX-induced effects.
In conclusion, the present analysis identifies restoration of the SARS-CoV-2 associated physiological balance of vWf and ADAMTS13 as a new rationale to consider PLEX as a therapeutic option in COVID-19. Moreover, it confirms previous findings on a reduction of inflammation and an improvement of ventilation parameters.
Methods
Patients
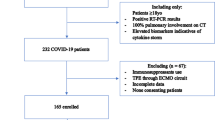
We analysed 25 patients from two university hospital intensive care units, who were tested positive for SARS-CoV-2 by real-time polymerase chain reaction (RT-PCR) analysis in respiratory tract specimen (nasopharyngeal swab test or bronchoalveolar lavage). Patients were recruited at the Ruhr-University Bochum (n = 19) and the Ruprecht-Karls University of Heidelberg (n = 6) in Germany. All patients suffered from a critical course of disease, as categorized by the Robert Koch Institute, including invasive and non-invasive mechanical ventilation and fulfilling parameters for the diagnosis of an acute respiratory distress syndrome27. Changes in vWf, ADAMTS13, cytokines, and key parameters of ventilation were defined as clinical endpoints. Retrieval of blood samples was performed as part of an investigation of immunological, inflammatory, and hemostaseological parameters in COVID-19 at Ruhr-University Bochum and in the context of a prospective register study at Heidelberg University Hospital. Informed consent was obtained from all subjects or their legal guardian. Both investigations were approved by the respective ethical committee of Ruhr-University Bochum (20-6886) and Ruprecht-Karls University Heidelberg (S-148/2020).
Clinical and biochemical parameters
PEEP, inspiratory pressure (Pinsp), and Horovitz index (partial pressure of oxygen [pO2]/fraction of inspired oxygen [FiO2]) were defined as key ventilatory parameters. Additionally, the need of vasopressors (noradrenaline) was documented. VWf, ADAMTS13, lactate dehydrogenase and platelets were assessed as markers of thrombotic microangiopathy. C-reactive protein and a cytokine panel were used as markers of inflammation: The LEGENDplex Human Inflammation Panel 1 (13-plex) (BioLegend, CA, USA) containing interleukin (IL)-1β, interferon (IFN)-α2, IFN-γ, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1 (CCL2), IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33 was used according to the manufacturer’s instruction. The concentration of the analytes was calculated using the LEGENDplex Cloud-based Data Analysis Software v.2021.07.01.
Plasma exchange (PLEX)
In both centers PLEX was clinically indicated as an ultima ratio therapy of COVID-19 associated ARDS with a Horovitz Quotient ≤ 300 mmHg. Patients were checked for remediable conditions like volume overload or superinfection. Thus, volume status was assessed clinically and via ultrasound and was excluded before therapy induction. The PLEX regimen consisted of three to six sessions with a turnover of one individualised plasma volume. In case of acute clinical amelioration during treatment (clear increase of Horovitz index), or for restricted capacity reasons (e.g. shortage of staff during critical phases of the pandemic), this kind of treatment was individually reconsidered and eventually discontinued. PLEX were performed on the Octo Nova (Diamed, Köln, Germany) with heparin as anticoagulation. Plasma separation was provided via Plasma Flux P2 Dry (Fresenius Medical Care, Germany) or using a Comtech centrifuge (Fresenius Medical Care AG & Co. KGaA, Bad Homburg, Germany). PLEX sessions had a maximum 48-h interval. In addition to retrieval of blood samples before and after the complete PLEX sequence (overall population), blood samples were obtained before and after individual PLEX sessions in a subset of 11 patients.
Measurement of ADAMTS13 activity and vWf antigen
VWf:Ag and ADAMTS13 activity were analyzed in all patients prior to the first PLEX and after a sequence of three to six PLEX, noteworthy the second specimen was obtained 24 h after the end of the PLEX series. Additionally, blood samples of 11 patients were obtained immediately prior and after a total of 35 individual PLEX sessions, allowing to assess an extinction rate of vWf:Ag and a restoration rate of ADAMTS13. ADAMTS13 activity (%) was analyzed from citrate-plasma using Technozym ADAMTS13 ELISA (Technoclone, Vienna, Austria)28. vWf:Ag (%) was measured using a sandwich ELISA with polyclonal antibodies29.
Statistics
Data were checked for Gaussian distribution by D’Agostino Pearson test and are presented as mean ± standard deviation. Changes in ADAMTS13 activity, vWf:Ag and ADAMTS13/vWf:Ag ratios right before and after PLEX were investigated by paired two-tailed t-test. Moreover, a paired two-tailed t-test was used to analyze the evolvement of clinical parameters (PEEP, Pinsp, Horovitz index, noradrenaline, LDH, CRP, lactate, thrombocytes, cytokines) as well as ADAMTS13 activity, vWf:Ag and ADAMTS13/vWf:Ag ratios before and after the complete sequence of PLEX. p < 0.05 was regarded significant. Statistical analyses were performed using Prism 9 (Graph Pad, San Diego, USA).
Ethical approval
Both investigations were approved by the respective ethical committee of Ruhr-University Bochum (20-6886) and Ruprecht-Karls University Heidelberg (S-148/2020) and performed in accordance with the relevant guidelines and regulations.
References
Bonaventura, A. et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329. https://doi.org/10.1038/s41577-021-00536-9 (2021).
Klok, F. A. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 191, 145–147. https://doi.org/10.1016/j.thromres.2020.04.013 (2020).
Oxley, T. J. et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N. Engl. J. Med. 382, e60. https://doi.org/10.1056/NEJMc2009787 (2020).
Bellosta, R. et al. Acute limb ischemia in patients with COVID-19 pneumonia. J. Vasc. Surg. https://doi.org/10.1016/j.jvs.2020.04.483 (2020).
Magro, C. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 220, 1–13. https://doi.org/10.1016/j.trsl.2020.04.007 (2020).
Connors, J. M. & Levy, J. H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. https://doi.org/10.1111/jth.14849 (2020).
Airoldi, A. et al. COVID-19-related thrombotic microangiopathy in a cirrhotic patient. Dig. Liver Dis. https://doi.org/10.1016/j.dld.2020.06.019 (2020).
Jhaveri, K. D. et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. https://doi.org/10.1016/j.kint.2020.05.025 (2020).
Doevelaar, A. A. N. et al. von Willebrand factor multimer formation contributes to immunothrombosis in coronavirus disease 2019. Crit. Care Med. 49, e512–e520. https://doi.org/10.1097/CCM.0000000000004918 (2021).
Kamran, S. M. et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome: A retrospective propensity matched control study. PLoS ONE 16, e0244853. https://doi.org/10.1371/journal.pone.0244853 (2021).
Patidar, G. K. et al. Understanding the role of therapeutic plasma exchange in COVID-19: Preliminary guidance and practices. Vox Sang. https://doi.org/10.1111/vox.13067 (2021).
Khamis, F. et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. 99, 214–218. https://doi.org/10.1016/j.ijid.2020.06.064 (2020).
Tabibi, S., Tabibi, T., Conic, R. R. Z., Banisaeed, N. & Streiff, M. B. Therapeutic plasma exchange: A potential management strategy for critically ill COVID-19 patients. J. Intens. Care Med. 35, 827–835. https://doi.org/10.1177/0885066620940259 (2020).
Morath, C. et al. Plasma exchange in critically ill COVID-19 patients. Crit. Care 24, 481. https://doi.org/10.1186/s13054-020-03171-3 (2020).
Varga, Z. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5 (2020).
Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 383, 120–128. https://doi.org/10.1056/NEJMoa2015432 (2020).
Yu, J. et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 136, 2080–2089. https://doi.org/10.1182/blood.2020008248 (2020).
Doevelaar, A. A. N., Bachmann, M., Holzer, B. & Seibert, F. Generation of inhibitory autoantibodies to ADAMTS13 in coronavirus disease 2019. MedRxiv. https://doi.org/10.1101/2021.03.18.21253869 (2021).
Fernandez, J. P. et al. Plasma exchange: An effective rescue therapy in critically ill patients with coronavirus disease 2019 infection. Crit. Care Med. https://doi.org/10.1097/CCM.0000000000004613 (2020).
Khamis, F. et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. https://doi.org/10.1016/j.ijid.2020.06.064 (2020).
Fernandez, J. et al. Plasma exchange: An effective rescue therapy in critically ill patients with coronavirus disease 2019 infection. Crit. Care Med. 48, e1350–e1355. https://doi.org/10.1097/CCM.0000000000004613 (2020).
Vincent, J. L. et al. Thrombocytopenia in the ICU: Disseminated intravascular coagulation and thrombotic microangiopathies-what intensivists need to know. Crit. Care 22, 158. https://doi.org/10.1186/s13054-018-2073-2 (2018).
Investigators, R.-C. et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N. Engl. J. Med. 385, 777–789. https://doi.org/10.1056/NEJMoa2103417 (2021).
Tavazzi, G. et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit. Care 24, 508. https://doi.org/10.1186/s13054-020-03222-9 (2020).
Ferrari, M. et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J. Crit. Care 60, 159–160. https://doi.org/10.1016/j.jcrc.2020.08.007 (2020).
Roach, A. et al. Lung transplantation for COVID-19-related respiratory failure in the United States. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2117024 (2022).
Force, A. D. T. et al. Acute respiratory distress syndrome: The Berlin definition. JAMA 307, 2526–2533. https://doi.org/10.1001/jama.2012.5669 (2012).
Miyata, T., Kokame, K. & Banno, F. Measurement of ADAMTS13 activity and inhibitors. Curr. Opin. Hematol. 12, 384–389. https://doi.org/10.1097/01.moh.0000169286.74464.3a (2005).
Cejka, J. Enzyme immunoassay for factor VIII-related antigen. Clin. Chem. 28, 1356–1358 (1982).
Funding
Open Access funding enabled and organized by Projekt DEAL. This is a self-funded research. All authors received no financial support for the research, authorship or publication of this article.
Author information
Authors and Affiliations
Contributions
F.S.S., B.H., T.H.W. designed the study, supervised the management, analysed and interpreted the data and wrote the manuscript. A.B., N.B., R.D., S.S., J.W., U.B. performed cytokine and hemostasiologic measurements. A.A.D., P.Z., C.N., U.M., C.M. were responsible for sample and data collection. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seibert, F.S., Blazquez-Navarro, A., Hölzer, B. et al. Effect of plasma exchange on COVID-19 associated excess of von Willebrand factor and inflammation in critically ill patients. Sci Rep 12, 4801 (2022). https://doi.org/10.1038/s41598-022-08853-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08853-2
- Springer Nature Limited
This article is cited by
-
Generation of potentially inhibitory autoantibodies to ADAMTS13 in coronavirus disease 2019
Scientific Reports (2023)