Abstract
Bacterial vaginosis (BV) is the most common vaginal infection in reproductive women, which is characterized by depleted level of lactic acid bacteria and overgrowth of anaerobes such as Gardnerella vaginalis spp. Lactic acid bacteria have been known to be beneficial for amelioration of BV, since they produce antimicrobial substances against G. vaginalis spp. The objectives of this study were to characterize different fractions of cell-free supernatant of Lactobacillus paracasei CH88 (LCFS) and investigate antibacterial activity of the LCFS fractions against G. vaginalis in-vitro and in-vivo. Antibacterial activity of the LCFS was stable during thermal treatment up to 120 °C for 30 min and maintained at pH ranging from 3.0 to 13.0 except pH 5.0. Fraction below 3 kDa of the LCFS partially lost its antibacterial activity after treatment with proteolytic enzymes. Precipitated protein fraction below 3 kDa of the LCFS (< 3 kDa LCFSP) inhibited the growth and biofilm formation of G. vaginalis. Treatment of L. paracasei CH88 or the < 3 kDa LCFSP attenuated G. vaginalis-induced BV in mice by inhibiting the growth of G. vaginalis, reducing exfoliation of vaginal epithelial cells, and regulating immune response. These results suggest that L. paracasei CH88 may have potential in ameliorating G. vaginalis-induced BV.
Similar content being viewed by others
Introduction
Bacterial vaginosis (BV) is the most prevalent vaginal infection in women of reproductive age1. Over 3 million women undergo BV annually in the United States2. BV is linked with increased risk of preterm birth, miscarriage, acquisition of human immunodeficiency virus, and sexually transmitted infections3,4,5,6. BV is commonly characterized by reduced level of lactobacilli, elevated vaginal pH, vaginal discharge, vaginal fluid fish odor, and overgrowth of pathogenic anaerobic bacteria such as Gardnerella vaginalis spp., Prevotella bivia, and Fannyhessea vaginae7.
G. vaginalis spp. are facultative anaerobes detected in up to 95% of BV cases8. G. vaginalis spp. have been regarded as one of the bacterial consortiums inducing BV that was best-studied species among the pathogenic anaerobic bacteria9,10. These bacteria adhere to vaginal epithelial cells and make an environment favorable for growth of other pathogenic anaerobes forming biofilm, leading to an induction of cytotoxicity on vaginal epithelial cells11. However, despite over 60 years of research, a definite etiology of BV has not been fully elucidated12.
The administration of antibiotics such as clindamycin and metronidazole has been recommended for treatment of BV6, since it showed 75–86% cure rates13. However, the use of antibiotics has limitations due to their side effects. About 52% of the patients with BV who were orally treated with metronidazole were associated with gastrointestinal complaints14. About 80% of the women with BV who were administered with clindamycin were reported to be exposed to pathogenic anaerobes which are resistant to this antibiotic15. Moreover, about 60% of women experienced recurrence of BV after 12 months of metronidazole treatment16.
To avoid the side effects of antibiotic treatments, there has been an increasing interest in administration of probiotic bacteria, instead of antibiotics, which can alleviate BV. Reid et al. demonstrated that vaginal microbial microbiota in the patients with BV were reinstated after oral administration of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14, which did not cause any adverse effect17. Moreover, oral administration of L. rhamnosus GR-1 and L. fermentum RC-14 caused a reduction in the levels of pathogenic bacteria and yeasts in vagina18. Intravaginal administration of L. rhamnosus GR-1 and L. fermentum RC-14 to the patients with BV suppressed recurrence of BV19. Intravaginal administration of L. crispatus CTV-05 to the patients with BV resulted in this bacterium colonizing the vagina, consequently restoring healthy vaginal microbiota in the patients20.
Although some reproductive women maintain a healthy vaginal environment without lactobacilli21, lactobacilli are still considered as the most common bacteria in vaginal tract of healthy reproductive-age women and play an important role in maintaining healthy vaginal mucosa22. Lactobacilli have been known to have protective effects against BV via production of H2O2 and lactic acid23,24,25. Moreover, they can produce antimicrobial compounds that inhibit growth of pathogens in the urogenital ecosystem26. Bacteriocin produced by L. acidophilus 160 had inhibitory activity against G. vaginalis27.
We found that cell-free supernatant of L. paracasei CH88 possessed antibacterial activity against G. vaginalis. Moreover, cell-free supernatant of L. paracasei CH88 that was adjusted to pH 7.0 (LCFS) also showed the antibacterial activity against G. vaginalis.
Thus, we hypothesized that the antibacterial activity of the LCFS (pH 7.0) may be attributed to antibacterial compounds other than lactic acid. The aim of this study was to characterize and fractionate the LCFS and to determine antibacterial effects of the LCFS fraction against G. vaginalis in-vitro and ameliorative effect on G. vaginalis-induced BV in mice.
Materials and methods
Bacterial strains and culture conditions
G. vaginalis KCTC 5097 was purchased from Korean Collection for Type Cultures (Jeongeup, Korea). G. vaginalis was inoculated at 1% (v/v) in brain heart infusion (BHI; Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA) broth supplemented with 1% (w/v) yeast extract (BD), 0.1% (w/v) maltose (Sigma-Aldrich Co., St. Louis, MO, USA), 0.1% (w/v) glucose (Sigma-Aldrich Co.), and 10% (v/v) horse serum (Thermo Fisher Scientific, Waltham, MA, USA), named as BHIS broth. G. vaginalis was cultured at 37 °C with 5% CO2 for 18 h and stored in 50% (v/v) glycerol at − 80 °C until further use.
L. paracasei CH88 was cultured in BIFIDO Co., Ltd. (Hongcheon, Korea). Briefly, L. paracasei CH88 was inoculated at 10% (v/v) in de Man, Rogosa, Sharpe (MRS; BD) broth in a fermentor (LiFlus GX, Hanil Science Co., Ltd., Daejeon, Korea) and was cultured under anaerobic condition (85% N2, 10% H2, and 5% CO2). The culture was kept at pH 5.5 and 37 °C with 150 rpm agitation for 15 h.
Preparation of the LCFS
Culture broth of L. paracasei CH88 was centrifuged at 10,000 × g for 30 min at 4 °C. After centrifugation, the supernatant was adjusted to pH 7.0 with 5 M NaOH and filtered using a sterilized bottle-top vacuum filter (0.22 μm; Merck, Darmstadt, Germany). The filtrate (LCFS) was stored at − 80 °C for further experiments. Prior to every experiment, we confirmed that no colony was observed when the LCFS was spread and incubated on MRS agar plate at 37 °C for 24 h.
Characterization of the LCFS
Thermal treatment and pH adjustment of the LCFS
To determine thermal stability on antibacterial activity of the LCFS against G. vaginalis, G. vaginalis was inoculated at 1% (v/v) in a 96-well plate with the BHIS broth containing 5% (v/v) LCFS heated for 30 min at 60, 80, 100, or 120 °C. G. vaginalis was inoculated in the BHIS broth containing 5% (v/v) MRS broth as control. The plate was incubated at 37 °C for 24 h and its optical density (OD) was measured at 600 nm using a microplate reader (SpectraMax 190, Molecular Devices, San Jose, CA, USA) under anaerobic condition (85% N2, 10% H2, and 5% CO2).
To evaluate the effect of pH on antibacterial activity of the LCFS against G. vaginalis, pH of the LCFS was adjusted to 3.0, 5.0, 7.0, 9.0, 11.0, or 13.0 with 5 M NaOH or 5 M HCl. G. vaginalis was inoculated at 1% (v/v) in a 96-well plate with the BHIS broth containing 5% (v/v) LCFS with different pH. Antibacterial activity of each sample was examined as described above.
Size-exclusion filtration of the LCFS
To determine molecular weight of the active fraction, Amicon Ultra-15 Centrifugal Filter (Merck) with molecular weight cut-offs (MWCO) of 3, 10, 30, 50, and 100 kDa was used. Fractions of over 100 kDa, 50–100 kDa, 30–50 kDa, 10–30 kDa, 3–10 kDa, and below 3 kDa were obtained according to the manufacturer’s instruction. G. vaginalis was inoculated at 1% (v/v) in a 96-well plate with the BHIS broth added with 5% (v/v) LCFS fractions with different molecular weights. The plate was incubated at 37 °C for 24 h and its OD was measured at 600 nm under anaerobic condition (85% N2, 10% H2, and 5% CO2). The fraction below 3 kDa of the LCFS (< 3 kDa LCFS), which had antibacterial activity, was used for further study.
Proteolytic enzyme treatment on the < 3 kDa LCFS
Proteolytic enzymes were treated on the < 3 kDa LCFS, according to the method of Lee et al. and Mun et al.27,28. Proteinase K (MGmed, Seoul, Korea) in 5 mM ethylenediaminetetraacetic acid containing 0.5% (w/v) sodium dodecyl sulfate solution, protease (EC 3.4.21.62, type VIII, Sigma-Aldrich Co.) in 50 mM hydroxymethylaminomethane-hydrochloric acid (Tris–HCl) buffer (pH 7.5), trypsin (EC 3.4.21.4, type II, Sigma-Aldrich Co.) in sterilized distilled water (pH 7.0), pepsin (EC 3.4.23.1, Sigma-Aldrich Co.) in 50 mM citrate buffer (pH 2.0), and α-chymotrypsin (EC 3.4.21.1, Sigma-Aldrich Co.) in 50 mM Tris–HCl/10 mM CaCl2 buffer (pH 7.5) were used. All enzyme reactions were performed in the < 3 kDa LCFS at a final enzyme concentration of 2 mg/mL. Proteinase K was incubated at 55 °C for 8 h and inactivated at 95 °C for 30 min. α-Chymotrypsin was activated at 25 °C for 6 h. The other enzymes were incubated at 37 °C for 6 h. G. vaginalis was inoculated in a 96-well plate with the BHIS broth added with 5% (v/v) < 3 kDa LCFS treated with different proteolytic enzymes. G. vaginalis was inoculated at 1% (v/v) in the BHIS broth containing 5% (v/v) fraction below 3 kDa of MRS broth as control. The plate was incubated at 37 °C for 24 h and its OD was measured at 600 nm under anaerobic condition (85% N2, 10% H2, and 5% CO2).
Protein precipitation and plate count
The LCFS was mixed with ammonium sulfate (70%, (w/v); Samchun Pure Chemicals, Pyeongtaek, Korea), followed by stirring the mixture for 12 h at 4 °C. The mixture was centrifuged at 10,000 × g at 4 °C for 1 h. Precipitated proteins were suspended with phosphate buffered saline (PBS) and then dialyzed for 48 h using Pur-A-Lyzer Mega Dialysis Kit (Sigma-Aldrich Co.) with a MWCO of 1 kDa in distilled water at 4 °C. Collected proteins were filtered using a 3 kDa MWCO Amicon Ultra Centrifugal Filter to obtain protein fraction over 3 kDa of the LCFS (> 3 kDa LCFSP) and protein fraction below 3 kDa of the LCFS (< 3 kDa LCFSP). The fractionated protein fractions were lyophilized and suspended with PBS at a concentration of 1 mg/mL. G. vaginalis (1.1 × 106 colony forming unit (CFU)/mL) was inoculated in a 24-well plate with the BHIS broth, in which each 200 μL protein fraction was added. G. vaginalis was inoculated in the BHIS broth, in which 200 μL precipitated protein fraction (1 mg/mL) of MRS broth was added as control. The plate was incubated for 24 h at 37 °C under anaerobic condition (85% N2, 10% H2, and 5% CO2). After incubation, the suspension was spread on tryptic soy broth (TSB; BD) supplemented with 1.5% (w/v) bacto agar (BD), 5% (v/v) horse blood (Synergy Innovation Co., Ltd., Seongnam, Korea), 0.02% (v/v) menadione solution (BDH Chemicals Ltd., Poole, UK), and 1% (v/v) hemin solution (Sigma-Aldrich Co.). The agar plate was cultured for 48 h at 37 °C with 5% CO2 and colonies of G. vaginalis were counted.
Evaluation of G. vaginalis biofilm formation
Inhibition of G. vaginalis biofilm formation with the < 3 kDa LCFSP was examined. The > 3 kDa LCFSP and < 3 kDa LCFSP were lyophilized and suspended in PBS at a concentration of 1 mg/mL. G. vaginalis (1.1 × 106 CFU/mL) was inoculated in a 24-well plate with the BHIS broth, in which each 200 μL LCFSP fraction was added. G. vaginalis was inoculated in the BHIS broth, in which 200 μL precipitated protein fraction (1 mg/mL) of MRS broth was added as control. The plate was incubated at 37 °C for 24 h with 5% CO2. Biofilm was washed with 1 mL PBS, and then 1 mL 0.1% (v/v) crystal violet solution (Sigma-Aldrich Co.) was added to stain the biofilm. Staining was done by mixing the plate at 300 rpm for 15 min using well plate mixer (MX-M, DLAB, Riverside, CA, USA). Excess stain was removed, and stained biofilm was washed with 1 mL PBS and air-dried for 15 min. Biofilm was solubilized in 600 μL 33% (v/v) acetic acid, followed by mixing at 300 rpm for 10 min using the well plate mixer. OD was measured at 570 nm.
Evaluation of ameliorative effect of L. paracasei CH88 and the < 3 kDa LCFSP on BV-induced mice
Induction of BV and intravaginal treatment of L. paracasei CH88 and < 3 kDa LCFSP
Female C57BL/6 mice (7 weeks, 17–19 g) were purchased from Daehan Bio Link Co., Ltd. (Eumsung, Korea). The animals were housed in wire cages under a cycle of 12 h light/12 h dark at 50 ± 10% humidity and 23 ± 3 °C. All mice were fed AIN-93G diet (Research Diets Inc., New Brunswick, NJ, USA) and allowed to access water ad libitum. All animal experimental protocol was approved by Institutional Biosafety Committee of Seoul National University (SNUIBC-R201228-1; date of approval: December 31, 2020). All animal experiments were performed in accordance with a Guidelines for the Care and Use of Laboratory Animals issued by the Institutional Animal Care and Use Committee of Seoul National University (SNU-201228–2-1; date of approval: Feburary 1, 2021) and the ARRIVE guidelines.
BV was induced in accordance to the method of Jang et al.29 with minor modifications. After 1-week acclimatization, mice were divided into five groups with 6 mice each and all treatments were executed for 10 days. β-Estradiol 3-benzoate (0.5 mg; Sigma-Aldrich Co.) was diluted in 100 μL filter-sterilized sesame oil (Sigma-Aldrich Co.). The β-estradiol 3-benzoate solution was injected intraperitoneally 72 h before inducing BV and administered weekly thereafter30. On the day of BV induced, G. vaginalis (1.1 × 108 CFU/20 μL PBS) was inoculated intravaginally. The control group was intravaginally treated with 20 μL PBS instead of G. vaginalis suspension. Each day for 6 days after infection, the control and G. vaginalis-infected group were intravaginally treated with 20 μL PBS. 100 μg metronidazole (Sigma-Aldrich Co.) was diluted in 20 μL sterilized PBS and injected intravaginally each day for 6 days after infection. L. paracasei CH88 (1.1 × 1010 CFU/20 μL PBS) was intravaginally injected each day for 6 days after infection. The lyophilized < 3 kDa LCFSP was diluted in sterilized PBS at a concentration of 1 mg/mL, 20 μL of which was then intravaginally injected each day for 6 days after infection. All mice were anesthetized with 4% isoflurane before intravaginal injection. Mice were euthanized with CO2 after 24 h of final administration. Vagina was washed with 100 μL sterilized PBS to obtain vaginal fluid and then excised. The excised vagina was fixed in 10% neutral buffered formalin solution (Sigma-Aldrich Co.) for histological examination and the other was stored at − 80 °C for enzyme-linked immunosorbent assay (ELISA) analysis.
Evaluation of G. vaginalis CFU in vaginal fluid
Obtained vaginal fluid was directly transferred to anaerobic chamber with GasPakTM EZ Anaerobe Container System (BD), and then serially 10-fold diluted. 100 μL of each diluted fluid was spread on TSB supplemented with 1.5% (w/v) bacto agar, 5% (v/v) horse blood, 0.02% (v/v) menadione solution, 1% (v/v) hemin solution, 4 mg/L gentamicin sulfate (Sigma-Aldrich Co.), 30 mg/L nalidixic acid (Sigma-Aldrich Co.), and 2 mg/L amphotericin B (Sigma-Aldrich Co.), followed by incubation for 48 h at 37 °C with 5% CO2. After the culture, colonies were counted. Colonies on TSB agar plate supplemented with antibiotics were confirmed to be G. vaginalis by gram-staining .
Histopathological examination
Fixed vaginal tissue was stained with hematoxylin–eosin (H&E) and then assessed under a light microscope (× 200). H&E staining was performed by the Department of Clinical Laboratory Science, Wonkwang Health Science University (Iksan, Korea).
Analysis of vaginal cytokine levels
ELISA kits (Thermo Fisher Scientific) were used to determine the levels of interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor-α (TNF-α) in the vaginal tissue. 100 mg excised vagina was homogenized in 1 mL ice-cold RIPA lysis buffer containing 1% (v/v) protease inhibitor cocktail (Thermo Fisher Scientific) and 1% (v/v) phosphatase inhibitor cocktail (Thermo Fisher Scientific)31. Homogenates were centrifuged at 13,000 × g at 4 °C for 30 min and cytokine levels of the supernatants were examined by measuring OD at 450 nm.
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics 26.0 (Chicago, IL, USA). Results were represented as means and standard errors of the means (SEM). One-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test (p < 0.05) or Duncan’s multiple range test (p < 0.05), and Kruskal–Wallis test with Dunn’s test (adjusted p < 0.05) were used to determine statistical significance between the groups.
Results
The viability of G. vaginalis was significantly reduced after 24 h incubation with the LCFS (Fig. 1a). Inhibitory activities of the LCFS against the growth of G. vaginalis depended on pH (Fig. 1b). The LCFS with pH 5.0 had the least activity. However, the growth curve of G. vaginalis treated with the LCFS with pH 5.0 exhibited delayed exponential phase compared to the control group, suggesting that the LCFS with pH 5.0 still had inhibitory effect (Supplementary Fig. 1b). The most active inhibition was observed in the LCFS with pH 11.0 and 13.0. These results suggest that antibacterial compound or compounds might be active potently at pH 11.0 and 13.0.
Optical density (OD) of Gardnerella vaginalis suspension incubated for 24 h with 5% (v/v) Lactobacillus paracasei CH88 cell-free supernatant treated at different temperatures for 30 min (a) and different pH (b). Bars are means and standard errors of the means (n = 3) of triplicate experiments. *Significant difference compared to the control (p < 0.05; one-way ANOVA with Dunnett’s multiple comparison test).
Among the fractions filtered with different MWCO membranes (< 3, 3–10, 10–30, 30–50, 50–100, and > 100 kDa), the < 3 kDa LCFS had the most antibacterial activity against G. vaginalis (Fig. 2). Furthermore, the < 3 kDa LCFS also delayed the exponential phase of G. vaginalis (Supplementary Fig. 2).
Optical density (OD) of Gardnerella vaginalis suspension incubated for 24 h with 5% (v/v) Lactobacillus paracasei CH88 cell-free supernatant fractions with different molecular weights. Bars are means and standard errors of the means (n = 3) of triplicate experiments. *Significant difference compared to the control (p < 0.05; one-way ANOVA with Dunnett’s multiple comparison test).
The < 3 kDa LCFS treated with protease, trypsin, and α-chymotrypsin showed a tendency to lose their antibacterial activity against G. vaginalis after 24 h incubation (Fig. 3). Among them, the < 3 kDa LCFS treated with trypsin or protease showed higher OD values in exponential phase than the others (Supplementary Fig. 3).
Optical density (OD) of Gardnerella vaginalis suspension incubated for 24 h with 5% (v/v) fraction below 3 kDa from Lactobacillus paracasei CH88 cell-free supernatant treated with different proteolytic enzymes. Bars are means and standard errors of the means (n = 3) of triplicate experiments. *Significant difference compared to the control (p < 0.05; one-way ANOVA with Dunnett’s multiple comparison test).
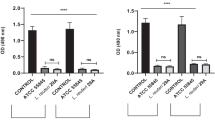
We investigated whether protein precipitates from the LCFS, including the > 3 kDa LCFSP and the < 3 kDa LCFSP, had antibacterial activity against G. vaginalis. The CFU of G. vaginalis in the < 3 kDa LCFSP-treated group was significantly lower than the control and > 3 kDa LCFSP-treated groups (p < 0.05) (Fig. 4a). Both of the LCFSP reduced the biofilm formation of G. vaginalis compared to the control group (Fig. 4b). Especially, the < 3 kDa LCFSP significantly more inhibited the biofilm formation than the > 3 kDa LCFSP (p < 0.05).
Colony forming unit (CFU) (a) and biofilm formation (b) of Gardnerella vaginalis treated with precipitated protein fraction of the Lactobacillus paracasei CH88 cell-free supernatant (LCFSP; 1 mg/mL). Bars are means and standard errors of the means (n = 3) of triplicate experiments. Different small letters indicate significant differences (p < 0.05; one-way ANOVA and Duncan’s multiple range test). (p < 0.05; one-way ANOVA and Duncan’s multiple range test).
The CFU of G. vaginalis were significantly reduced in vaginal fluid of the mice to which L. paracasei CH88, < 3 kDa LCFSP, or metronidazole was intravaginally administered (Fig. 5). Vaginal tissue of the G. vaginalis group showed increased exfoliation of vaginal epithelial cells and thickness of transitional epithelium in H&E staining (Fig. 6). On the other hand, the L. paracasei CH88 or < 3 kDa LCFSP group showed less vaginal epithelial cell exfoliation and thinner transitional epithelium than the G. vaginalis group. Intravaginal treatment of L. paracasei CH88 or the < 3 kDa LCFSP tended to reduce the levels of IL-1β, IL-6, and TNF-α in vaginal tissue of the mice (Fig. 7). IL-10 production was higher in the CH88 group than in the G. vaginalis and metronidazole groups.
Colony forming unit (CFU) of Gardnerella vaginalis in vaginal fluid of the mice intravaginally treated with phosphate-buffered saline (20 μL), G. vaginalis (1 × 108 CFU), metronidazole (100 μg), Lactobacillus paracasei CH88 (1 × 1010 CFU), and protein fraction below 3 kDa of L. paracasei CH88 cell-free supernatant (20 μg; < 3 kDa LCFSP). Bars are means and standard errors of the means (n = 6). Different small letters indicate significant differences (p < 0.05; one-way ANOVA and Duncan’s multiple range test).
Hematoxylin–eosin staining of the murine vaginal tissue intravaginally treated with phosphate-buffered saline (20 μL), G. vaginalis (1 × 108 CFU), metronidazole (100 μg), Lactobacillus paracasei CH88 (1 × 1010 CFU), and protein fraction below 3 kDa of L. paracasei CH88 cell-free supernatant (20 μg; < 3 kDa LCFSP).
Cytokines in the murine vagina intravaginally treated with phosphate-buffered saline (20 μL), G. vaginalis (1 × 108 CFU), metronidazole (100 μg), Lactobacillus paracasei CH88 (1 × 1010 CFU), and protein fraction below 3 kDa of L. paracasei CH88 cell-free supernatant (20 μg; < 3 kDa LCFSP). Bars are means and standard errors of the means (n = 6) of duplicate experiments. *Significant difference (adjusted p < 0.05; Kruskal–Wallis test with Dunn’s test).
Discussion
Antibacterial effect of cell-free supernatant fractions of L. paracasei CH88 against G. vaginalis was investigated and characterized in-vitro and in-vivo. The LCFS still had antibacterial activity after thermal treatment at the various temperatures, indicating that antibacterial compound or compounds in the LCFS were stably active even when treated at 120 °C for 30 min (Supplementary Fig. 1a). Bacteriocin fraction from L. paracasei SD1 cell-free supernatant lost their activity completely after thermal treatment at 120 °C for 20 min32. However, L. paracasei HD1-7 cell-free supernatant maintained their activity after thermal treatment at 121 °C for 20 min33. Class II bacteriocin, one of food preservatives, is known to be stable during thermal food processing34. The LCFS showed the highest antibacterial activity against G. vaginalis at pH 11.0 and pH 13.0 in this study. Antibacterial activity of L. paracasei ZFM54 bacteriocin was significantly reduced at pH 5.0 [e], which was consistent with our result. However, bacteriocin fraction from L. paracasei SD1 cell-free supernatant showed the highest antibacterial activity at pH 5.0 and 6.032. The most active inhibition of L. paracasei HD1-7 cell-free supernatant was observed at pH 3.0, but its antibacterial activity was inactivated at pH 9.033.
The < 3 kDa LCFS had the most antibacterial activity against G. vaginalis in this study, suggesting that the antibacterial compound or compounds in the LCFS pass through 3 kDa MWCO membrane. Antibacterial compounds below 10 kDa, such as class II bacteriocins and bacteriocin from Lactobacillus spp., have been reported to easily permeate cell wall causing cell wall leakage35,36,37, leading to irreversible alteration of the cellular membrane.
After proteolytic enzyme treatment on the < 3 kDa LCFS, antibacterial activity against G. vaginalis was reduced. These results suggest that antibacterial compound or compounds in the < 3 kDa LCFS might be mainly composed of proteins and easily hydrolyzed by trypsin or protease, which prevents its accumulation in body unlike antibiotics38.
Among the LCFSP, < 3 kDa LCFSP had antibacterial activity in this study. Numerous studies identified inhibitory activity of protein precipitates secreted by various bacteria. Protein precipitates from cell-free supernatant of L. acidophilus KS400, Bacillus amyloliquefaciens, and B. coagulans had inhibitory activities against the growth of G. vaginalis and their molecular weights ranged from 3 kDa to 7.5 kDa39,40,41. In this study, antibiofilm compound or compounds in the LCFSP mainly existed in the < 3 kDa LCFSP. G. vaginalis develops a dense biofilm adherent to vaginal epithelium in the women who suffer from BV42. Biofilm offers G. vaginalis a favorable environment providing nutrients and protecting it from antimicrobial agents43. Therefore, preventing the biofilm formation of G. vaginalis might be a primary strategy to treat BV and to prevent its recurrence43. However, it was reported that antibiotics such as metronidazole and tobramycin have no significant disruptive effect on G. vaginalis biofilm in-vitro44. Saunders et al. reported that L. reuteri RC-14 and L. rhamnosus GR-1 could reduce G. vaginalis biofilm, suggesting that biosurfactants produced by these lactobacilli might contribute to inhibiting biofilm formation45. It was also reported that L. delbrueckii DM8909, Lactiplantibacillus plantarum ATCC14917, and Lactiplantibacillus plantarum ZX27 had antibiofilm abilities against G. vaginalis46. It can be concluded that the < 3 kDa LCFSP might have a potency to prevent the formation of G. vaginalis biofilm. However, further study should be performed to identify antibiofilm agent(s) in the < 3 kDa LCFSP.
Intravaginal treatment of metronidazole, L. paracasei CH88, and < 3 kDa LCFSP significantly reduced CFU of G. vaginalis in vaginal fluid. Previous studies also reported that intravaginal and oral administration of lactobacilli such as L. johnsonii HY7042, L. rhamnosus HN001, and L. acidophilus GLa-14 could ameliorate BV via reducing G. vaginalis level in vagina29,31. Jang et al. reported that intravaginal administration of cell-free supernatant from L. fermentum SNUV175 and L. crispatus SNUV220 had ameliorative effects on vulvovaginal candidiasis in murine model by reducing the CFU of Candida albicans, which is also known as a representative pathogenic yeast causing vaginal disorder47. Lactobacilli have been known to be able to inhibit the growth of G. vaginalis by producing antimicrobial compounds as well as lactic acid23,24. Since pH of the < 3 kDa LCFSP was set at 7.0, it could be presumed that antibacterial activity of L. paracasei CH88 against G. vaginalis might be mainly attributed to unidentified compounds in the < 3 kDa LCFSP. Intravaginal treatment of G. vaginalis increased thickness of vaginal transitional epithelium in this study. Increased thickness of transitional epithelium in vagina was reported to be related to epithelial proliferation, which results in cell exfoliation48. Exfoliated vaginal epithelial cells covered with adherent anaerobic bacteria have been commonly observed in vaginal fluid of the women who have BV, but not in the women who do not49. Our results suggest that L. paracasei CH88 or the < 3 kDa LCFSP reduced G. vaginalis-induced vaginal epithelial cell exfoliation. Intravaginal inoculation of G. vaginalis was reported to induce immune response, resulting in elevating the levels of IL-1β, IL-6, IL-8, and IL-10 in cervicovaginal fluid50. L. paracasei CH88 and < 3 kDa LCFSP-treated group showed lower IL-1β, IL-6, TNF-α level compared to G. vaginalis group. Previous studies also reported that intravaginal and oral administration of L. johnsonii HY7042, L. rhamnosus HN001, and L. acidophilus GLa-14 tended to reduce the levels of IL-1β, IL-6, and TNF-α, while increasing IL-10 production in mice with G. vaginalis-induced BV29,31, which were consistent with our results.
Conclusion
The antibacterial effect of L. paracasei CH88 may be due to unidentified proteins in the fraction below 3 kDa of L. paracasei CH88 cell-free supernatant. L. paracasei CH88 or < 3 kDa LCFSP could ameliorate BV via not only inhibiting the growth of G. vaginalis and its biofilm formation, but also reducing exfoliation of vaginal cells and production of pro-inflammatory cytokines. However, this study has some limitations that in-vitro and in-vivo antibacterial experiments against F. vaginae and P. bivia should be followed since BV is a polymicrobial infection. Moreover, safety assessment must be followed to apply this treatment to humans.
Data availability
The data presented in this study are available on reasonable request and for non-commercial purposes.
References
Schwebke, J. R., Muzny, C. A. & Josey, W. E. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 210, 338–343 (2014).
Wang, J. Bacterial vaginosis. Prim Care Update Ob Gyns 7, 181–185 (2000).
Laxmi, U., Agrawal, S., Raghunandan, C., Randhawa, V. S. & Saili, A. Association of bacterial vaginosis with adverse fetomaternal outcome in women with spontaneous preterm labor: a prospective cohort study. J. Matern-Fetal. Neonatal. Med. 25, 64–67 (2012).
Hay, P. E. et al. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 308, 295–298 (1994).
Jamieson, D. J. et al. Longitudinal analysis of bacterial vaginosis: Findings from the HIV epidemiology research study. Obstet. Gynecol. 98, 656–663 (2001).
Workowski, K. A. & Bolan, G. A. Sexually transmitted diseases treatment guidelines. MMWR 64, 110–112 (2015).
Menard, J. P. Antibacterial treatment of bacterial vaginosis: current and emerging therapies. Int. J. Womens Health 3, 295 (2011).
Catlin, B. W. Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin. Microbiol. Rev. 5, 213–237 (1992).
Morrill, S., Gilbert, N. M. & Lewis, A. L. Gardnerella vaginalis as a cause of bacterial vaginosis: appraisal of the evidence from in vivo models. Front. Cell Infect. Microbiol. 10, 168 (2020).
Castro, J. et al. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci. Rep. 5, 11640 (2015).
Muzny, C. A. et al. An updated conceptual model on the pathogenesis of bacterial vaginosis. J. Infect. Dis. 220, 1399–1405 (2019).
Ferris, D. G., Litaker, M. S., Woodward, L., Mathis, D. & Hendrich, J. Treatment of bacterial vaginosis: a comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. Fam. Pract. 41, 443–450 (1995).
Hanson, J. M. et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. JRM 45, 889–896 (2000).
Beigi, R. H., Austin, M. N., Meyn, L. A., Krohn, M. A. & Hillier, S. L. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am. J. Obstet. Gynecol. 191, 1124–1129 (2004).
Bradshaw, C. S. et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193, 1478–1486 (2006).
Reid, G., Beuerman, D., Heinemann, C. & Bruce, A. W. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol. 32, 37–41 (2001).
Reid, G. et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol. 35, 131–134 (2003).
Anukam, K. C. et al. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microb. Infect. 8, 2772–2776 (2006).
Hemmerling, A. et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. STD 37, 745–750 (2010).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. PNAS 108, 4680–4687 (2011).
Nunn, K. L. & Forney, L. J. Focus: microbiome: unraveling the dynamics of the human vaginal microbiome. Y JBM 89, 331 (2016).
Klebanoff, S. J., Hillier, S. L., Eschenbach, D. A. & Waltersdorph, A. M. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J. Infect. Dis. 164, 94–100 (1991).
Boskey, E. R., Cone, R. A., Whaley, K. J. & Moench, T. R. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 16, 1809–1813 (2001).
Sabia, C. et al. Detection and partial characterization of a bacteriocin-like substance produced by Lactobacillus fermentum CS57 isolated from human vaginal secretions. Anaerobe 26, 41–45 (2014).
Voravuthikunchai, S. P., Bilasoi, S. & Supamala, O. Antagonistic activity against pathogenic bacteria by human vaginal lactobacilli. Anaerobe 12, 221–226 (2006).
Aroutcheva, A. A., Simoes, J. A. & Faro, S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect. Dis. Obstet. Gynecol. 9, 33–39 (2001).
Lee, S. G. & Chang, H. C. Purification and characterization of mejucin, a new bacteriocin produced by Bacillus subtilis SN7. LWT-Food Sci. Technol. 87, 8–15 (2018).
Mun, S. Y., Kim, S. K., Woo, E. R. & Chang, H. C. Purification and characterization of an antimicrobial compound produced by Lactobacillus plantarum EM showing both antifungal and antibacterial activities. LWT-Food Sci. Technol. 114, 108403 (2019).
Jang, S. E. et al. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 attenuate Gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients 9, 531 (2017).
Yano, J. & Fidel, P. L. Jr. Protocols for vaginal inoculation and sample collection in the experimental mouse model of Candida vaginitis. J. Vis. Exp. 58, e3382 (2011).
Joo, H. M. et al. Lactobacillus johnsonii HY7042 ameliorates Gardnerella vaginalis-induced vaginosis by killing Gardnerella vaginalis and inhibiting NF-κB activation. Int. Immunopharmacol. 11, 1758–1765 (2011).
Wannun, P., Piwat, S. & Teanpaisan, R. Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe 27, 17–21 (2014).
Ge, J., Sun, Y., Xin, X., Wang, Y. & Ping, W. Purification and partial characterization of a novel bacteriocin synthesized by Lactobacillus paracasei HD1-7 isolated from Chinese sauerkraut juice. Sci. Rep. 6, 1–7 (2016).
And, H. C. & Hoover, D. G. Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2, 82–100 (2003).
Ye, P., Wang, J., Liu, M., Li, P. & Gu, Q. Purification and characterization of a novel bacteriocin from Lactobacillus paracasei ZFM54. LWT-Food Sci. Technol. 143, 111125 (2021).
Héchard, Y. & Sahl, H. G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84, 545–557 (2002).
Bendjeddou, K., Fons, M., Strocker, P. & Sadoun, D. Characterization and purification of a bacteriocin from Lactobacillus paracasei subsp. paracasei BMK2005, an intestinal isolate active against multidrug-resistant pathogens. World J. Microbiol. Biotechnol. 28, 1543–1552 (2012).
Perez, R. H., Zendo, T. & Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb. Cell Fact. 13, 1–13 (2014).
Gaspar, C. et al. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express 8, 1–8 (2018).
Noll, K. S., Sinko, P. J. & Chikindas, M. L. Elucidation of the molecular mechanisms of action of the natural antimicrobial peptide subtilosin against the bacterial vaginosis-associated pathogen Gardnerella vaginalis. Probiotics Antimicrob. Proteins 3, 41–47 (2011).
Riazi, S., Dover, S. E. & Chikindas, M. L. Mode of action and safety of lactosporin, a novel antimicrobial protein produced by Bacillus coagulans ATCC 7050. J. Appl. Microbiol. 113, 714–722 (2012).
Swidsinski, A. et al. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 106, 1013–1023 (2005).
Marrazzo, J. M. Vaginal biofilms and bacterial vaginosis: of mice and women. J. Infect. Dis. 207, 1481–1483 (2013).
Gottschick, C. et al. Screening of compounds against Gardnerella vaginalis biofilms. PLoS ONE 11, e0154086 (2016).
Saunders, S., Bocking, A., Challis, J. & Reid, G. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf. B 55, 138–142 (2007).
Qian, Z. et al. Probiotic Lactobacillus sp. strains inhibit growth, adhesion, biofilm formation, and gene expression of bacterial vaginosis-inducing Gardnerella vaginalis. Microorganisms 9, 728 (2021).
Jang, S. J., Lee, K., Kwon, B., You, H. J. & Ko, G. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci. Rep. 9, 1–9 (2019).
Gilbert, N. M., Lewis, W. G. & Lewis, A. L. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS ONE 8, e59539 (2013).
Gardner, H. L. & Dukes, C. D. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified “nonspecific” vaginitis. Am. J. Obstet. Gynecol. 69, 962–976 (1955).
Sierra, L. J. et al. Colonization of the cervicovaginal space with Gardnerella vaginalis leads to local inflammation and cervical remodeling in pregnant mice. PLoS ONE 13, e0191524 (2018).
Funding
This research was financially supported by the Gang-neung Science & Industry Promotion Agency (GSIPA), Korea, under the “Regional development investment agreement pilot project”.
Author information
Authors and Affiliations
Contributions
Conceptualization: [E.C.M., M.S.P., G.E.J., and K.T.H.], methodology: [E.C.M., M.S.P. and S.Y.K.], data curation: [E.C.M.], writing-original draft preparation: [E.C.M.], writing-review and editing: [T.L., R.H.K., and K.T.H.], supervision: [K.T.H.], funding acquisition: [G.E.J.]. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
MSP, GEJ and KTH hold BIFIDO Ltd. stocks. Other authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moon, E.C., Park, M.S., Lim, T. et al. Antibacterial effect of cell-free supernatant fraction from Lactobacillus paracasei CH88 against Gardnerella vaginalis. Sci Rep 12, 4763 (2022). https://doi.org/10.1038/s41598-022-08808-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08808-7
- Springer Nature Limited
This article is cited by
-
Lactobacillus plantarum LPYC225 mixture partially modulates the vaginal bacterial community of Gardnerella vaginalis-infected bacterial vaginosis in mice
Food Science and Biotechnology (2024)
-
Management of Cardiovascular Diseases by Short-Chain Fatty Acid Postbiotics
Current Nutrition Reports (2024)
-
Lactobacillus helveticus HY7801 ameliorates bacterial vaginosis by inhibiting biofilm formation and epithelial cell adhesion of Gardnerella vaginalis
Food Science and Biotechnology (2023)
-
Screening, Characterization and Optimization of Bioactive Peptides with Antibacterial Activities Against Multi-Drug Resistant Pathogens, Produced by Bacillus safensis Strain MK-12.1
International Journal of Peptide Research and Therapeutics (2022)