Abstract
Animal migration contributes largely to the seasonal dynamics of High Arctic ecosystems, linking distant habitats and impacting ecosystem structure and function. In polar deserts, Arctic hares are abundant herbivores and important components of food webs. Their annual migrations have long been suspected, but never confirmed. We tracked 25 individuals with Argos satellite telemetry to investigate the existence of migration in a population living at Alert (Ellesmere Island, Nunavut, Canada). During fall, 21 hares undertook directional, long-distance movements in a southwestern direction towards Lake Hazen. Daily movement rates averaged 1.3 ± 0.5 km, 4.3 ± 1.6 km, and 1.7 ± 0.9 km before, during, and after relocation, respectively. Straight-line and minimum cumulative distances traveled averaged 98 ± 18 km (range: 72–148 km) and 198 ± 62 km (range: 113–388 km), respectively. This is the first report of large-scale seasonal movements in Arctic hares and, surprisingly, in any lagomorph species. These movements may be part of an annual migratory pattern. Our results redefine our understanding of the spatial ecology of Arctic hares, demonstrate unsuspected mobility capacities in lagomorphs, and open new perspectives regarding the ecological dynamics of the northern polar deserts.
Similar content being viewed by others
Introduction
Long-distance movements of animals are widespread and have far-reaching implications for the dynamics of ecosystems1,2,3. For example, individuals moving across ecosystem boundaries may transport nutrients, parasites, and pathogens1,4,5. The mass arrival of new animals in a system may also alter local food webs by introducing new predators6 or prey7. Identifying the extent of movements exhibited by species or populations can therefore provide critical ecological insight and yield new knowledge pertinent to management or conservation8.
Three broad categories of long-distance movements are generally recognized9. Dispersal occurs when an animal leaves a previously used area to move to another area that will be used for breeding10. Migration is a periodic movement between two distinct areas, such as a summer and a winter range10,11. Finally, nomadism involves movements that may be highly variable in their timing and direction within and between years10,11.
Migration and nomadism are common in strongly seasonal environments, since moving animals can benefit from tracking the fluctuations of resources and weather10,12. Northern polar deserts represent extreme environments defined by low temperatures and precipitation13,14 which, combined with nutrient-poor soils, result in low plant productivity15,16. Winters are extremely cold (temperatures routinely reach − 40 °C), and access to vegetation may be reduced by hard layers of snow such as hard wind slabs and melt-freeze crusts17. To cope with the high seasonality of this environment, Arctic species use multiple movement strategies, ranging from range residency18 to migration19 and nomadism20. While the long migrations of Arctic birds are well known, at least 18 of the 70 species of Arctic terrestrial mammals also have migratory individuals21. More generally, migration is diffusely spread throughout the Mammal class (found in 12 of 27 orders)22, with concentrations of migratory species within Cetacea, Artiodactyla, and Chiroptera12,22, and suggested benefits belonging to four categories: increased energy intake, decreased energy expenditure, decreased predation, and increased mating opportunities12.
The Arctic hare (Lepus arcticus) is one of the largest lagomorphs and an important component of the polar desert food web23. It is omnipresent in the diet of multiple predators, such as Arctic wolves (Canis lupus arctos)24,25 and Arctic foxes (Vulpes lagopus)23. Arctic hares are also generalist herbivores that are active year-round and can reach high densities23. Their movements may therefore influence both upper (predators) and lower (plants) levels of the High Arctic food web. While the species range encompasses the whole Canadian Arctic and a portion of coastal Greenland, their spatial ecology has only been investigated in Newfoundland, Canada26,27,28. A recurrent debate about Arctic hare ecology at the highest latitudes is whether they migrate seasonally. Harper29 observed in southwestern Keewatin (Nunavut), that hares disappeared during the summer and returned in November. While Harper29 believed that Arctic hares migrated, no empirical evidence supports this claim and Dalerum et al.30 recently questioned whether lagomorphs possess the locomotion abilities required to accomplish large-scale movements. Current literature suggests that Arctic hares are a sedentary species with little dispersal capacity28.
Despite the extraordinary techniques now available to track wildlife, Arctic hare movements have never been studied in the High Arctic. Very little is therefore known on this topic, mostly due to the logistic constraints associated with capturing many individuals in difficult-to-reach locations. In addition to increasing our understanding of navigation and locomotion in animals, our improved ability to track individuals precisely and over long periods has facilitated the integration of movement ecology with wildlife management and conservation31. While rapid global changes significantly alter landscapes at large scales, species status assessments increasingly use data on species mobility to evaluate conservation threats31. Currently, Arctic hares have no legal conservation status in Canada due to lack of data, but their range is restricted to the Arctic, a region strongly exposed to climate change and increasing human activities32. Better knowledge of Arctic hare movements is therefore necessary to close knowledge gaps regarding both their movement ecology and their conservation.
Here, we used satellite tracking to investigate seasonal movements of Arctic hares on Ellesmere Island, Nunavut, in the polar desert of the Canadian High Arctic. Our objectives were to (i) test the hypothesis that migration occurs in an Arctic hare population at the species northern distribution limit, (ii) characterize seasonal movement metrics, including timing of movements, and (iii) identify areas of seasonal residency. We report relatively synchronized and directional long-distance movements of Arctic hares during the fall. The scale of these movements, reaching several hundred kilometers, has never been observed in any lagomorph species.
Methods
Study area
We worked in a 170-km2 study area surrounding Canadian Forces Station Alert, Ellesmere Island, Nunavut (82°30′N, 62°20′W; Fig. 1a). The landscape encompasses multiple hills and creeks, four lakes, and several ponds33. With only 156 mm of precipitation per year on average, and temperatures reaching − 40 °C in winter, the environment is a polar desert33. Due to the short growing season (60–70 days) and dry soils poor in nutrients, only 77 species of vascular plants grow at Alert33. The most common species are saxifrages (Saxifraga sp.), Arctic poppies (Papaver sp.), Arctic willows (Salix arctica), and several species of grasses33. The main herbivores at Alert are the Arctic hare, the collared lemming (Dicrostonyx groenlandicus), the muskox (Ovibos moschatus), the Peary caribou (Rangifer tarandus pearyi), and the rock ptarmigan (Lagopus muta). At Alert, predators of young and adult hares include the Arctic wolf, the Arctic fox, and the snowy owl (Bubo scandiacus). Ermines (Mustela erminea) and glaucous gulls (Larus hyperboreus) may also predate leverets at Alert.
Location of study area and spatial extent of Arctic hare movements at the northeastern tip of Ellesmere Island. (a) Location of Alert (red dot) on Ellesmere Island, west of Greenland. The grey rectangle is enlarged in (b). (b) Locations of 25 hares, obtained by Argos telemetry between 15 June 2019–31 May 2020, are represented by green circles (summer locations), orange circles (fall relocation), and blue circles (winter locations). (c) Summer range (green), relocation path (orange dots and line) and winter range (blue) of an Arctic hare collared at Alert and wintering in the Lake Hazen area. The boundaries of the Quttinirpaaq National Park of Canada are represented by a black line. This map was created using QGIS 3.8.340. Satellite imagery was obtained from: Esri, USGS | Esri, HERE, Garmin, FAO, NOAA, USGS, NRCan, Parks Canada | Earthstar Geographics.
Captures and satellite tracking
Arctic hares were captured using Tomahawk cage traps (102 cm × 38 cm × 38 cm, model 208, Tomahawk Live Trap Co, Tomahawk, WI, USA) and custom-made drop cages (95 cm × 95 cm × 45 cm). Cages were baited with peanuts and commercial bird seeds and checked every 2–4 h. Capture effort occurred from 15 May to 26 July 2019 and was irregular through space and time, depending on weather conditions, opportunistic hare observations, and time constraints. Upon capture, we determined sex, mass (nearest 50 g, Pesola spring scale 10 kg), age class based on body size (juvenile or ≥ 1 year old, the latter being referred to as “adult”) and, for females, reproductive status (pregnant, lactating, or undetermined). Pregnancy was assessed by examination and palpation of the abdomen, and lactation by squeezing milk from the teats34. Adults were ear-tagged using four numbered metal bands (Jiffy Wing Bands—Style 893, National Band & Tag Company) to which were attached custom-made plastic color tags (1.5 cm × 2.5 cm) providing unique color codes allowing individual identification at a distance or during recaptures. From 14 June to 26 July 2019, 25 hares were fitted with an Argos Platform Terminal Transmitter (PTT, model KiwiSat 303, Lotek, Newmarket, Ontario, Canada; 115 g; 2–3.1% of body mass) with a temperature sensor. Capture and handling techniques were approved by the Animal Care Committee of Université du Québec à Rimouski (CAC-68-17-184) and the Government of Nunavut (Permit number WL 2018-020). The dataset generated and analyzed for this study (Fig. 2a) is part of the Arctic Animal Movement Archive35, is freely available in MoveBank36, and is stored in the MoveBank Data Repository37.
Methodological workflow for the identification of fall departure and arrival dates of Arctic hares tracked at Alert (Nunavut, Canada). After acquisition and filtering of location data (steps a–b, white boxes), departure dates (steps c–f, green boxes) and arrival dates (steps g–j, blue boxes) were obtained through a multi-step process involving visualization of individual trajectories and delineation of individual summer and winter grounds.
Argos location filter
PTT collars were programmed to transmit daily between 10:00–13:00 (local time) with a repetition rate of 60 s. To maximize both accuracy and number of days with locations for each hare, we filtered positions (Fig. 2b) with a Location Class 3, 2, 1, and A, using a speed filter implemented in R 3.6.238 (see S1 in Christin et al.39). The speed filter removed locations that were likely erroneous based on a > 5 km/h cruising speed, with possible acceleration bouts of 10 km/h for locations less than 10 min apart. We set these values based on preliminary data analysis. We then kept for further analyzes one location per day per hare, based on the smallest location error. A visual check of the data confirmed that no outlier locations remained.
Determination of departure date from summer ground
Our visual inspection of individual trajectories (QGIS 3.8.340, Fig. 2c) showed that no hare left its summer grounds before 11 August. We subsequently determined departure dates of individuals in two steps. First, we delineated for each hare the 95% minimum convex polygon (MCP) of the area used after collaring but before 11 August (Fig. 2d,e). For most individuals, locations were too few (median: 32, range: 12–54) for this area to reach an asymptotic size41 and be considered a home range. Second, we identified the first day with a location outside of, and with no subsequent return to this area. We identified this day as the departure date from summer grounds (Fig. 2f). All hares departing then traveled > 80 km, which prevented any ambiguity regarding departure date. Hares that did not leave their summer grounds were classified as residents.
Determination of arrival date on winter ground
Terrestrial migratory herbivores usually show much higher movement rates during migration than before or after migration42,43,44,45,46. We therefore expected that hares leaving their summer grounds would show high movement rates during relocation. We define here relocation as the action of moving to a new area, happening between departure from summer grounds and arrival to winter grounds. To confirm high movement rates during relocation, we calculated daily individual movement rates for the entire (filtered) dataset generated for each hare. Daily movement rates are defined as the straight-line distance between daily locations or, in rare cases when some daily locations were missing, the straight-line distance between locations divided by the number of days elapsed between locations. Accordingly, average daily movement rate of individuals dramatically increased after they departed from their summer grounds, remained high during ca. 50 days (the relocation period of the population), then returned to values observed during summer when most individuals had reached their winter grounds (see “Results”, Fig. 3). Using our estimate of the average length of the relocation period (Fig. 2g), we determined arrival dates of individuals on winter grounds in two steps. First, using locations collected > 50 days after their departure (Fig. 2h), we delineated for each relocated hare the 95% MCP used after the relocation period of the population (Fig. 2i). Second, we identified the arrival date of each hare on its winter grounds as the day it entered the above area and remained in it for ≥ 2 consecutive days (Fig. 2j).
Variation through time of the mean movement rate and mean net squared displacement (NSD) of Arctic hares equipped with Argos satellite collars at Alert (Nunavut, Canada). Departure dates of individuals from their summer grounds ranged from 11 August 2019 to 17 September 2019 (on average 26 August 2019) and were considered as day 0 before plotting. Mean daily movement rates (blue line) and 5-day moving average of daily movement rates (orange line) are shown from day − 75 (on average 27 June 2019) to day 266 (on average 30 May 2020). Mean net squared displacement (NSD, green line) is shown for the same period. The number of hares contributing data to a given day is shown as a gray bar. Vertical dashed lines indicate the average start (day 0) and end (day 50, on average 15 October 2019) of relocation of tracked hares. Note the sharp increase of movement rates and NSD when relocation begins, but the more gradual change when relocation ends, due to individual differences in length of the relocation period.
Determination and statistical testing of movement metrics describing hare residency and relocation
We used 10 variables to describe residency and relocation of Arctic hares (Table 1). Of those, three describe the timing of relocation: departure date from summer grounds, arrival date to winter grounds, duration of relocation (time elapsed between start and end of relocation). Four variables describe the traveling behaviour of hares: minimum cumulative traveled distance from start to end of tracking (sum of the distances between consecutive daily locations of a hare path), minimum cumulative relocation distance (same as above but from start to end of relocation), straight line distance between summer and winter grounds (minimum distance between centroids of summer and winter MCPs calculated below), orientation of relocation travel (bearing of the line joining the centroids of summer and winter MCPs calculated below). Finally, three surface variables describe areas used by tracked hares: size of summer and winter grounds for relocating hares (summer 95% MCP calculated from locations collected between collaring date and departure date, and winter 95% MCP calculated from locations collected between arrival date and end of tracking), and size of annual grounds for resident hares (95% MCP calculated over entire tracking period). Note that summer and winter MCPs were calculated for each hare using individual departure and arrival dates, and can thus slightly differ from MCPs calculated at steps e and i of Fig. 2. For each individual, we displayed the movement path, along with the net squared displacement (NSD) curve starting from the capture location (Supplementary material S1).
We used a linear mixed-effect model in the lme4 package (version 1.1-27.1)47 in R to verify that movement rates differed significantly across the three movement phases (summer residency, fall relocation, and winter residency). We also included in the model the synchronised time as fixed effect and individual ID as random effect, to account for the unequal number of observations between individuals. We log-transformed (log10) movement rates to respect the assumptions of variance homogeneity and normality of residuals. Given that movement phase had a significant effect, we conducted a pairwise comparison between phases using Tukey’s method.
Mapping of summer and winter grounds
We identified the summer and winter grounds at population level by creating kernel density surfaces using fixed kernel density estimation (KDE) with plug-in bandwidth selection (hplug-in) implemented in the ks package (version 1.13.0)48 in R (data projection: arctic polar stereographic; grid size: 500 × 500 m)49,50. To account for the varying number of locations per animal, we delineated summer grounds using the last 45 consecutive locations (corresponding to approximately 1.5 month of data) obtained before the start of autumn relocation for each hare. For resident hares, we included the last 45 consecutive locations collected before 11 August. Since 16 hares were captured less than 1.5 month before their departure date (or 11 August for residents), the mean number of locations used to delineate summer grounds was 35 ± 11 (median = 42, range: 12–45, n = 25). Similarly, we delineated winter grounds using the first 45 consecutive locations obtained after the arrival of each relocating hare. Winter grounds of resident hares were not delineated. Since six relocating hares died less than 1.5 month after their arrival, the mean number of locations used to delineate winter grounds of relocating hares was 40 ± 9 (median = 45, range: 17–45, n = 19). We extracted percent volume contours (50%, 75%, 95% and 99%) for each seasonal population-level KDEs to represent Arctic hares’ utilization distribution.
All results are expressed as mean ± SD.
Results
A total of 21 adult females (2 pregnant, 15 lactating, 4 unknown) and four adult males received a satellite collar (Table 1). Body mass of individuals at capture averaged 4.69 ± 0.38 kg for females and 3.90 ± 0.16 kg for males. We obtained 4139 locations (after filtering) with an average of 165 ± 75 locations (range: 33–333) per individual. On average, each hare was monitored for 180 ± 79 days (range: 48–346).
Fall relocation
Twenty-one hares (19 F, 2 M) moved from their summer to winter grounds (Fig. 1b,c; Supplementary material S1). Summer MCPs of relocating individuals averaged 10.4 ± 13.3 km2 (F: 10.3 ± 14.0 km2, n = 19; M: 10.7 ± 2.1 km2, n = 2) while the MCPs of resident individuals averaged 14.1 ± 4.1 km2 (F: 12.8 ± 0.2 km2, n = 2; M: 15.4 ± 6.5 km2, n = 2). Relocating hares left their summer grounds between 11 August and 17 September (26 August ± 12 days) and arrived on their winter grounds from 10 September to 5 November (6 October ± 13 days). Departures from summer grounds were relatively synchronized, with 80% (17/21 hares) of relocating hares leaving within 25 days (11 Aug–4 Sep). Arrivals on winter grounds were also relatively synchronized with 71% (15/21 hares) arriving within 21 days (22 Sep–12 Oct). Daily hare movement rates differed between the summer residency, fall relocation and winter residency movement phases (F = 66.93, p < 0.001). Before relocation, daily hare movement rates averaged 1.3 ± 0.5 km (n = 1–19) while they increased to 4.3 ± 1.6 km during relocation (n = 15–21, Fig. 3, p < 0.001). A maximum daily average of 11.6 km was observed on day 33 of the relocation period, when seven hares traveled > 15 km. After day 50, hare movement rates decreased, averaging 1.7 ± 0.9 km (n = 1–19), similar to values observed before fall relocation (p = 0.287). The daily movement rate of resident hares during the relocation period averaged 1.2 ± 0.5 km (n = 3–4).
The mean duration of fall relocation was 42 ± 10 days (range: 15–55, n = 21) while the minimum cumulative distance traveled during relocation averaged 198 ± 62 km (range: 113–388, n = 21). Some hares made temporary stopovers while relocating between summer and winter grounds (see e.g. F1, F2, F5 in Supplementary material S1).
Winter residency
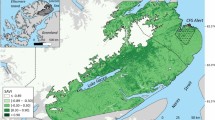
Kernel densities indicated that the main winter grounds of Artic hares captured at Alert were located within the Lake Hazen basin in Quttinirpaaq National Park (Fig. 4). One female initially headed towards Lake Hazen but then turned back and settled close to Alert (F9 in Supplementary material S1). Considering that five hares remained near Alert (including F9), and one died during relocation (F8), the kernel density area for the Lake Hazen basin winter grounds included 19 individuals. The 75% kernel density contour for the Lake Hazen winter grounds covered 283.8 km2, which is 12 times larger than the same contour for their summer grounds (23.8 km2). Upon arrival, Arctic hares congregated mainly in two areas, one group (68%, n = 13) in a valley at the southern end of Piper Pass (75% kernel density contour = 184.9 km2) and another group (16%, n = 3) near the northern tip of Lake Hazen (75% kernel density contour = 42.7 km2) (Fig. 4). Only three hares were not spatially connected to these two groups: F16 went further south, F6 settled near the coast, and F17 went to the other side of the ice cap and glaciers (Fig. 4 and Supplementary material S1). Winter MCPs averaged 83.4 ± 118.7 km2 (F: 90.9 ± 123.1 km2, n = 18; M: 15.5 ± 3.9 km2, n = 2). The straight-line distance between summer and winter grounds averaged 98 ± 18 km (range: 72–148 km, n = 19) once F9 was excluded, and 94 ± 26 km (range: 8–148 km, n = 20) if included. Finally, winter grounds were generally located southwest (− 112° ± 0.2°; range: − 92.4° to − 131.0°, n = 19) of summer grounds.
Kernel density distributions of Arctic hares, showing residency areas during summer (green shades, n = 25) and winter (blue shades, n = 19). Kernel density contours at the 50%, 75%, 95% and 99% levels are shown for each season. White numbers indicate the number of individuals present in the smoothed polygons of the 75% kernel density contours. The northeastern boundaries of Quttinirpaaq National Park of Canada are represented by a black line. This map was created using QGIS 3.8.340. Satellite imagery was obtained from: Esri, USGS | Esri, HERE, Garmin, FAO, NOAA, USGS, NRCan, Parks Canada | Earthstar Geographics.
Two collars stopped transmitting while hares were still alive (F12 and M2) while 23 were still functional when hares died. Most mortalities (n = 17) occurred from November to February, with a peak in November (n = 8). Cause of death was unknown. Individual F12 was the last individual to be tracked and she was moving away from her winter grounds, towards the east, when her collar stopped transmitting on 20 May 2020 (Supplementary material S1).
Discussion
We observed long-distance movements of Arctic hares during fall. Movements were synchronized, directional and resulted in the use of two distant seasonal ranges. Most (84%) of 25 collared hares undertook these movements, suggesting a population-wide phenomenon. No individual was tracked until the next summer, due to winter mortality or collar failure, so we cannot confirm any spring return to summer grounds.
Migration in Arctic hares
In addition to demonstrating synchronized, directional, and long-distance fall movements, our results provide detailed movement metrics such as speed and timing of relocation. During their fall relocation, the cumulative distance traveled by hares largely exceeded 150 km in most cases, with some individuals traveling > 300 km. These are minimum estimates based on only one location per day. Daily movement rates during relocation were twice as high than during the summer or winter sedentary phases. Following relocation, hares settled for the winter at about 100 km from their summer grounds. All the above contrasts strongly with our current understanding of the spatial ecology of lagomorphs, including Arctic hares. Most lagomorphs are considered sedentary, despite a few accounts of relatively long-distance dispersal in some species like the snowshoe (Lepus americanus) and the mountain hare (Lepus timidus)51,52. However, these dispersals rarely exceed 30 km. Suggestions of long migrations have been made for mountain hares in Russia53, but empirical evidence is weak. The only possible migrant lagomorph is the black-tailed jackrabbit (Lepus californicus), but observed movements were ≤ 35 km and migration over a full annual cycle was never observed54.
Three lines of evidence suggest that the observed movement pattern reflects migratory behavior. First, the fall departure of adults from their summer grounds was both massive and synchronized, as is often observed in migrations55,56,57. We know of no instance of adult dispersal in mammals that is characterized by such frequency of occurrence and such synchronicity. Although several hare species have been tracked, recorded movements do not usually exceed a few tens of kilometers, and adult movements do not occur massively and do not present such synchronicity58. Second, migrations are highly directional movements59, as observed in this study. It would be surprising to observe such directionality if breeding dispersal was at play, although local geography and topography did constrain the direction of movements. Third, the presence of distinct seasonal ranges used alternatively during the year is the true hallmark of migrations10,11 and we did observe that hares relocating from Alert used during the winter a common area in the Lake Hazen basin.
We note that four hares showed range residency at Alert, and one (F9) reversed her relocation path to settle ca. 8 km from her summer grounds, indicating potential within-population variation in migratory tactics60. Variation in migration patterns has been observed in other mammalian herbivores, including white-tailed deer (Odocoileus virginianus)44, caribou (Rangifer tarandus)43 and moose (Alces alces)61. Such variability often challenges characterization of movement strategies and movement modes from telemetry data. Considering that long-distance movement behavior was never characterized in Arctic hares and that many movement patterns, including in well-known migratory species, do not perfectly fit conceptualized (modelled) movement strategies43,62, we chose not to rely on a published approach such as NSD to separate the residency periods from relocation, and rather used the alternative approach described in Fig. 2. Further research relying on GPS technology allowing a more precise and frequent sampling of individual trajectories may allow the implementation of new methods in the future.
Since we could not observe a complete annual track, we cannot determine fidelity of hares to seasonal grounds. Site fidelity to both seasonal grounds could occur, or it could be limited to only the summer or winter grounds, or fidelity to seasonal grounds may not occur at all (nomadism). Individuals showed correlated fall movements and most of them aggregated in the same winter grounds, so the observed movement pattern could also fit the definition of type II nomadism (sensu Mueller and Fagan63). Considering that it is between-year regularity that ultimately determines if a movement pattern is migratory or nomadic10, tracking individuals over several years will be necessary to determine the type of movement displayed by Arctic hares reproducing at Alert.
Interestingly, we observed that 92% (23/25) of tracked individuals died during the 11 months that elapsed between the first hare was collared at Alert on 14 June 2019 and the last one was tracked near Lake Hazen on 20 May 2020. All but one of these mortalities occurred on winter grounds rather than on summer grounds or during fall relocation. The longevity of Arctic hares is unknown23 and, to our knowledge, annual adult survival was only estimated once (0.78), from extrapolation of daily survival rates and in the most southern part of the species range28. High adult mortality rate is not uncommon in the genus Lepus. For example, a 99.5% annual adult mortality rate was observed during the declining phase of a cyclic snowshoe hare population at Kluane, Yukon64. Observations at Alert that are independent from the current study also suggest a high mortality rate in the studied population. First, visual counts of Arctic hares from observation points at Alert strongly decreased from 2017 to 2019 (D. Berteaux, unpublished data), potentially indicating a population crash. Second, re-sightings of ear-tagged hares were rare during that period; of 28 hares ear-tagged (and not collared) in 2018, only two were re-sighted in 2019. We also note that dramatic density fluctuations of Arctic hares have previously been observed at Eureka, 775 km southwest of our study site25. Radio-collars have long been used to track several hare species26,52,65,66, including under Arctic climates67. Our collars weighed 2–3.1% of hare body mass, well in line with studies of snowshoe hares (< 3%68, < 5%51), European hares (Lepus europaeus) (< 3%69, 2.1%66), and mountain hares (< 3%69, 3%52). Therefore, we hypothesize that radio-collaring did not affect survival, but further monitoring of the study population is needed to confirm this.
Understanding animal movements in the High Arctic
New research avenues emerge from our results and can be broadly split into three categories, that is (1) further characterization of the movement strategy of Arctic hares, (2) understanding of the drivers of Arctic hare movements, and (3) theoretical and practical implications. Describing annual movements of a large sample of individuals in the polar desert is a prerequisite to assess the movement strategy of Arctic hares at their northern distribution limit. Critical questions are: 1—do the fall movements observed in 2019 occur every year, 2—do individuals surviving the winter come back to Alert, 3—what proportion of the population regularly undertakes long-distance movements, 4—do annual movements differ according to age and sex, and 5—to what extent do annual movements influence individual fitness.
Deciphering the drivers of Arctic hare movements in the polar desert will require a good understanding of how external factors (e.g., food, predators, snow conditions, access to social information) interact with the internal state (e.g., body condition, physiology, genetically inherited behavioral traits), the navigation capacity and the motion capacity of individuals70. A first step involves testing hypotheses about how the distribution of resources in the spatiotemporal landscape correlates with observed movements63,71. We hypothesize that hares overwintering in the Lake Hazen basin can access better food conditions and more favorable microclimates than those not doing so. Indeed, the geographical configuration of the terrain around Lake Hazen makes it a polar thermal oasis with milder temperatures during winter, a longer frost-free period and a higher plant productivity than the surrounding region72. The above, however, would not explain why individuals spend the summer at Alert. Given that most captured females were pregnant or lactating, a complementary hypothesis is therefore that Alert provides a refuge against predation for females raising leverets. Preliminary evidence suggests that Arctic wolves may be more abundant in Quttinirpaaq National Park than at Alert. Indeed, high concentrations of muskoxen have been reported on the Lake Hazen-Alert Plateau, a low-lying plateau extending north from Lake Hazen73. Arctic wolves rely heavily on muskoxen in the polar desert74 and it is often assumed that high muskoxen numbers support higher wolf numbers75. Arctic hares may be moving to poorer foraging areas during the breeding season as a predator-avoidance strategy, as seen in ungulates such as moose and caribou76,77.
The theoretical and practical implications of our results are many. Given the important role of Arctic hares in the polar desert, both as prey25 and herbivores78, and their large numbers at high latitudes23, Arctic hare movements have the potential to impact deeply the seasonal dynamics of local ecosystems. Herds of 100–300 hares are routinely reported in the literature23, groups of 1000 individuals have been repeatedly observed on Ellesmere Island79, and a biologist referred to the “moving hillside” phenomenon (R.I.G. Morrison, pers. com.) after observing large groups of hares running across slopes in our study area. A large population of moving herbivores could strongly affect plant communities, other herbivores, and predators. Given the above ecological implications, resolving Arctic hare movements would be a major addition to our understanding of regional ecosystems.
A unified understanding of why animals migrate necessitates comparative studies across taxa while accounting for phylogeny22. This requires data spanning multiple taxa, whereas published information is, in mammals, severely biased toward Cetacea and Artiodactyla22. If confirmed, migration in Arctic hares would thus indicate development of long-distance migration in the order Lagomorpha, a useful addition to the growing database of migrating mammals. A practical implication of our work involves biodiversity conservation at the very northern margin of the Americas. Hares crossed the boundaries of Quttinirpaaq National Park during their relocation. While the Lake Hazen basin constitutes an area with a high degree of protection80, it is also important to recognize that movements extend beyond park boundaries and may necessitate an integrative conservation strategy. Finally, the increasing pressure of climate change on Arctic ecosystems32 may modify not only the environmental cues used to trigger movements (e.g., spring snow melt or fall snow establishment), but also the spatiotemporal variability of vegetation growth and the extent of the snow-free season, potentially leading to altered movements or a loss of migratory behavior81,82.
Conclusion
We provide the first evidence that Arctic hares are capable of seasonal long-distance movement. Individuals can routinely cover distances exceeding by far any previously reported in the order lagomorph. This new knowledge opens avenues for future research in several fields ranging from animal behavior to ecosystem ecology and conservation biology.
References
Jeltsch, F. et al. Integrating movement ecology with biodiversity research—Exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 1, 6 (2013).
Dingle, H. Migration: The Biology of Life on the Move Migration (Oxford University Press, 2014).
Joly, K. et al. Longest terrestrial migrations and movements around the world. Sci. Rep. 9, 15333 (2019).
Lundberg, J. & Moberg, F. Mobile link organisms and ecosystem functioning: Implications for ecosystem resilience and management. Ecosystems 6, 0087–0098 (2003).
Bauer, S. & Hoye, B. J. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552 (2014).
Nifong, J. C., Layman, C. A. & Silliman, B. R. Size, sex and individual-level behaviour drive intrapopulation variation in cross-ecosystem foraging of a top-predator. J. Anim. Ecol. 84, 35–48 (2015).
Giroux, M.-A. et al. Benefiting from a migratory prey: Spatio-temporal patterns in allochthonous subsidization of an arctic predator. J. Anim. Ecol. 81, 533–542 (2012).
Allen, A. M. & Singh, N. J. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 3, 155 (2016).
Bunnefeld, N. et al. A model-driven approach to quantify migration patterns: Individual, regional and yearly differences. J. Anim. Ecol. 80, 466–476 (2011).
Teitelbaum, C. S. & Mueller, T. Beyond migration: Causes and consequences of nomadic animal movements. Trends Ecol. Evol. 34, 569–581 (2019).
Berg, J. E., Hebblewhite, M., St. Clair, C. C. & Merrill, E. H. Prevalence and mechanisms of partial migration in ungulates. Front. Ecol. Evol. 7, 325 (2019).
Avgar, T., Street, G. & Fryxell, J. M. On the adaptive benefits of mammal migration. Can. J. Zool. 92, 481–490 (2014).
Barbour, M. G. & Billings, W. D. North American Terrestrial Vegetation (Cambridge University Press, 2000).
Smith, S. L., Throop, J. & Lewkowicz, A. G. Recent changes in climate and permafrost temperatures at forested and polar desert sites in northern Canada. Can. J. Earth Sci. 49, 914–924 (2012).
Lévesque, E. Plant Distribution and Colonization in Extreme Polar Deserts, Ellesmere Island, Canada (University of Toronto, 1997).
Bliss, L. C., Svoboda, J. & Bliss, D. I. Polar deserts, their plant cover and plant production in the Canadian High Arctic. Holarctic Ecol. 7, 305–324 (1984).
Berteaux, D. et al. Effects of changing permafrost and snow conditions on tundra wildlife: Critical places and times. Arctic Sci. 3, 65–90 (2017).
Duchesne, D., Gauthier, G. & Berteaux, D. Habitat selection, reproduction and predation of wintering lemmings in the Arctic. Oecologia 167, 967–980 (2011).
Fuglei, E., Blanchet, M.-A., Unander, S., Ims, R. A. & Pedersen, Å. Ø. Hidden in the darkness of the Polar night: A first glimpse into winter migration of the Svalbard rock ptarmigan. Wildl. Biol. 2017, SP1 (2017).
Schmidt, N. M. et al. Ungulate movement in an extreme seasonal environment: Year-round movement patterns of high-arctic muskoxen. Wildl. Biol. 22, 253–267 (2016).
Berteaux, D. & Lai, S. Walking on water: Terrestrial mammal migrations in the warming Arctic. Anim. Migr. 8, 65–73 (2021).
Gnanadesikan, G. E., Pearse, W. D. & Shaw, A. K. Evolution of mammalian migrations for refuge, breeding, and food. Ecol. Evol. 7, 5891–5900 (2017).
Best, T. L. & Henry, T. H. Lepus arcticus. Mamm. Species 1–9 (1994).
Dalerum, F. et al. Exploring the diet of arctic wolves (Canis lupus arctos) at their northern range limit. Can. J. Zool. 96, 277–281 (2018).
Mech, L. D. Annual arctic wolf pack size related to arctic hare numbers. Arctic 60, 309–311 (2007).
Small, R. J., Keith, L. B. & Barta, R. M. Demographic responses of Arctic hares Lepus arcticus placed on two predominantly forested islands in Newfoundland. Ecography 15, 161–165 (1992).
Small, R. J., Keith, L. B. & Barta, R. M. Dispersion of introduced arctic hares (Lepus arcticus) on islands off Newfoundland’s south coast. Can. J. Zool. 69, 2618–2623 (1991).
Hearn, B. J., Keith, L. B. & Rongstad, O. J. Demography and ecology of the arctic hare (Lepus arcticus) in southwestern Newfoundland. Can. J. Zool. 65, 852–861 (1987).
Harper, F. The Mammals of Keewatin Vol. 12 (Miscellaneaous Publications, Museum of Natural History, University of Kansas, 1956).
Dalerum, F. et al. Spatial variation in Arctic hare (Lepus arcticus) populations around the Hall Basin. Polar Biol. 40, 2113–2118 (2017).
Fraser, K. C. et al. Tracking the conservation promise of movement ecology. Front. Ecol. Evol. 6, 150 (2018).
CAFF. Arctic Biodiversity Assessment. Status and trends in Arctic biodiversity. Conservation of Arctic Flora and Fauna, Akureyri (2013).
Desjardins, É. et al. Survey of the vascular plants of Alert (Ellesmere Island, Canada), a polar desert at the northern tip of the Americas. CheckList 17, 181–225 (2021).
Keith, L. B., Meslow, E. C. & Rongstad, O. J. Techniques for snowshoe hare population studies. J. Wildl. Manag. 32, 801–812 (1968).
Davidson, S. C. et al. Ecological insights from three decades of animal movement tracking across a changing Arctic. Science 370, 712–715 (2020).
Wikelski, M., Davidson, S. C. & Kays, R. Movebank: Archive, analysis and sharing of animal movement data. Hosted by the Max Planck Institute of Animal Behavior. http://www.movebank.org (2021).
Berteaux, D. Data from: Study ‘Arctic hare Alert—Argos tracking’. MoveBank Data Repository https://doi.org/10.5441/001/1.d5d912c4 (2021).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2021).
Christin, S., St-Laurent, M.-H. & Berteaux, D. Evaluation of Argos telemetry accuracy in the High-Arctic and implications for the estimation of home-range size. PLoS One 10, e0141999 (2015).
QGIS Association. QGIS Geographic Information System (2021).
Harris, S. et al. Home-range analysis using radio-tracking data? A review of problems and techniques particularly as applied to the study of mammals. Mamm. Rev. 20, 97–123 (1990).
Le Corre, M., Dussault, C. & Côté, S. D. Detecting changes in the annual movements of terrestrial migratory species: Using the first-passage time to document the spring migration of caribou. Mov. Ecol. 2, 19 (2014).
Nicholson, K. L., Arthur, S. M., Horne, J. S., Garton, E. O. & Vecchio, P. A. D. Modeling caribou movements: Seasonal ranges and migration routes of the central Arctic herd. PLoS One 11, e0150333 (2016).
Nelson, M. E., Mech, L. D. & Frame, P. F. Tracking of white-tailed deer migration by global positioning system. J. Mammal. 85, 505–510 (2004).
Singh, N. J. & Ericsson, G. Changing motivations during migration: Linking movement speed to reproductive status in a migratory large mammal. Biol. Lett. 10, 20140379 (2014).
Jakes, A. F. et al. Classifying the migration behaviors of pronghorn on their northern range. J. Wildl. Manag. 82, 1229–1242 (2018).
Bates, D., Maechler, M., Bolker, B. & Walker, S. lme4: Linear mixed-effects models using Eigen and S4. (2015).
Duong, T. ks: Kernel density estimation and kernel discriminant analysis for multivariate data in R. J. Stat. Softw. 21, 1–16 (2007).
Gitzen, R. A., Millspaugh, J. J. & Kernohan, B. J. Bandwidth selection for fixed-kernel analysis of animal utilization distributions. J. Wildl. Manag. 70, 1334–1344 (2006).
Austin, R. E. et al. Patterns of at-sea behaviour at a hybrid zone between two threatened seabirds. Sci. Rep. 9, 14720 (2019).
Gillis, E. A. & Krebs, C. J. Natal dispersal of snowshoe hares during a cyclic population increase. J. Mammal. 80, 933–939 (1999).
Dahl, F. & Willebrand, T. Natal dispersal, adult home ranges and site fidelity of mountain hares (Lepus timidus) in the boreal forest of Sweden. Wildl. Biol. 11, 309–317 (2005).
Angerbjörn, A. & Flux, J. E. C. Lepus timidus. Mamm. Species 495, 1–11 (1995).
Smith, G. W., Stoddart, L. C. & Knowlton, F. F. Long-distance movements of black-tailed jackrabbits. J. Wildl. Manag. 66, 463 (2002).
Cote, J. et al. Behavioural synchronization of large-scale animal movements—Disperse alone, but migrate together?. Biol. Rev. 92, 1275–1296 (2017).
Bauer, S., McNamara, J. M. & Barta, Z. Environmental variability, reliability of information and the timing of migration. Proc. R. Soc. B 287, 20200622 (2020).
Couzin, I. D. Collective animal migration. Curr. Biol. 28, R976–R980 (2018).
Lai, S. et al. Unsuspected mobility of Arctic hares revealed by longest journey ever recorded in a lagomorph. Ecology 103(3), e3620 https://doi.org/10.1002/ecy.3620 (2022).
Abrahms, B. et al. Suite of simple metrics reveals common movement syndromes across vertebrate taxa. Mov. Ecol. 5, 12 (2017).
Chapman, B. B., Brönmark, C., Nilsson, J. -Å. & Hansson, L.-A. The ecology and evolution of partial migration. Oikos 120, 1764–1775 (2011).
Singh, N. J., Börger, L., Dettki, H., Bunnefeld, N. & Ericsson, G. From migration to nomadism: Movement variability in a northern ungulate across its latitudinal range. Ecol. Appl. 22, 2007–2020 (2012).
Bastille-Rousseau, G. et al. Flexible characterization of animal movement pattern using net squared displacement and a latent state model. Mov. Ecol. 4, 15 (2016).
Mueller, T. & Fagan, W. F. Search and navigation in dynamic environments—From individual behaviors to population distributions. Oikos 117, 654–664 (2008).
Krebs, C. J., Boonstra, R. & Boutin, S. Using experimentation to understand the 10-year snowshoe hare cycle in the boreal forest of North America. J. Anim. Ecol. 87, 87–100 (2018).
Reid, N. & Harrison, A. Post-release GPS tracking of hand-reared Irish hare Lepus timidus hibernicus leverets, Slemish, Co. Antrim, Northern Ireland. J. Wildl. Rehabil. 31, 25 (2011).
Weterings, M. J. A. et al. Strong reactive movement response of the medium-sized European hare to elevated predation risk in short vegetation. Anim. Behav. 115, 107–114 (2016).
Krebs, C. J., Boutin, S. & Boonstra, R. Ecosystem Dynamics of the Boreal Forest: The Kluane Project (Oxford University Press, 2001).
Feierabend, D. & Kielland, K. Movements, activity patterns, and habitat use of snowshoe hares (Lepus americanus) in interior Alaska. J. Mammal. 95, 525–533 (2014).
Levänen, R., Pohjoismäki, J. L. O. & Kunnasranta, M. Home ranges of semi-urban brown hares (Lepus europaeus) and mountain hares (Lepus timidus) at northern latitudes. Ann. Zool. Fenn. 56, 107–120 (2019).
Nathan, R. et al. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. U.S.A. 105, 19052–19059 (2008).
Abrahms, B. et al. Emerging perspectives on resource tracking and animal movement ecology. Trends Ecol. Evol. 36, 308–320 (2021).
France, R. L. The Lake Hazen trough: A late winter oasis in a polar desert. Biol. Conserv. 63, 149–151 (1993).
Jenkins, D. A., Campbell, M., Hope, G., Goorts, J. & McLoughlin, P. Recent trends in abundance of Peary caribou (Rangifer tarandus pearyi) and muskoxen (Ovibos moschatus) in the Canadian Arctic Archipelago, Nunavut 233.
Mech, L. Proportion of calves and adult muskoxen, Ovibos moschatus killed by gray wolves, Canis lupus, in July on Ellesmere Island (USGS Northern Prairie Wildlife Research Center, 2010).
Gunn, A., Miller, F., Barry, S. & Buchan, A. A near-total decline in caribou on Prince of Wales, Somerset, and Russell Islands, Canadian Arctic. Arctic 59, 1–13 (2006).
Edwards, J. Diet shifts in moose due to predator avoidance. Oecologia 60, 185–189 (1983).
Gustine, D. D., Parker, K. L., Lay, R. J., Gillingham, M. P. & Heard, D. C. Calf survival of woodland caribou in a multi-predator ecosystem. Wildl. Monogr. 165, 1–32 (2006).
Klein, D. & Bay, C. Diet selection by vertebrate herbivores in the High Arctic of Greenland. Ecography 14, 152–155 (1991).
Parks Canada. Resource Description and Analysis—Ellesmere Island National Park Reserve Vol. 1 (Natural Resource Conservation Section, Parks Canada, Department of Canadian Heritage, 1994).
Parks Canada. Quttinirpaaq National Park of Canada: Management plan 76. https://www.pc.gc.ca/en/pn-np/nu/quttinirpaaq/info/index/gestion-management-2009 (2009).
Winkler, D. W. et al. Cues, strategies, and outcomes: How migrating vertebrates track environmental change. Mov. Ecol. 2, 10 (2014).
Robinson, R. et al. Travelling through a warming world: Climate change and migratory species. Endang. Species Res. 7, 87–99 (2009).
Acknowledgements
We thank field assistants and CFS Alert personnel for their support. Three reviewers and the Editor provided very helpful comments. This study was financially supported by: Canada Research Chairs Program, Department of National Defence of Canada, Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation (Project #38881), Network of Centers of Excellence of Canada ArcticNet, Northern Scientific Training Program (Polar Knowledge Canada), BIOS2 NSERC CREATE program (BIOS2 NSERC CREATE 509948-2018), and The Kenneth M. Molson Foundation.
Author information
Authors and Affiliations
Contributions
D.B., J.C.C., S.L., F.V. and A.T. contributed to the study design. J.C.C., S.L. and D.B. conducted the field work, with the aid of F.V. and A.T. J.C.C. analyzed the movement data and J.C.C., S.L. and D.B. created the main figures. J.C.C., S.L. and D.B. wrote the text and all authors reviewed and contributed to the final text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caron-Carrier, J., Lai, S., Vézina, F. et al. Long-distance, synchronized and directional fall movements suggest migration in Arctic hares on Ellesmere Island (Canada). Sci Rep 12, 5003 (2022). https://doi.org/10.1038/s41598-022-08347-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08347-1
- Springer Nature Limited