Abstract
With the increasing use of computed tomography, bronchiectasis has become a common finding in patients with chronic obstructive pulmonary disease (COPD). However, the clinical aspects and medical utilization of COPD with bronchiectasis (BE) remain unclear. We aimed to investigate the BE effect on prognosis and medical utilization in patients with COPD. Among 263,747 COPD patients, we excluded patients lacking chest X-ray, CT, or pulmonary function test codes and classified 2583 GOLD-C/D patients matched according to age, sex, and medical aid as having COPD-BE (447 [17.3%]) and COPD without BE (2136 [82.7%]). Patients with COPD-BE showed a higher rate of acute exacerbation requiring antibiotics than those without BE. Moreover, multivariable analysis showed that BE co-existence was a crucial factor for moderate-to-severe exacerbation (incidence rate ratio [IRR] 1.071; 95% CI 1.012–1.134; p = 0.019). Patients with COPD-BE had a significantly higher rate of exacerbations requiring antibiotics, as well as treatment cost and duration (meant as number of days using hospitalization plus outpatient appointment), than those with COPD without BE (52.64 ± 65.29 vs. 40.19 ± 50.02 days, p < 0.001; 5984.08 ± 8316.96 vs. 4453.40 ± 7291.03 USD, p < 0.001). Compared with patients with COPD without BE, patients with COPD-BE experienced more exacerbations requiring antibiotics, more hospitalizations, and a higher medical cost.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation and is comprised of a complex disease group with varying effects and pathophysiologies. In patients with COPD, the bronchiectatic feature of airway wall thickening and dilatation is positively correlated with ageing and airflow obstruction severity1,2. With the increased use of computed tomography (CT), bronchiectasis (BE) has become a common finding among patients with COPD3,4. Moreover, a recent study using national patient sample data reported that 19.3% of Korean patients with BE presented with COPD as a comorbidity5.
It has been found that the prevalence of BE with COPD (COPD-BE) ranges from 25.6 to 69%4,6,7,8,9,10,11, with patients with COPD-BE presenting more exacerbations and worse lung function than those without BE. Further, a study on the Canadian Cohort of Obstructive Lung Disease (CanCOLD) reported that the BE prevalence in patients with mild-to-moderate and severe COPD was 14.1–22.2% and 35.1%, respectively12. In the CanCOLD cohort, COPD-BE was not associated with an increased risk of acute exacerbation; however, it was associated with dyspnoea and other respiratory symptoms. Other small-scale studies on COPD-BE have reported a wide prevalence range and varying characteristics with respect to exacerbation episodes and respiratory symptoms4,12,13. There is a need for a nationwide large-scale study to elucidate the clinical aspects, prognosis, and medical utilization in patients with COPD-BE.
Therefore, this nation-wide representative study aimed to investigate BE-associated outcomes and medical utilization in patients with COPD.
Results
Baseline characteristics of study population
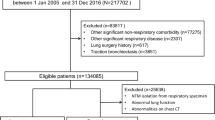
Among 263,747 patients with COPD, we excluded 243,247 participants who did not undergo chest X-ray/CT examination or a PFT and 16,754 participants without ≥ 2 exacerbations or ≥ 1 hospitalization within previous 1 year. Consequently, we enrolled the remaining 3746 GOLD C/D patients. Among them, 447 patients with COPD-BE underwent 1:5 PS matching according to age, sex, and medical aid while 2136 patients with COPD without BE were matched (Fig. 1). After PS matching, we analysed 2583 patients with GOLD C/D COPD. There was no significant between-group difference in age, sex, and medical aid. Table 1 presents the baseline characteristics of the two groups. The use of secondary (43.6% vs. 38.0%; p = 0.027) or tertiary hospitals (86.4% vs. 73.0%; p < 0.001) was higher in the COPD-BE group than in the COPD without BE group. Moreover, compared with the COPD without BE group, the COPD-BE group showed a higher number of moderate-to-severe exacerbations within the previous year (6.08 ± 6.73 vs. 5.42 ± 6.28; p = 0.047), prevalence of gastro-oesophageal reflux disease (43.6% vs. 38.2%; p = 0.031) and anaemia (10.3% vs. 7.3%; p = 0.032). Supplementary Table 1 showed the prescribed medications of each group.
Exacerbation related clinical outcomes
Table 2 presents the between-group differences in the rate of moderate-to-severe exacerbation. Compared with the COPD without BE group, the COPD-BE group showed a significantly higher percentage of patients with moderate exacerbation who were prescribed with systemic steroids or antibiotics (84.8% vs. 77.7%, p < 0.001). Notably, compared with the COPD without BE group, the COPD-BE group showed a significantly higher percentage of patients with exacerbation who were only prescribed with antibiotics (51.7% vs. 38.0%, p < 0.001). Compared with the COPD without BE group, the COPD-BE group showed a higher percentage of patients with severe exacerbation requiring hospitalization and prescriptions of systemic steroids or antibiotics (76.5% vs. 63.0%, p < 0.001 for steroid/antibiotic prescriptions; 38.9% vs. 28.8%, p < 0.001 only antibiotic prescriptions). There were significant between-group differences in the percentage of patients with severe exacerbation requiring ICU care who were prescribed either systemic steroids or antibiotics (14.1% vs. 10.4%, p = 0.023) (Table 2). Multivariable analysis revealed that BE co-existence was a crucial factor for moderate-to-severe exacerbation (incidence rate ratio [IRR] 1.071, 95% CI 1.012–1.134, p = 0.019) (Table 3).
Medical utilization
Compared with the COPD without BE group, the COPD-BE group showed significantly more overall days of medical utilization (52.64 ± 65.29 vs. 40.19 ± 50.02 days, p < 0.001), which was mainly attributed to a high number of hospitalization days (39.14 ± 64.09 vs. 27.91 ± 49.70 days, p < 0.001) compared with the number of outpatient visits (13.50 ± 15.03 vs. 12.28 ± 13.54 days, p = 0.115). There was a similar between-group trend in the medical cost of hospitalization and outpatient visits (5586.30 ± 8232.74 vs. 4115.46 ± 7220.81 USD, p < 0.001; 397.78 ± 586.11 vs. 337.93 ± 697.80 USD, p = 0.058) (Table 4). Regression analysis revealed that gender (male), medical aid, visits to a tertiary hospital, and medications were significant factors (Table 5). Multivariable analysis of medical utilization revealed that co-existing BE was a significant factor in increasing medical use days (β = 7.304, p = 0.006) (Table 6).
Discussion
This study investigated the effect of comorbid BE on clinical outcomes and medical utilization in patients with COPD. Our study yielded two main findings. First, compared with the COPD without BE group, the COPD-BE group showed has an increased risk of moderate-to-severe exacerbation and severe exacerbation requiring ICU admission. Second, the cost of medical utilization was significantly higher in patients with COPD with BE compared with patients without BE.
An emerging approach for COPD treatment is the determination of specific phenotypes. BE features in radiologic imaging is among the most prominent phenotypes and is frequently identified using the diagnostic assessment of chest CT scans. Previous studies have reported a COPD-BE prevalence widely ranging from 25.6 to 69%4; moreover, COPD-BE has revealed frequent exacerbations and poor prognoses4,7,14. However, most of these were small-scale studies with others not investigating the significance of COPD exacerbation with BE7. To our knowledge, this is the first study to analyse the relationship between COPD and BE using large-scale national claim data. The COPD-BE group showed an increased rate of moderate-to-severe exacerbation, especially severe exacerbation requiring hospitalizations and ICU admission. Moreover, BE coexistence was a significant risk factor for moderate-to-severe exacerbations.
Acute COPD exacerbation is generally caused by bacterial infections15. Ni et al.4 reported that the structural abnormalities of BE in patients with COPD can result in chronic colonization with potentially pathogenic micro-organisms, which causes recurrent infection, frequent exacerbations, and poorer prognosis. This can be prevented by the administration of effective antibiotics as additional treatment in patients with COPD-BE16,17. In our study, moderate and severe exacerbation events resulted in a significant increase in the rate of oral antibiotic prescription, which was consistent with previous findings11.
The present study investigated access to BE-related medical costs and clinical outcomes. A matched-cohort study on US health-care claim data analysed health care utilization in patients with COPD-BE18 and found that COPD-BE treatment involved greater medical resources (USD 15,685 [14,693–16,678]) than only treating COPD (USD 6262 [5655–6868]). These expenses were related to acute care management or the prescription of antibiotics, steroids, or bronchodilators at outpatient visits. In our study, we monitored medications, including ICS, bronchodilators, and oral medications, with the COPD-BE group showing a higher prescription than the COPD without BE group. Additionally, the percentage of prescribed systemic steroids or antibiotics was high in patients with hospitalizations, including ER visits and hospitalizations requiring ICU care. BE contributed to higher medical costs, particularly related to hospitalization. Additionally, factors associated with increased medical costs were tertiary hospital visits, as well as the prescription of ICS, bronchodilators, and mucolytic medications.
In our study, there was a significant occurrence of anaemia and GERD in the COPD-BE group. A previous study reported that the GERD prevalence was higher in the BE group, especially when accompanied with nontuberculous mycobacterial (NTM) disease19. Although the causal relationship remains unclear, one explainable hypothesis is that recurrent acid reflux irritates the airway, which causes NTM lung infection that leads to BE. Furthermore, in patients with COPD-BE, anaemia might result from systemic inflammation or erythropoiesis20,21. The anaemia prevalence is much higher in patients with COPD-BE due to the synergic effect of systemic inflammation.
This study has several limitations. First, COPD is diagnosed as having a postbronchodilator FEV1/FVC < 0.7 in the spirometry test15. However, this was a national cohort study using claim data and we could not determine the COPD diagnosis through lung function results. To make up for this limitation, the study population was defined using the operational definitions (ICD-10 code for COPD plus COPD drug prescription history), which were validated from previous studies using Korean National Health Insurance data22,23,24,25,26,27,28,29,30,31,32,33,34. Moreover, the inclusion of patients who underwent PFT and radiological examinations, including chest X-ray/CT, allowed more precise definition of the COPD and BE diagnoses. Previous studies have indicated that BE was more common among elderly patients with COPD with lower FEV14,35. Since we could not determine each individual’s FEV1, we could not adjust the values. In case the COPD-BE group had lower FEV1 values than the COPD without BE group, we could not determine the causal effect of BE coexistence on increased exacerbation since the effect could have resulted from lower FEV1 values rather than BE coexistence in the COPD-BE group. Nonetheless, we attempted to adjust disease severity by including only GOLD C/D patients with COPD with exacerbations confirmed by ≥ 2 prescriptions of systemic steroids or antibiotics over one year. In the additional sensitivity analysis, even after including all patients with COPD, the COPD-BE group showed higher exacerbations and was an independent risk factor in multivariate analysis (data not shown). Second, potentially pathogenic micro-organisms can cause recurrent pulmonary infection4,11. Our study used Korean claim data; therefore, we could not determine the colonized pathogen in the COPD-BE group. Third, regression analysis revealed that any ICS or mucolytic use was associated with exacerbations. However, given that this was an observational study with cross-sectional analysis of 1-year insurance database rather than a cohort study with long-term follow-up, the ICS or mucolytics could have been prescribed more in patients with frequent exacerbations regardless of BE coexistence, which represents as a reverse causality issue. Traditionally, clinicians prescribe ICS and mucolytics for patients with a high exacerbation risk and bronchitis, respectively.
In conclusion, this study showed that patients with COPD-BE experienced a higher frequency of moderate-to-severe exacerbations than those with COPD only. Specifically, the COPD-BE group showed a higher rate of exacerbations requiring hospitalization, including ER visits and ICU care, which led to a high hospitalization rate with subsequently increased medical costs.
Methods
Study design and study population
We selected patients with COPD from the 2012 Health Insurance Review and Assessment (HIRA) database. COPD was identified as a major or minor diagnosis based on the International Classification of Disease Tenth Revision (ICD-10) code and defined according to the following criteria: (1) age ≥ 40 years; (2) ICD-10 codes for COPD (J43.x-J44.x, except J430) as the principal or secondary (within the fourth position) diagnosis; (3) use of > 1 medication for COPD at least twice per year [long-acting muscarinic antagonist (LAMA), long-acting beta-2 agonist (LABA), inhaled corticosteroids (ICS) plus LABA (ICS + LABA), LABA plus LAMA (LABA + LAMA), short-acting muscarinic antagonist (SAMA), short-acting beta-2 agonist (SABA), SABA plus SAMA (SABA + SAMA), phosphodiesterase-4 inhibitor (PDE4-I), systemic bronchodilator, or theophylline]. To increase the credibility of the recruited group, we identified GOLD C/D patients who underwent the pulmonary function test (PFT). Moreover, we performed propensity-score (PS) matching between the patients with COPD with and without BE according to age, sex, and medical aid covering patients with low socioeconomic status. BE was defined as at least one claim under the J47 ICD-10 code (except E84 [cystic fibrosis]) with the inclusion of only patients who underwent chest X-ray or chest CT examination to ensure BE diagnosis accuracy. The patients with COPD were allocated to the COPD- BE group and COPD without BE group. Additionally, comorbidities were defined using the following ICD-10 diagnosis codes: ischemic heart disease [angina pectoris (I20.x), myocardial infarction (I21.x, I22.x, or I25.2)], congestive heart disease (I50.x), hypertension (I10.x-I15.x), diabetes mellitus (E10.x-E14.x), hyperlipidaemia (E78.x), osteoporosis (M80.x-M81.x), depressive disorder (F32.x, F33.x), gastro-oesophageal reflux disorder [GERD] (K21.x), pneumothorax (J93.9), arthritis [osteoarthritis (M15.x-M19.x), rheumatoid arthritis (M05.x, M06.x)], and anaemia (D50.x). The frequency of moderate-to-severe exacerbation within the previous year was counted using 2011 HIRA data.
Clinical outcomes and medical utilization measurement
In this study, the primary outcome was moderate-to-severe exacerbation. Moderate exacerbation was defined as an outpatient clinic visit with ICD-10 code for COPD (J43.x-J44.x, except J430) and prescription of systemic steroids and/or antibiotics. Severe exacerbation was defined as exacerbation requiring hospitalization or emergency room (ER) visits and prescription of systemic steroids and/or antibiotics. Additionally, severe exacerbation with a worsening condition resulting in admission to the Intensive care unit (ICU) was considered as severe exacerbation with ICU care.
We analyzed medical utilization and cost based on the 2012 HIRA database with the exclusion of medical utilization or costs not associated with COPD. We excluded patients with > 1 reimbursement per year for cancer (ICD-10 code: C00.x-C97.x), renal failure (N17.x-N19.x), and/or cerebrovascular disease (I60.x-I69.x) given the difficulty in distinguishing the significant expenses reimbursed for treatment of these diseases from those of COPD-related medical services. Regarding medication utilization and costs, we only analyzed COPD-related medications (LAMA, LABA, LABA + LAMA, ICS, ICS + LABA, SAMA, SABA, theophylline, LTRA, SABA + SAMA, PDE4-I, systemic steroids, systemic beta-agonist, mucolytics, and antitussive agent).
Regarding outpatient services, we only analyzed visits with a principal or secondary (within the fourth position) COPD diagnosis (J43.x-J44.x, except J430). For inpatient services, the analysis was limited to admission when the principal or secondary (within the fourth position) diagnosis was COPD (J43.x-J44.x, except J430) or a COPD-related disease (pneumonia: J12.x-J17.x; pulmonary thromboembolism: I26, I26.0, and I26.9; dyspnea: R06.0; or acute respiratory distress syndrome: J80). We collected data regarding age, sex, co-morbidity, hospitalizations, ER visits, ICU admissions, medical costs, and medication use. Further, we separately analysed the number of days that a particular outpatient and inpatient service was used (“used days”). All costs were presented in US dollars (USD) with an exchange rate of 1 USD for 1100 Korean won (exchange rate based on April 2018). This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (X-1801-447-907), which waived the requirement for informed consent since this study was based on anonymous health claim data. All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
The selected patients were allocated to the COPD-BE group and COPD without BE group; moreover, we performed 1:5 PS matching according to age, sex, and socioeconomic status (insurance type). Between-group differences in categorical and continuous variables were assessed using the chi-square test and Student’s t-test, respectively.
To assess factors associated with moderate-to-severe exacerbations, we calculated the incidence rate using negative binomial regression and used the adjusting variables as age, sex, medical aid, type of hospital use (tertiary), number of moderate to severe exacerbation in previous year, coexistence with BE, any ICS use, any bronchodilators use, and any steine use. We performed multiple linear regression analysis to identify factors affecting medical costs by using the adjusting variables as age, sex, medical aid, type of hospital use (tertiary), number of moderate to severe exacerbation in previous year, coexistence with BE, any ICS use, any bronchodilators use, and any steine use. For linear regression analysis, we log-transformed the dependent variables since health care utilization variables are usually right-skewed (i.e., distributed with a long and heavy right tail). All tests were two-sided with statistical significance being set at p < 0.05. Data were expressed as mean ± standard deviation. All statistical analyses were performed using SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA).
References
Hurst, J. R., Elborn, J. S., De Soyza, A., Consortium, B.-U. COPD-bronchiectasis overlap syndrome. Eur. Respir. J. 45, 310–313. https://doi.org/10.1183/09031936.00170014 (2015).
O’Brien, C., Guest, P. J., Hill, S. L. & Stockley, R. A. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 55, 635–642. https://doi.org/10.1136/thorax.55.8.635 (2000).
Stockley, R. A. Bronchiectasis; a progressive phenotype of chronic obstructive pulmonary disease. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa073 (2020).
Ni, Y. et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: A systemic review and meta-analysis. Int. J. Chron. Obstruct. Pulm. Dis. 10, 1465–1475. https://doi.org/10.2147/COPD.S83910 (2015).
Choi, H. et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J. 54, 1900194. https://doi.org/10.1183/13993003.00194-2019 (2019).
Bafadhel, M. et al. The role of CT scanning in multidimensional phenotyping of COPD. Chest 140, 634–642. https://doi.org/10.1378/chest.10-3007 (2011).
Gatheral, T. et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD 11, 605–614. https://doi.org/10.3109/15412555.2014.922174 (2014).
Martinez-Garcia, M. A. et al. Factors associated with bronchiectasis in patients with COPD. Chest 140, 1130–1137. https://doi.org/10.1378/chest.10-1758 (2011).
Fujimoto, K., Kitaguchi, Y., Kubo, K. & Honda, T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology 11, 731–740. https://doi.org/10.1111/j.1440-1843.2006.00930.x (2006).
Tulek, B., Kivrak, A. S., Ozbek, S., Kanat, F. & Suerdem, M. Phenotyping of chronic obstructive pulmonary disease using the modified Bhalla scoring system for high-resolution computed tomography. Can. Respir. J. 20, 91–96. https://doi.org/10.1155/2013/727523 (2013).
Martinez-Garcia, M. A. et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 823–831. https://doi.org/10.1164/rccm.201208-1518OC (2013).
Tan, W. C. et al. Findings on thoracic computed tomography scans and respiratory outcomes in persons with and without chronic obstructive pulmonary disease: A population-based cohort study. PLoS ONE 11, e0166745. https://doi.org/10.1371/journal.pone.0166745 (2016).
Agusti, A. et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 11, 122. https://doi.org/10.1186/1465-9921-11-122 (2010).
Du, Q., Jin, J., Liu, X. & Sun, Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: A systematic review and meta-analysis. PLoS ONE 11, e0150532. https://doi.org/10.1371/journal.pone.0150532 (2016).
Singh, D. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 53, 1900164. https://doi.org/10.1183/13993003.00164-2019 (2019).
Murray, M. P., Turnbull, K., Macquarrie, S. & Hill, A. T. Assessing response to treatment of exacerbations of bronchiectasis in adults. Eur. Respir. J. 33, 312–318. https://doi.org/10.1183/09031936.00122508 (2009).
Wilson, R. et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur. Respir. J. 40, 17–27. https://doi.org/10.1183/09031936.00090311 (2012).
Seifer, F. D., Hansen, G. & Weycker, D. Health-care utilization and expenditures among patients with comorbid bronchiectasis and chronic obstructive pulmonary disease in US clinical practice. Chron. Respir. Dis. 16, 1479973119839961. https://doi.org/10.1177/1479973119839961 (2019).
Koh, W. J. et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest 131, 1825–1830. https://doi.org/10.1378/chest.06-2280 (2007).
John, M. et al. Anemia and inflammation in COPD. Chest 127, 825–829. https://doi.org/10.1378/chest.127.3.825 (2005).
McDonnell, M. J. et al. Comorbidities and the risk of mortality in patients with bronchiectasis: An international multicentre cohort study. Lancet Respir. Med. 4, 969–979. https://doi.org/10.1016/S2213-2600(16)30320-4 (2016).
Rhee, C. K. et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD 11, 163–170. https://doi.org/10.3109/15412555.2013.831061 (2014).
Lee, J., Lee, J. H., Kim, J. A. & Rhee, C. K. Trend of cost and utilization of COPD medication in Korea. Int. J. Chron. Obstruct. Pulm. Dis. 12, 27–33. https://doi.org/10.2147/COPD.S121687 (2017).
Kim, J. A., Lim, M. K., Kim, K., Park, J. & Rhee, C. K. Adherence to inhaled medications and its effect on healthcare utilization and costs among high-grade chronic obstructive pulmonary disease patients. Clin. Drug Investig. 38, 333–340. https://doi.org/10.1007/s40261-017-0612-2 (2018).
Park, S. C. et al. Hemoglobin and mortality in patients with COPD: A nationwide population-based cohort study. Int. J. Chron. Obstruct. Pulm. Dis. 13, 1599–1605. https://doi.org/10.2147/COPD.S159249 (2018).
Kim, J. et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: A national cross-sectional cohort study. BMC Pulm. Med. 13, 51. https://doi.org/10.1186/1471-2466-13-51 (2013).
Park, S. C. et al. Mortality of patients with chronic obstructive pulmonary disease: A nationwide population based cohort study. Korean J. Intern. Med. 34, 1272–1278. https://doi.org/10.3904/kjim.2017.428 (2019).
Jo, Y. S., Rhee, C. K., Kim, K. J., Yoo, K. H. & Park, Y. B. Risk factors for early readmission after acute exacerbation of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 14, 1753466620961688. https://doi.org/10.1177/1753466620961688 (2020).
Jo, Y. S. et al. Relationship between changes in inhalation treatment level and exacerbation of chronic obstructive pulmonary disease: Nationwide the health insurance and assessment service database. Int. J. Chron. Obstruct. Pulm. Dis. 15, 1367–1375. https://doi.org/10.2147/COPD.S248616 (2020).
Park, H. J. et al. Frequent outpatient visits prevent exacerbation of chronic obstructive pulmonary disease. Sci. Rep. 10, 6049. https://doi.org/10.1038/s41598-020-63064-x (2020).
Park, H. Y. et al. Impact of chronic obstructive pulmonary disease on mortality: A large national cohort study. Respirology 25, 726–734. https://doi.org/10.1111/resp.13678 (2020).
Park, H. Y. et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: A cohort study. Thorax 75, 506–509. https://doi.org/10.1136/thoraxjnl-2019-213732 (2020).
Kim, J. et al. The health care burden of high grade chronic obstructive pulmonary disease in Korea: Analysis of the Korean Health Insurance Review and Assessment Service data. Int. J. Chron. Obstruct. Pulm. Dis. 8, 561–568. https://doi.org/10.2147/COPD.S48577 (2013).
Kim, C. et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int. J. Tuberc. Lung Dis. 18, 737–743. https://doi.org/10.5588/ijtld.13.0634 (2014).
Patel, I. S. et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 170, 400–407. https://doi.org/10.1164/rccm.200305-648OC (2004).
Funding
This study used HIRA research data (M20180126888) made by Health Insurance Review & Assessment Service (HIRA). This research was supported by a grant from the Korea Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI18C0522).
Author information
Authors and Affiliations
Contributions
Y.K. contributed to the conception of the study, data interpretation, and drafted the manuscript. K.K. contributed to data collection and data analysis. S.W.R. and C.K.R. contributed to the conception of the study, data interpretation, and revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y., Kim, K., Rhee, C.K. et al. Increased hospitalizations and economic burden in COPD with bronchiectasis: a nationwide representative study. Sci Rep 12, 3829 (2022). https://doi.org/10.1038/s41598-022-07772-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07772-6
- Springer Nature Limited
This article is cited by
-
Clinical significance of chronic bronchitis in different racial groups
BMC Pulmonary Medicine (2024)
-
Effect of Broncho-Vaxom (OM-85) on the frequency of chronic obstructive pulmonary disease (COPD) exacerbations
BMC Pulmonary Medicine (2023)