Abstract
The effect of a hydrolyzed protein diet on the fecal microbiota has not been studied in feline chronic enteropathy (CE). Our study aimed to (1) compare the fecal microbiota of cats with CE to control cats with no gastrointestinal signs and (2) determine the effect of a hydrolyzed protein diet on the fecal microbiota of cats with CE and whether this differs between dietary responders and non-responders. The fecal microbiome of cats with CE (n = 36) showed decreased α-diversity in terms of genus richness (P = 0.04) and increased β-diversity in terms of Bray–Curtis Dissimilarity (P < 0.001) compared to control cats (n = 14). Clostridium was the only genera significantly over-represented in cats with CE compared to control cats (adjusted P < 0.1). After 6-weeks of feeding the diet, fifteen cats were classified as responders and 18 as non-responders, based on clinical signs. At the genus level, α-diversity was increased in non-responders versus responders at diagnosis, but decreased after dietary intervention in both groups (P < 0.05). At the family level, non-responders became increasingly dissimilar after dietary intervention (P = 0.012). In general, the abundance of bacteria decreased with feeding a hydrolyzed diet, with the genera most significantly affected being more frequently observed in non-responders. Bifidobacterium was the only genus that increased significantly in abundance post-diet and this effect was observed in both responders and non-responders. Both Oscillibacter and Desulfovibrionaceae_unclassified were most abundant in non-responders at diagnosis but were rarely observed post diet in neither responders nor non-responders. Cats with CE had similar microbiota changes to those described in human inflammatory bowel disease. Whether the presence of Oscillibacter and Desulfovibrionaceae_unclassified are indicators of non-response to the diet at diagnosis requires further investigation. Despite the hydrolyzed diet reducing α-diversity in all cats with CE, this did not resolve gastrointestinal signs in some cats. However, responders metabolized the diet in a similar manner, reflected by sustained β-diversity, while the microbiome of non-responders became increasingly dissimilar compared to diagnosis at the family level. Therefore, the microbiome may not be as tightly regulated in cats with CE that are non-responders and therefore, these cats would require additional therapy for remission of clinical signs.
Similar content being viewed by others
Introduction
Feline chronic enteropathy (CE) describes a spectrum of diseases resulting in idiopathic chronic gastrointestinal (GI) signs1. Although, the pathophysiology is not well established, it is hypothesized to involve the interplay of four key components; genetic susceptibility, environmental risk factors, altered GI mucosal immune response and aberrant gut microbial composition2.
Gut microbial disruptions have been documented in humans with inflammatory bowel disease (IBD) and dogs with CE3,4,5,6,7,8. However, it is currently unknown if these changes are the cause or consequence of the chronic inflammation. Although, studies in cats have characterized the fecal microbiome, most have been in cats with acute or chronic diarrhea, where the underlying disease process was not confirmed9,10. However, one recent study in cats with confirmed CE documented the presence of fecal dysbiosis, with changes similar to those found in humans with IBD11. Therefore, further studies analyzing the fecal microbiota of cats with CE are needed to allow for a more comprehensive understanding of the GI environment and the potential benefit of dietary therapies aimed at modulating this intestinal population.
Currently, the definitive diagnosis of CE in cats requires intestinal histopathology and treatment often requires a sequential or combination approach with diet, antibiotics and immunosuppressive drugs depending on the severity of the disease2. Cats with chronic gastrointestinal signs without gastrointestinal biopsies performed, but where diagnostic investigations do not reveal a cause are commonly referred to as suspected CE and treated empirically. Cats with confirmed or suspected CE that respond to dietary therapy alone are described as having a food-responsive enteropathy (FRE) and are thought to comprise the majority of CE cases. Commercial hydrolyzed protein, limited ingredient novel protein, highly digestible or high fiber diets have all been used successfully for feline CE12,13,14,15,16,17,18,19. Although, only one small published study specifically evaluated the response to hydrolyzed protein diets in cats with CE19, this category of diets is frequently used for CE in cats20. Remission induced by a hydrolyzed protein diet in dogs with CE is accompanied by alterations in microbial community structure marked by a decreased abundance of pathobionts (e.g., Escherichia coli and Clostridium perfringens) and reduced severity of dysbiosis21. Similar studies are unavailable for cats with CE. As such, determining the microbial and metabolic effects of a hydrolyzed protein diet in cats with CE may provide insight into diet-induced remission of GI disease in this species, which could then help to guide the development of more effective therapeutic diets.
Unfortunately, cats with FRE cannot be differentiated from CE requiring steroid treatment (SRE) based on history, clinical signs, laboratory parameters or the severity of GI endoscopic or intestinal histopathologic lesions22. Therefore, a therapeutic food trial is currently needed to differentiate the two conditions. The ability to differentiate FRE from SRE in cats at the time of diagnosis or shortly after may help to avoid unnecessary exclusion diet trials and delay in effective treatment to which the cat may respond. In pediatric patients with Crohn’s disease, the composition of the fecal microbiota was found to differ between responders and non-responders one week after exclusive enteral nutrition23. This highlights the promise of the microbiota as early predictors of response to dietary therapy. Therefore, further studies are needed to determine if the fecal microbiota at diagnosis or during dietary therapy may help to predict responders versus non-responders to a therapeutic hydrolyzed protein diet trial in cats with CE. This study sought to compare the fecal microbiota of cats with CE to the fecal microbiota of control cats with no GI signs and to determine the effect of a hydrolyzed protein diet on the fecal microbiota of cats with CE. The potential of the pre therapeutic diet fecal microbiota to discriminate between dietary responders and non-responders was also explored.
Materials and methods
Recruitment of case and control cats
Cats referred to the Langford Vets Small Animal Hospital, University of Bristol and Royal Veterinary College (RVC), University of London for persistent or intermittent GI signs (vomiting and/or diarrhea) of at least 2 weeks duration and where CE was suspected based on thorough investigation were considered for the study. Those cats where a commercial therapeutic hydrolyzed protein diet (Royal Canin Veterinary Diet Feline Hypoallergenic dry food (composition: rice, hydrolysed soya protein isolate, animal fats, vegetable fibers, minerals, hydrolysed poultry liver, soya oil, beet pulp, fish oil, fructo-oligosaccharides, borage oil, marigold extract (source of lutein). Key values per 100 g as fed: protein 25.5 g, fat 20 g, carbohydrate 34.5 g, dietary fiber 8.2 g, metabolizable energy 410 kcal)) trial was then used for treatment without concurrent antimicrobial and immunosuppressive therapy were prospectively recruited. All cats underwent the same diagnostic investigations consisting of a minimum of complete blood count, serum biochemistry with electrolytes, serum cobalamin (with or without folate) concentration and trans-abdominal ultrasound examination. Feline pancreatic lipase immunoreactivity (PLI, n = 15), trypsin-like immunoreactivity (TLI, n = 24), serum thyroxine (total T4, n = 18), basal cortisol (n = 0), pre- or pre and post-prandial bile acid (n = 16) concentrations, fecal parasitology using saturated zinc sulfate flotation (n = 17), fecal culture (for Salmonella, Campylobacter and Clostridium difficile, n = 9), empirical deworming (n = 9), polymerase chain reaction for Tritrichomonas foetus (n = 15) and feline leukemia and feline immunodeficiency virus testing (n = 13) were performed in some cats as indicated by the history, physical examination and ultrasound examination findings. Cats that had histopathologic confirmation of CE via intestinal biopsy specimens collected via endoscopy or exploratory laparotomy were included as confirmed CE, whereas those that had no intestinal biopsies performed, but investigations described above revealed no underlying cause for the chronic GI signs were included as suspected CE. The feline chronic enteropathy activity index (FCEAI) was calculated for all cats with suspected or confirmed CE24.
Adult cats (at least 1 year of age) without a history of GI signs (n = 14) and owned by staff members were recruited as controls.
The University of Bristol and Royal Veterinary College granted ethical approval for the study (VIN/14/017 and URN 2018 1837-3, respectively). Written informed consent for participation into the study was obtained from all owners of cats.
Dietary recommendations for cats with CE
All cats with suspected or confirmed CE were discharged from the hospital with the same therapeutic hydrolyzed protein diet (Royal Canin Veterinary Diet Feline Hypoallergenic dry food). All owners were instructed to feed the therapeutic diet exclusively for 6 weeks and were specifically instructed against feeding treats and other foods. However, owners were not specifically instructed against the use of oral flavoured medication, such as deworming medication or toothpaste. Owners were asked to follow the feeding instructions on the pet food label according to current body weight and to adjust daily calories to maintain current body weight and condition if the cat was at an ideal condition or over-conditioned and to achieve and then maintain an ideal body condition if the body condition score was 3/9 or below25. Control cats were not given any dietary intervention.
Fecal collection
For each cat with suspected or confirmed CE, naturally voided feces were collected during hospitalization and frozen immediately. The owners of all cats with suspected or confirmed CE were asked to schedule a recheck appointment after receiving the hydrolyzed protein diet for 6 weeks or were asked to collect feces at home at that time point if they were unable to return to the referral hospital. For those cats that returned, the owner brought naturally voided feces from the cat to the appointment, which they had frozen immediately and then transported, on ice packs to the referral hospital. For those cats that were unable to return, but owners were able to collect feces at home, samples were stored in multiple plastic bags in the freezer at the owners’ homes and transported to their local veterinary practice on ice for collection by one of the authors (A.K.). All samples were transported back to the referral hospital on dry ice.
For the control cats, all owners collected feces at home. They were frozen immediately and brought to the referral practice using ice packs.
Assessing response to hydrolyzed protein diet in cats with CE
Owners of cats with suspected or confirmed CE were contacted via email, telephone or in person at their scheduled 6 week recheck visit to assess the response of the cat to the hydrolyzed protein diet after 6 weeks of feeding. Responders were those cats where the owners reported a significant reduction in GI signs, which did not warrant any change or additional therapy. Non-responders included those cats that were reported to have no or partial response necessitating discontinuation of the hydrolyzed protein diet with a change to a different therapeutic diet or continuation of the hydrolyzed protein diet with the addition of prednisolone, or further diagnostic investigations, such as GI biopsy if not previously performed.
16S rRNA gene amplicon sequencing of fecal microbiota
Fecal samples were thawed and extracted using the Qiagen PowerFecal Pro DNA kit (Qiagen #51804) according to the manufacturer’s instructions. Sample homogenization was performed using a Qiagen Vortex adapter coupled to a Vortex Genie 2 for 20 min at maximum speed. Initial deoxyribonucleic acid (DNA) quantification was performed on a NanoDrop (Thermo Scientific, Leicestershire, U.K.) and DNA concentration was adjusted to within the desired mass range for sequencing and plated on a 96 well, low profile, skirted polymerase chain reaction (PCR) plate (4titude, Surrey, U.K.) for sequencing submission. For bacterial 16S rRNA gene amplicon sequencing, the variable V3 and V4 regions of the 16S rRNA gene were amplified from genomic DNA using the primers (standard IUPAC nucleotide nomenclature): Forward Primer = 5' TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAGTCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG, Reverse Primer = 5’ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC.
The amplicons were then attached with indices and Illumina sequencing adapters using the Nextera XT index kit. The 16S amplicon libraries were pooled and sequenced in an Illumina MiSeq v3 flowcell as 300 paired-end reads. Library preparation and sequencing was performed at the Oxford Genomics Centre.
Sequence processing
Quality of raw sequencing data was assessed using FastQC (v0.11.07). Following quality assessment, downstream processing was performed using dada2 (PMID:27214047) which was implemented in pipeline_dada2.py from the OCMS_16S repository (https://github.com/OxfordCMS/OCMS_16S). Given the drop in quality at ~ 200 bp, we decided to proceed with the analysis using the first read in the pair (V3 region). The filterAndTrim function in dada2 was used to truncate the reads to 200 bp. Retained primer sequences were removed from reads (17 bp) which resulted in final read lengths of 183 bp. Error learning, de-replication and sample inference were performed using dada2 with default parameters. Taxonomy was assigned to amplicon sequence variants (ASVs) using the assignTaxonomy function in dada2 and the Genome Taxonomy Database (GTDB) training data (https://zenodo.org/record/2541239/files/GTDB_bac-arc_ssu_r86.fa.gz). ASVs were retained if they made up at least 0.001% of the data set total read count in at least 5% of samples. ASVs were aggregated at either the genus or family level, depending on the analysis conducted.
Microbiome analysis and statistics
Batch effects, potentially introduced by the veterinary center from which cats were recruited or by DNA extraction batch, were assessed using PERMANOVA on Bray–Curtis distances calculated from relative abundance of family-level taxa. This revealed that extraction batch did not have any significance, while the veterinary center did have a significant batch effect (data not shown). ComBat-Seq was used to correct for this batch effect, and batch-corrected counts were used in all subsequent analyses26.
α-Diversity was measured using Shannon’s diversity index and richness and β-diversity was measured using Bray–Curtis dissimilarity distance, which assess sample dissimilarity in a pairwise manner. Shannon’s diversity index, richness, and Bray–Curtis dissimilarity were calculated at the genus and family levels, using the vegan R package27. All pairwise significance testing was conducted using Wilcoxon Rank Sum test, with p-values adjusted by the Benjamini Hochberg (BH) method, and a significance threshold set at p-adjusted ≤ 0.05.
Microbial profiles were analyzed at the genus level, and aggregated counts were normalized using total sum-scaling and transformed by log10. Normalized, transformed, genus-level microbiome profiles were fitted to linear fixed effects modeling, with health status (healthy control vs. CE) as a fixed effect when comparing control and CE cats at baseline. When assessing the effect of diet in CE cats, a linear mixed effects model was used, where fixed effects were diet (pre vs. post), diet response (non-responder vs. responder), and diet-response interaction (change in non-responders vs. change in responders). Individual cat participants were included in the model as a random effect. All linear modeling were performed using Maaslin228. Significance was determined using BH-adjusted p-value (q ≤ 0.1).
Microbiome profiles were converted into presence-absence data to assess the prevalence of each genus across groups. Contingency tables were created for each genus to assess the frequency observed in each group condition. Significance of contingency tables were assessed by Fisher’s Exact Test to estimate the probability of observing prevalence deviating from a perfectly even distribution across groups. For example, in control vs. CE, the null hypothesis was observation of 50% prevalence in both control and CE groups. Significance was determined using BH-adjusted p-value (q ≤ 0.1).
When assessing associations between clinical severity of CE and microbiome, FCEAI (1–9) and diet response (non-responder vs. responder) and FCEAI-response interaction (severity of non-responders vs. severity of responders) were used as fixed effects.
Logistic regression analysis
Univariable binary logistic regression models were used to evaluate: 1. Presence or absence of fecal Clostridium in cats with CE and 2. Response or non-response to a hydrolyzed protein diet in cats with CE to determine if there were any associations with clinical measures. The clinical measures assessed were: referral center, signalment, duration and nature of signs, body condition score, laboratory parameters (serum albumin, cobalamin and folate concentrations and alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities), results from abdominal imaging and intestinal histopathology if available and FCEAI. Variables associated with the presence of fecal Clostridium or response to a hydrolyzed protein diet with P < 0.2 in simple logistic regression were subsequently entered into multivariable analyses. In the multivariable regression models, analyses were performed in a backward stepwise manner. All variables were initially included and the variable with the highest p-value was removed until all remaining variables had a P < 0.05. Analyses were performed using a computer software package (IBM SPSS Statistics Version 23).
Ethical approval and consent to participate
The University of Bristol and Royal Veterinary College granted ethical approval for the study (VIN/14/017 and URN 2018 1837-3, respectively).
Results
Cats with confirmed or suspected CE (n = 42) were prospectively enrolled from 2 referral veterinary hospitals in the U.K. Twenty-five cases were enrolled from the University of Bristol and 17 from the RVC. Three cases were excluded, as naturally voided feces could not be collected during hospitalization. The fecal microbiota were characterized in samples collected from the time of diagnostic investigations (n = 39) and after receiving a minimum of 6 weeks of the same commercial hydrolyzed protein diet (n = 25) in cats with CE and in control cats (n = 14).
Three cats in the CE group were subsequently excluded due to consequent diagnosis of small cell lymphoma (n = 2) or feline infectious peritonitis (n = 1). Thirty-six cats with suspected or confirmed CE were included in the study: 24 neutered male, 1 intact female and 11 neutered female. The age of the cats ranged from 0.8 years to 19 years, with a median age of 7 years. Eighteen cats were domestic shorthair, 5 domestic longhair, 4 Bengal, 3 Ragdoll, 2 Siamese and 1 each of the following breeds: Maine Coon, Abyssinian, Burmese and British Shorthair. Body condition score ranged from 2/9 to 9/9, with a median of 5/9.
The duration of GI signs for all cats in the CE group ranged between 0.5 months to 142 months (median, 6 months).
The control cats consisted of 14 staff owned cats: 10 male neutered, 3 female neutered and 1 female intact. The age of the cats ranged from 1 to 15 years with a median of 7 years. Breeds included 5 Domestic shorthair, 5 Domestic longhair and one each of the following breeds: British shorthair, Maine Coon, Russian Blue and Bengal. No control cats had a previous or current history of GI signs.
Histologic diagnosis
Ten cats had intestinal biopsies performed, which found lymphoplasmacytic enteritis in 3 cats, lymphoplasmacytic and eosinophilic enteritis in 2 cats, lymphoplasmacytic and neutrophilic enteritis in 2 cats, mixed enteritis in 1 cat, lymphoplasmacytic and neutrophilic colitis in 1 cat and no abnormalities in 1 cat.
FCEAI
The median FCEAI for all cats was 4, with a range of 1–9. For those cats that did not have endoscopy performed, a score of 0 was given for the variable endoscopic lesions24.
Response to therapeutic hydrolyzed protein diet
Three cats did not eat the diet and were excluded from responder/non-responder analysis. Fifteen cats (45%) had full remission of signs (responders) and 18 cats (55%) had partial/no response (non-responders) after 6 weeks of feeding.
Microbial communities: CE versus controls
The fecal microbiome of cats with CE showed decreased α-diversity in terms of genus richness (P = 0.04) and increased β-diversity in terms of Bray–Curtis Dissimilarity (P < 0.001) compared to control cats (Fig. 1a and b).
Diversity measures of control and chronic enteropathy (CE) cats at the genus level. (a) Genus richness (left panel) is significantly lower in CE cats at time of diagnosis as compared to control cats. Diversity as measured by Shannon’s Diversity Index (right panel) is not significant between CE and control cats at time of diagnosis. (b) Sample dissimilarity in control and CE cats shows that CE cats tend to be more dissimilar from other CE cats as compared to the dissimilarity of control cats from other cats in the same group.
Based on the linear model and contingency analyses, most genera are over-represented in control cats as compared to CE cats (Supplementary Fig. 1). This is consistent with the α-diversity and sample dissimilarity results described above. Bacterial genera associated with CE in dogs and cats and humans with IBD in the existing literature (Enterococcus, Escherichia and Clostridium) were also over-represented in the cats with CE as compared to control cats, albeit only Clostridium was statistically significant (adjusted p-value < 0.1, Table 1 and Fig. 2a and b).
Comparison of selected bacterial genera associated with chronic enteropathy (CE) in dogs and cats and humans with inflammatory bowel disease in the existing literature (Enterococcus, Escherichia and Clostridium) in cats with CE as compared to control cats. Although, all 3 selected genera are over-represented in cats with CE as compared to control cats, only Clostridium was statistically significant (q < 0.1). (a) Normalized abundance of Enterococcus, Escherichia, and Clostridium in control and CE cats at time of diagnosis. Statistical testing performed on linear fixed model, with significance at q < 0.1. (b) Contingency analysis showing prevalence of a given genus in control and CE cats.
Effect of diet on the fecal microbiota of cats with CE
Shannon’s Diversity Index for α-diversity decreased over time in both responders and non-responders (P < 0.05) at both the genus and family level (Figs. 3 and 4). At the genus level, β-diversity was different between the responders and non-responders at diagnosis (P = 0.004, Fig. 3), but not between the 2 groups after dietary intervention (P = 0.35, Fig. 3). However, at the family level, non-responders became increasingly dissimilar after dietary intervention (β-diversity, P = 0.012, Fig. 4).
Diversity measures at the genus level of chronic enteropathy (CE) cats at time of diagnosis and post dietary intervention. NR = non-responder, R = responder, 0 = time of diagnosis, 1 = post dietary intervention (a) Genus richness (left panel) and diversity measured by Shannon’s Diversity Index (right panel) are lower in responders than non-responders at time of diagnosis. Dietary intervention resulted in decreased richness and diversity in both groups such that post-treatment diversity measures are not significantly different between responders and non-responders. Adjusted p-value < 0.0001 represented with ***. Adjusted p-value < 0.001 represented with **. Adjusted p-value < 0.01 represented with *. Adjusted p-value > 0.05 represented with ns. (b) Sample dissimilarity shows that responders have greater within-group dissimilarity than non-responders at the time of diagnosis. Following dietary intervention, non-responders and responders have equivalent within-group dissimilarities.
Diversity measures at the family level of chronic enteropathy (CE) cats at time of diagnosis and post dietary intervention. NR = non-responder, R = responder, 0 = time of diagnosis, 1 = post dietary intervention (a) Family richness (left panel) is decreased in responders but remains unchanged in non-responders. Diversity as measured by Shannon’s Diversity Index (right panel) is decreased after dietary treatment, regardless of clinical response to the dietary intervention. Adjusted p-value < 0.0001 represented with ***. Adjusted p-value < 0.001 represented with **. Adjusted p-value < 0.01 represented with *. Adjusted P-value > 0.05 represented with ns. (b) Sample dissimilarity shows that non-responders become more dissimilar after dietary intervention. On the other hand, responders maintain the same level of sample dissimilarity after dietary intervention, even though responders are more heterogeneous in β-diversity than non-responders at the time of diagnosis.
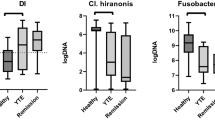
Fecal bacterial genera that were significantly associated with diet alone, response alone, and interaction of diet with response are shown in Table 2 and summarized in Fig. 5. The majority of the significant features identified by the model are due to the effect of the diet alone (Table 2 and Fig. 5). In general, the abundance of bacteria decreases with diet. In particular, the bacteria most significantly affected by diet (adjusted p-value < 0.1) were more frequently observed in non-responders (dark purple circles in panel a of Fig. 6). For example, Oscillibacter, Sutterella and Fusobacterium were all present in higher frequency in non-responders at baseline as compared to responders (Fig. 6, panel b).
Dietary response analysis in cats with chronic enteropathy (CE). Significance of feature coefficients in linear model where fixed effects are diet, response, and interaction of the two (ResponseDiet). q < 0.1 shown with a star. P-values > 0.05 are colored in grey. Association is -log(q-value)*sign(coefficient).
The effect of diet in cats with chronic enteropathy (CE). The majority of the significant features identified by the model are due to the effect of diet. In general, the abundance of bacteria decreases with diet. In particular, the bacteria most significantly affected by diet (q < 0.1) were more frequently observed in non-responders (dark purple circles in panel A). Bifidobacterium was the only significant genus that increased in abundance post-diet, an effect that was observed in both responders and non-responders.
Bifidobacterium was the only significant genus that increased in abundance post-diet, an effect that was observed in both responders and non-responders (Table 2, Figs. 5 and 6).
The interaction term describes if responders respond differently to the diet than non-responders. As seen in Fig. 5, there are a limited number of significant interaction terms, where the only significant genera are Oscillibacter and Desulfoviberionaceae_unclassified. Both are most abundant in non-responders at baseline and are rarely observed post diet in both non-responders and responders (Fig. 7).
Differing effects of diet for responders and non-responders in cats with chronic enteropathy (CE). There are a limited number of significant interaction terms, where the only significant genera are Oscillibacter and Desulfoviberionaceae_unclassified. Both are most prevalent in non-responders at baseline and are rarely observed post diet in both non-responders and responders.
Effect of clinical severity (FCEAI) on fecal microbiome
Linear fixed effect modeling was assessed at every taxonomic level where fixed effects are response, clinical severity (FCEAI), and interaction of two variables (FCEAI_Response). No terms were significant at the Genus level. However, the following taxa were significant in either the clinical severity or the response_severity coefficients: Campylobacterota, Campylobacteria, Tannerellaceae (Supplementary Table 1 and Supplementary Fig. 2). Although these taxa were significant, their average abundance was < 0.5%.
The families Tannerellaceae (q = 0.07) and Helicobacteraceae (q = 0.15) both decreased in abundance with increasing severity in non-responders (Supplementary Table 1). On the other hand, abundance in responders is agnostic to CE severity, as either because it is absent or because levels remain consistent (Supplementary Fig. 3). Even though the response-severity interaction for Helicobacteraceae was not statistically significant, the trend was significantly observed at its class and phylum levels, Campylobacteria (q = 0.061) and Campylobacterota (q = 0.056), respectively. Similarly, Tannerellaceae’s response-severity interaction was recapitulated at the class (Bacteroidia) and phylum (Bacteroidota) level (Supplementary Table 2).
Logistic regression analysis for presence or absence of fecal Clostridium species in CE
Univariable logistic regression models showed no significant association between the presence of fecal Clostridium in cats with CE and referral center, signalment, duration and nature of signs, body condition score, laboratory parameters (albumin, cobalamin, folate, ALT and ALP), or results from abdominal imaging and intestinal histopathology and FCEAI (P > 0.338).
Logistic regression analysis for response or non-response to therapeutic hydrolyzed protein diet
Univariable logistic regression models showed no significant association between response to a hydrolyzed protein diet in cats with CE and referral center, signalment, duration and nature of signs, body condition score, laboratory parameters (albumin, cobalamin, folate, ALT and ALP), or results from imaging and intestinal histopathology and FCEAI (P > 0.117).
Discussion
This study identified that at the genus level the fecal microbiota of cats with CE had a lower α-diversity and higher β-diversity than healthy control cats. This is consistent with previous reports comparing the fecal microbiota of cats with CE and clinically healthy cats11 and in dogs with CE6,7,8 and humans with IBD3,4,5. Although, it is unknown if these changes are the cause or consequence of the intestinal inflammation, studies in rodent models of IBD have shown that the microbiome is essential for the initiation and perpetuation of mucosal inflammation, as intestinal changes are absent when these models are raised in a germ-free environment29,30. Additionally, intestinal inflammation can be elicited in healthy rodents following transfer of the microbiome from rodents with IBD31,32.
In this study, the genus Clostridium was significantly higher in the feces of cats with CE compared to those of control cats at diagnosis. Several studies in human IBD have documented decreased abundance of Clostridium33, with one study documenting a negative correlation with disease severity34. A systematic review showed that people with active IBD had lower abundance of Clostridium coccoides and Clostridium leptum35, whereas Clostridium difficile has been shown to be prevalent in patients with ulcerative colitis36,37. Similar to humans with IBD, dogs with CE have been shown to have decreased species of Clostridium (e.g. C. hiranosis) but an increase in other species (e.g. C. perfringens)7,38. In this study, although Clostridium was more abundant in cats with CE, further studies are needed to determine the species involved, as a previous study showed that mucosally associated Clostridium spp. in cats with CE correlated with abnormalities in mucosal architecture, upregulation of cytokine mRNA and the number of clinical signs exhibited by the cats39.
The bacterial genera, Enterococcus and Escherichia have been associated with CE in dogs and cats and with IBD in humans5,11,21,39,40,41. These two genera were also over-represented in cats with CE as compared to control cats in our study. Although, neither reached significance after multiple comparison testing, the comparatively smaller number of cats in our control group may have reduced the power of detecting a significant difference. Therefore, studies incorporating a larger number of cats in both groups are needed to confirm or refute the role of Enterococcus and Escherichia in the pathogenesis of feline CE.
Interestingly, we noted a bimodal distribution of our dataset with regards to the fecal microbiota. We observed the feline microbiome to be sparse, such that microbiome profiles tended to be binary, where genera could be described as either present or absent. This type of distribution is important for the research community to be aware of, so they can account for the sparse nature of the feline fecal microbiome profiles. Analyses that account for the prevalence of genera, such as contingency testing, can be used to complement linear microbiome modeling methods when assessing differential abundances.
The rate of clinical remission achieved with a hydrolyzed protein diet in our study was only 45%. Alpha-diversity decreased over time in both dietary responders and non-responders, with the abundance of bacteria decreasing with the hydrolyzed protein diet. In particular, the bacteria most significantly affected by diet were more frequently observed in cats in the non-responder group. Therefore, despite a reduction in α-diversity and frequently present bacteria in the non-responder group, these cats still had persistent GI signs. This suggests that these cats likely require a different dietary strategy or medication to help improve their clinical signs, due to the differing etiopathogenesis of their disease. This is also exemplified by the fact that the β-diversity at the family level became increasingly dissimilar after dietary intervention in the non-responders. Therefore, dietary responders metabolize the diet similarly due to their sustained β-diversity following dietary intervention, whereas cats in the non-responder group likely represent a different group of cats with regards to the etiopathogenesis of their CE that requires a different therapeutic dietary strategy or medication for clinical remission. Further investigation would be needed to determine whether cats in the non-responder group are still food-responsive, potentially responding to another dietary strategy, such as limited-ingredient novel protein diet, or are steroid-responsive. If these cats are steroid-responsive they may have a specific aberration in their immune system, which results in a less tightly controlled microbiota, allowing for a more diverse response following dietary intervention. The gastrointestinal mucosal immune system is vital to homeostasis with regards to the microbiota and is home to a unique set of complex immunoregulatory mechanisms that help provide an appropriate immune response to commensal microorganisms42. Therefore, disruption of this highly complex network of immunological pathways may lead to chronic inflammation, which may alter their microbiological response to diet. Consequently, these cats may require additional therapy, such as glucocorticoids, to reduce the aberrant immune response, allowing them to enter clinical remission. Identifying the underlying reason for the increased β-diversity in non-responders following intervention with a hydrolyzed protein diet will help to advance our knowledge of the etiopathogenesis of the different sub-types of this disease and therefore guide the use of the most effective treatment for these cats.
Fecal Bifidobacterium was higher following dietary intervention in both the responder and non-responder groups. A previous study identified decreased Bifidobacterium species in the feces of cats with CE43. Therefore, an increase in this genus following dietary intervention may have helped the cats in the responder group enter remission. Bifidobacterium is considered beneficial in the gut due to its role in fermentation of fiber resulting in the production of the short-chain fatty acid butyrate. Butyrate is a major fuel source for colonocytes44 and helps to keep the intestine anaerobic, thereby inhibiting the growth of pathogenic bacteria. Butyrate also has direct effects on intestinal barrier function and the intestinal immune system45,46,47. Previous studies utilizing fructooligosaccharide (FOS) supplementation in cats demonstrated that these cats had higher levels of Bifidobacteria and increased levels of fecal butyrate48. The hydrolyzed protein diet utilized in our study contains FOS and is, therefore, the likely source for the increase in this bacterial genus. However, the reason for non-response in some cats following intervention with this diet, despite an increase in Bifidobacterium requires further investigation. One possible reason could be the predominant location of inflammation within the GI tract, with large intestinal disease more likely responding to the beneficial effects of FOS. Therefore, further studies correlating fecal microbiota changes with localization of inflammation within the GI tract will help to tease out the phenotype of CE cats that are more likely to benefit from a probiotic or higher dietary fiber strategy.
Oscillibacter and Desulfoviberionaceae_unclassified were most abundant in non-responders at diagnosis compared to responders but were rarely observed post diet in either group. In a mouse model of ulcerative colitis, Oscillibacter abundance was significantly positively associated with the pro-inflammatory cytokines interleukin (IL)-6 and IL-1 β and pathological scores49. This same genus was shown to increase transepithelial resistance of the proximal colon and reduce mRNA expression of tight junction protein, zona occludens 1, in the colon of obese mice50. This suggests that the increased abundance of this genus increases inflammation and compromises intestinal barrier function in dietary non-responders at diagnosis and may explain why additional treatment may be warranted to reduce GI signs in these cats. However, the hydrolyzed diet did reduce the abundance of this genus in the feces of non-responders, which suggests that other mechanisms may need to be targeted in addition to resolve GI signs in this cohort of cats. However, further studies assessing the effect of diet on immunological and barrier function in these cats will help to determine the mechanism of action of these diets and whether on a sub-clinical level their continued use is warranted in these cats, together with additional treatment.
Desulfovibrio spp., which are producers of toxic sulfides are significantly more abundant in cats with CE and humans with IBD43,51. Hydrogen sulfide is a highly toxic metabolic end product of Desulfovibrio spp. and is thought to inhibit oxidation of butyrate52. Indeed, in vitro, hydrogen sulfide selectively inhibited oxidation of butyrate by colonocytes, which led to lesions associated with colitis53. Also, Desulfovibrio spp., may produce immunogenic compounds that may result in an immune response in susceptible individuals54. Therefore, their increased abundance in non-responders at diagnosis may reflect their role in the pathogenesis of CE in these cats. The abundance of this genus did decrease following dietary intervention in non-responders. Interestingly, short-chain fatty acids produced by Bifidobacteria may inhibit the growth of Desulfovibrio spp. due to their effect on luminal pH, the direct inhibitory effect of short-chain fatty acids or production of antimicrobial peptides targeted towards these bacteria55. Such inter-microbial interactions may be a participating mechanism for the decrease in abundance of the genus Desulfoviberionaceae_unclassified in our study. However, despite the decrease in this genus following dietary intervention, cats in the non-responder group did not enter clinical remission, again suggesting that additional changes in the microbiota or immune system of these cats are needed to bring about remission. Assessing the metabolome of cats in the non-responder group before and after dietary intervention and comparing this to responder cats will help to further highlight the pathways involved in the pathogenesis of the different subtypes of this disease and the mechanism of action of this dietary intervention in these two group of cats. Further studies are also needed to help determine whether the presence of Oscillibacter and Desulfoviberionaceae_unclassified at diagnosis of CE in cats helps to predict subsequent response to hydrolyzed protein dietary intervention.
Interestingly, non-responders had reduced levels of the significant taxa; Campylobacterota, Campylobacteria and Tannerellaceae with increasing FCEAI, but this trend was not seen in the responders. One possibility could be that more severe CE results in more differing ecological niches that are less favorable for the growth of those organisms. Therefore, microbiota and histopathological characterization from intestinal mucosal biopsy specimens from all responders and non-responders would give us better insight into whether they differ in their inflammatory landscape to explain why the two groups follow different severity vs. abundance trends.
Although our data suggests that a hydrolyzed protein diet is able to modify the microbiome, the diet may induce clinical remission via mechanisms independent of the microbiome and, therefore, the alterations in the microbiota seen in our study may be a consequence rather than the direct cause of remission. Further studies assessing how dietary therapy may help to improve remission rates in cats with CE, by assessing the metabolome are therefore needed, as this will help to guide evidence-based design of more efficacious therapeutic diets.
Our study had the following limitations: although cats in the control group did not have any reported GI signs, full physical examination and laboratory blood work was not performed to ensure subclinical GI disease was not present nor was a full history collected to rule out other diseases or current medications. The control cats did not receive dietary intervention with the hydrolysed protein diet and therefore, repeat fecal samples were not collected for comparisons. Acquiring this information could have helped to provide more context for the changes seen in our study and therefore should be addressed in future studies assessing the impact of dietary intervention on the fecal microbiome in cats. Similarly, our study did not include a control diet to help compare the specific effects of the hydrolysed diet against and as such future studies should also focus on a group of cats with chronic enteropathy consuming a control diet, such as a commercial therapeutic gastrointestinal highly digestible diet. In addition, the number of control cats in our study was relatively small and therefore future studies should focus on recruiting a larger population of control cats. Although, all owners were specifically instructed to feed the diet exclusively, which included the use of no treats or additional foods, flavored medication such as deworming medication or toothpaste was not specifically advised against. An owner completed daily diary to verify all oral intake would have helped to confirm exclusive feeding and should be considered for future studies. In addition, the indoor/outside status of the cat and access to additional foods outside and as part of a multicat household was not specifically taken into consideration. However, a recent study suggested that dogs with food-responsive enteropathy did not need to receive these diets exclusively for effectiveness at controlling clinical signs56.The fecal microbiota might not accurately represent the mucosa-associated microbiota57 and therefore assessing the latter might have helped to identify additional changes between the groups. Finally, not all cats had GI biopsies performed to confirm an underlying diagnosis of chronic inflammatory enteropathy. Although two cats were excluded due to alimentary small cell lymphoma (SCL) on subsequent GI biopsy, additional cats may have been excluded if they all had this procedure performed. Nevertheless, a recent study did not find any bacterial taxa that differed between cats with chronic inflammatory enteropathy and SCL and concluded that their findings might lend support to the hypothesis that these two diseases are not different but rather a continuum11.
Conclusion
This study showed that the fecal microbiota of cats diagnosed with CE is significantly different from control cats without GI signs, and that these cats had similar changes to those described in human IBD. Despite the hydrolyzed protein diet reducing α-diversity in all cats with CE, this did not resolve GI signs in some cats. However, the microbiome of non-responders became increasingly dissimilar compared to diagnosis at the family level. Therefore, the microbiome may not be as tightly regulated by the immune system in cats with CE that are non-responders and therefore, may be the reason these cats require additional therapy for remission of GI signs. Whether Oscillibacter and Desulfovibrionaceae_unclassified are indicators of non-response to the diet at diagnosis requires further investigation. Further studies determining the metabolomic pathways for dietary responders and non-responders will enable the development of more efficacious therapeutic diets that might help all cats to attain remission with dietary therapy alone.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ASVs:
-
Amplicon sequence variants
- BH:
-
Benjamini Hochberg
- CE:
-
Chronic enteropathy
- DNA:
-
Deoxyribonucleic acid
- FCEAI:
-
Feline chronic enteropathy activity index
- FOS:
-
Fructooligosaccharide
- FRE:
-
Food responsive enteropathy
- GI:
-
Gastrointestinal
- GTDB:
-
Genome Taxonomy Database
- IBD:
-
Inflammatory bowel disease
- IL:
-
Interleukin
- NR:
-
Non-responder
- PCR:
-
Polymerase chain reaction
- PLI:
-
Pancreatic lipase immunoreactivity
- R:
-
Responder
- RVC:
-
Royal Veterinary College
- SCL:
-
Small cell lymphoma
- SRE:
-
Steroid responsive enteropathy
- T4:
-
Thyroxine
- TLI:
-
Trypsin-like immunoreactivity
References
Willard, M. D. Feline inflammatory bowel disease: A review. J. Feline Med. Surg. 1, 155–164. https://doi.org/10.1016/S1098-612X(99)90204-8 (1999).
Jergens, A. E. Feline idiopathic inflammatory bowel disease: What we know and what remains to be unraveled. J. Feline Med. Surg. 14, 445–458. https://doi.org/10.1177/1098612X12451548 (2012).
Frank, D. N., Zhu, W., Sartor, R. B. & Li, E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 19, 427–434. https://doi.org/10.1016/j.tim.2011.06.005 (2011).
Tamboli, C. P., Neut, C., Desreumaux, P. & Colombel, J. F. Dysbiosis in inflammatory bowel disease. Gut 53, 1–4. https://doi.org/10.1136/gut.53.1.1 (2004).
Walker, A. W. & Lawley, T. D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 69, 75–86. https://doi.org/10.1016/j.phrs.2012.09.008 (2013).
Minamoto, Y. et al. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 6, 33–47. https://doi.org/10.1080/19490976.2014.997612 (2015).
AlShawaqfeh, M. K. et al. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 93, 136. https://doi.org/10.1093/femsec/fix136 (2017).
Suchodolski, J. S. et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 7, e51907. https://doi.org/10.1371/journal.pone.0051907 (2012).
Ramadan, Z. et al. Fecal microbiota of cats with naturally occurring chronic diarrhea assessed using 16S rRNA gene 454-pyrosequencing before and after dietary treatment. J. Vet. Intern. Med. 28, 59–65 (2014).
Suchodolski, J. S. et al. The fecal microbiome in cats with diarrhea. PLoS ONE 10, e0127378. https://doi.org/10.1371/journal.pone.0127378 (2015).
Marsilio, S. et al. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 9, 19208. https://doi.org/10.1038/s41598-019-55691-w (2019).
Dennis, J. S., Kruger, J. M. & Mullaney, T. P. Lymphocytic/plasmacytic gastroenteritis in cats: 14 cases (1985–1990). J. Am. Vet. Med. Assoc. 200, 1712–1718 (1992).
Dennis, J. S., Kruger, J. M. & Mullaney, T. P. Lymphocytic/plasmacytic colitis in cats: 14 cases (1985–1990). J. Am. Vet. Med. Assoc. 202, 313–318 (1993).
Nelson, R. W., Dimperio, M. E. & Long, G. G. Lymphocytic-plasmacytic colitis in the cat. J. Am. Vet. Med. Assoc. 184, 1133–1135 (1984).
Guilford, W. G. et al. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J. Vet. Intern. Med. 15, 7–13. https://doi.org/10.1892/0891-6640(2001)015%3c0007:fsicwc%3e2.3.co;2 (2001).
Laflamme, D. P. et al. Evaluation of canned therapeutic diets for the management of cats with naturally occurring chronic diarrhea. J. Feline Med. Surg. 14, 669–677. https://doi.org/10.1177/1098612X12446906 (2012).
Perea, S. C., Marks, S. L., Daristotle, L., Koochaki, P. E. & Haydock, R. Evaluation of two dry commercial therapeutic diets for the management of feline chronic gastroenteropathy. Front. Vet. Sci. 4, 69. https://doi.org/10.3389/fvets.2017.00069 (2017).
Hart, J. R., Shaker, E., Patnaik, A. K. & Garvey, M. S. Lymphocytic-plasmacytic enterocolitis in cats - 60 cases. J. Am. Anim. Hosp. Assoc. 30, 505–514 (1993).
Mandigers, P. J., Biourge, V. & German, A. J. Efficacy of a commercial hydrolysate diet in eight cats suffering from inflammatory bowel disease or adverse reaction to food. Tijdschr Diergeneeskd 135, 668–672 (2010).
Kathrani, A., Church, D. B., Brodbelt, D. C., Pegram, C. & O’Neill, D. G. The use of hydrolysed diets for vomiting and/or diarrhoea in cats in primary veterinary practice. J. Small Anim. Pract. 61, 723–731. https://doi.org/10.1111/jsap.13214 (2020).
Wang, S. et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome 7, 126. https://doi.org/10.1186/s40168-019-0740-4 (2019).
Gaschen, F. P. & Merchant, S. R. Adverse food reactions in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 41, 361–379. https://doi.org/10.1016/j.cvsm.2011.02.005 (2011).
Lewis, J. D. et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host. Microbe 22, 247. https://doi.org/10.1016/j.chom.2017.07.011 (2017).
Jergens, A. E. et al. A clinical index for disease activity in cats with chronic enteropathy. J. Vet. Intern. Med. 24, 1027–1033. https://doi.org/10.1111/j.1939-1676.2010.0549.x (2010).
Laflamme, D. Development and validation of a body condition score system for cats: A clinical tool. Feline Pract. 25, 13–18 (1997).
Zhang, Y., Parmigiani, G. & Johnson, W. E. ComBat-seq: Batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform. 2, lqaa078. https://doi.org/10.1093/nargab/lqaa078 (2020).
Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.5-7 (2020).
Mallick, H. et al. Multivariable Association in Population-scale Meta'omic Surveys (2020).
Sellon, R. K. et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66, 5224–5231. https://doi.org/10.1128/IAI.66.11.5224-5231.1998 (1998).
Dianda, L. et al. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am. J. Pathol. 150, 91–97 (1997).
Garrett, W. S. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host. Microbe 8, 292–300. https://doi.org/10.1016/j.chom.2010.08.004 (2010).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757. https://doi.org/10.1016/j.cell.2011.04.022 (2011).
Zhuang, X. et al. Gut microbiota profile in pediatric patients with inflammatory bowel disease: A systematic review. Front. Paediatrics 9, 626232. https://doi.org/10.3389/fped.2021.626232 (2021).
Mottawea, W. et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 7, 13419. https://doi.org/10.1038/ncomms13419 (2016).
Prosberg, M., Bendtsen, F., Vind, I., Petersen, A. M. & Gluud, L. L. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 51, 1407–1415. https://doi.org/10.1080/00365521.2016.1216587 (2016).
Shoaei, P. et al. Clostridium difficile isolated from faecal samples in patients with ulcerative colitis. BMC Infect. Dis. 19, 361. https://doi.org/10.1186/s12879-019-3965-8 (2019).
Clayton, E. M. et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: An assessment of carriage in an outpatient setting among patients in remission. Am. J. Gastroenterol. 104, 1162–1169. https://doi.org/10.1038/ajg.2009.4 (2009).
Minamoto, Y., Dhanani, N., Markel, M. E., Steiner, J. M. & Suchodolski, J. S. Prevalence of Clostridium perfringens, Clostridium perfringens enterotoxin and dysbiosis in fecal samples of dogs with diarrhea. Vet. Microbiol. 174, 463–473. https://doi.org/10.1016/j.vetmic.2014.10.005 (2014).
Janeczko, S. et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet. Microbiol. 128, 178–193. https://doi.org/10.1016/j.vetmic.2007.10.014 (2008).
Khan, I. et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 8, 8030126. https://doi.org/10.3390/pathogens8030126 (2019).
Barko, P. C., McMichael, M. A., Swanson, K. S. & Williams, D. A. The gastrointestinal microbiome: A review. J. Vet. Intern. Med. 32, 9–25. https://doi.org/10.1111/jvim.14875 (2018).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141. https://doi.org/10.1016/j.cell.2014.03.011 (2014).
Inness, V. L., McCartney, A. L., Khoo, C., Gross, K. L. & Gibson, G. R. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridisation with special reference to Desulfovibrio spp. J. Anim. Physiol. Anim. Nutr. (Berl) 91, 48–53. https://doi.org/10.1111/j.1439-0396.2006.00640.x (2007).
Robles Alonso, V. & Guarner, F. Linking the gut microbiota to human health. Br. J. Nutr. 109(Suppl 2), S21–S26. https://doi.org/10.1017/S0007114512005235 (2003).
Cai, Y., Folkerts, J., Folkerts, G., Maurer, M. & Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 177, 1363–1381. https://doi.org/10.1111/bph.14871 (2020).
Raqib, R. et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. U S A 103, 9178–9183. https://doi.org/10.1073/pnas.0602888103 (2006).
Goncalves, P., Araujo, J. R. & Di Santo, J. P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 24, 558–572. https://doi.org/10.1093/ibd/izx029 (2018).
Barry, K. A. et al. Dietary cellulose, fructooligosaccharides, and pectin modify fecal protein catabolites and microbial populations in adult cats. J. Anim. Sci. 88, 2978–2987. https://doi.org/10.2527/jas.2009-2464 (2010).
Wu, M. et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 150, 104489. https://doi.org/10.1016/j.phrs.2019.104489 (2019).
Lam, Y. Y. et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 7, e34233. https://doi.org/10.1371/journal.pone.0034233 (2012).
Berry, D. & Reinisch, W. Intestinal microbiota: A source of novel biomarkers in inflammatory bowel diseases?. Best Pract. Res. Clin. Gastroenterol. 27, 47–58. https://doi.org/10.1016/j.bpg.2013.03.005 (2013).
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. https://doi.org/10.1136/gut.28.10.1221 (1987).
Roediger, W. E., Duncan, A., Kapaniris, O. & Millard, S. Reducing sulfur compounds of the colon impair colonocyte nutrition: Implications for ulcerative colitis. Gastroenterology 104, 802–809. https://doi.org/10.1016/0016-5085(93)91016-b (1993).
Sartor, R. B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am. J. Gastroenterol. 92, 5S-11S (1997).
Steer, T., Carpenter, H., Tuohy, K. & Gibson, G. R. Perspectives on the role of the human gut microbiota and its modulation by pro- and prebiotics. Nutr. Res. Rev. 13, 229–254. https://doi.org/10.1079/095442200108729089 (2000).
Allenspach, K., Culverwell, C. & Chan, D. Long-term outcome in dogs with chronic enteropathies: 203 cases. Vet. Rec. 178, 368. https://doi.org/10.1136/vr.103557 (2016).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32. https://doi.org/10.1038/nrmicro3552 (2016).
Acknowledgements
The authors thank Christina Maunder, Angela Hibbert, Victoria Black, Sophie Keyte and Vanessa Woolhead.
Funding
Grant from the American Academy of Veterinary Nutrition and Waltham.
Author information
Authors and Affiliations
Contributions
A.K. was involved in hypothesis generation and designed, conducted and analyzed all patient data. S.Y. analyzed and interpreted all fecal microbiota data. J.S. and E.H. were involved with hypothesis generation and design of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kathrani, A., Yen, S., Swann, J.R. et al. The effect of a hydrolyzed protein diet on the fecal microbiota in cats with chronic enteropathy. Sci Rep 12, 2746 (2022). https://doi.org/10.1038/s41598-022-06576-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06576-y
- Springer Nature Limited