Abstract
It is unclear as to whether Wilms’ tumor 1-associated protein (WTAP) promotes or suppresses breast cancer. This immunohistochemistry analysis explored levels of WTAP expression in 347 cases of breast cancer and analyzed the relationship between WTAP expression and the clinicopathological characteristics and prognosis of breast cancer patients. The rate of high WTAP expression was significantly higher in breast cancer tissue than in adjacent normal breast tissue (37.5% vs 0.0%; P < 0.001). WTAP expression was positively associated with tumor size and grade, and negatively associated with axillary lymph node metastasis, estrogen and progesterone receptor status. Rates of high WTAP expression were 66.1% in triple-negative breast cancer (TNBC) tissue and 31.3% in non-TNBC tissue. In multiple logistic regression analysis, independent predictors of WTAP expression in breast cancer included larger tumor size (odds ratio = 1.907; 95% confidence interval: 1.185–3.067; P = 0.008), lymph node metastasis (0.597; 0.373–0.956; P = 0.032) and TNBC status (3.735; 2.056–6.784; P < 0.001). No clear relationship was observed between patient prognosis and WTAP expression. We suggest that WTAP expression is upregulated in breast cancer and appears to both promote tumor growth and inhibit lymph node metastasis.
Similar content being viewed by others
Introduction
Female breast cancer is the most commonly diagnosed cancer worldwide and is the leading cause of cancer-related death among women1,2. Wilms’ tumor 1-associated protein (WTAP) is a key component of the methyltransferase complex, mediating in the deposition of cellular N6-methyladenosine (m6A), which is intimately involved in the initiation and progression of various types of human cancers3,4. In particular, WTAP contributes to aggressive features of malignant tumors. For example, WTAP overexpression facilitates the progression of hepatocellular carcinoma via the HuR-ETS proto-oncogene 1 (ETS1) axis5 and promotes osteosarcoma tumorigenesis by repressing HMBOX1 expression6. WTAP is also an oncogene in endometrial cancer7, gastric cancer8, ovarian cancer9 and bladder cancer10, which are all associated with poor prognosis.
Research on the expression of WTAP in breast cancer tissue and its clinical significance is only available from individual case reports, and contrasting findings have been reported from analyses of the expression and function of WTAP11,12. In an analysis of three human cancer databases11 that examined the relationship between WTAP expression and the prognosis of breast cancer patients, reduced WTAP expression was associated with poor survival in one database, and increased WTAP expression was associated with poor survival in another one database, while the results of the two microarrays in the third database are opposite, showing the same result of the first and second database above, respectively. In another analysis, WTAP expression was reduced in breast cancer samples compared with normal tissue derived from The Cancer Genome Atlas (TCGA) data12. Thus, further research is needed to better understand the expression and function of WTAP in breast cancer.
This study performed an immunohistochemistry (IHC) analysis of WTAP expression in breast cancer tissue samples obtained from 347 Chinese Han women, to clarify the expression of WTAP in breast cancer and its clinicopathological and prognostic significance.
Results
WTAP expression in breast tissue and its relationship with clinicopathological characteristics
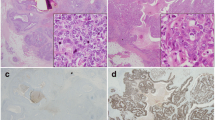
WTAP was expressed in the nuclei of breast cancer cells. The proportion of high WTAP expression in breast cancer tissue specimens was 37.5% (130/347), compared with 0.0% (0/23) of normal breast tissue specimens (Fig. 1); the expression of WTAP in breast cancer was significantly higher than that in normal breast tissue (P < 0.001) (Table 1).
As shown in Table 2, high WTAP expression was identified in 44.7% (84/188) of cases with tumors larger than 2 cm and in 28.9% (46/159) of tumors ≤ 2 cm; the between-group difference was highly significant (P = 0.003). High WTAP expression was identified in 54.9% (56/102) of grade III tumor tissue, in 30.7% (70/228) of grade II tumor tissue and in 23.5% (4/17) of grade I tumor tissue (P < 0.001). In patients with axillary lymph node metastasis and in those whose tumors were ER- or PR-positive, rates of high WTAP expression were 31.6% (54/171), 31.6% (68/215) and 29.6% (53/179), respectively, all of which were significantly lower than the corresponding rates among patients without lymph node metastasis (43.2%, 76/176), samples of ER-negative tissue (47.0%, 62/132) and PR-negative tissue (45.8%, 77/168) (P = 0.026, P = 0.004 and P = 0.002, respectively). Among the molecular subtypes that were examined, the rate of WTAP expression was 66.1% (41/62) in TNBC tissue compared with around one-second of non-TNBC tissue (31.3%, 88/281).
In logistic regression multivariate analysis, independent predictors of WTAP expression in breast cancer included larger tumor size (odds ratio = 1.907; 95% confidence interval: 1.185–3.067; P = 0.008), lymph node metastasis (0.597; 0.373–0.956; P = 0.032) and TNBC subtype (3.735; 2.056–6.784; P < 0.001).
We further analyze the relationship between WTAP expression and lymphatic invasion. The proportion of lymphovascular invasion (LVI) in breast cancer tissue specimens was 22.4% (34/152). High WTAP expression was identified in 32.4% (11/34) of LVI-positive tumor tissue and in 51.7% (61/118) of LVI-negative tumor tissue (P = 0.047). Spearman correlation analysis revealed a significantly negative correlation between WTAP expression and LVI in breast cancer tissue specimens (r = –0.161, P = 0.047).
No association between WTAP expression and survival
To assess the potential impact of WTAP expression on patient survival, we analyzed WTAP expression in relation to relapse-free survival (RFS), overall survival (OS) and distant metastasis-free survival (DMFS) rates. Five-year RFS, OS and DMFS rates were 79.0%, 88.0% and 80.0%, respectively. Local recurrence, regional recurrence, and distant metastasis occurred in 6 (3.0%), 2 (1.0%), and 34 (17.0%) patients, respectively. As shown in Fig. 2A–C, no clear associations were observed between WTAP expression and survival. The 59 patients with high levels of WTAP expression had a mean RFS of 53.3 months and an estimated 5-year RFS rate of 78.0%; corresponding values in the 141 patients whose tumors expressed low levels of WTAP were 53.8 months and 79.4%, respectively (P = 0.821; Fig. 2A). Mean OS was 57.3 months (with an estimated 5-year OS rate of 88.1%) in the patients with high levels of WTAP expression and 57.7 months (with an estimated 5-year OS rate of 87.9%) in those with low levels of WTAP (P = 0.995; Fig. 2B). Mean DMFS was 53.8 months (with an estimated 5-year OS rate of 79.7%) in the patients with high levels of WTAP expression and 54.1 months (with an estimated 5-year OS rate of 80.1%) in those with low levels of WTAP (P = 0.932; Fig. 2C).
(A-C) The associations of WTAP expression with relapse-free survival (RFS) (A), overall survival (OS) (B) and distant metastasis-free survival (DMFS) (C). (D-F) The associations of WTAP expression with RFS (D), OS (E) and DMFS (F) of patients with non-TNBC. (G-I) The associations of WTAP expression with RFS (G), OS (H) and DMFS (I) of patients with TNBC. P-values were calculated using the Mantel-Cox log-rank test.
We analyzed the effect of WTAP expression on the prognosis of non-TNBC or TNBC. As shown in Fig. 2D–F, in non-TNBC, the prognosis of tumors that were high WTAP expression did not differ significantly from that of low WTAP group. Similarly, in TNBC, the prognosis of tumors that were high WTAP expression did not differ significantly from that of low WTAP group (Fig. 2G–I).
Discussion
Several studies have reported that WTAP plays a important role in promoting the occurrence and development of malignant tumors5,6,7,8,9,10. However, scant research is available regarding WTAP expression and function in breast cancer, with only two published reports11,12. Both examined human cancer databases to analyze the expression of WTAP in breast cancer and its effect on prognosis in breast cancer, but the results of these analyses are controversial11,12. Thus, the role and function of WTAP in breast cancer needs to be clarified with urgency.
Our findings revealed significantly upregulated WTAP expression in breast cancer tissue compared with noncancerous breast tissue, in larger- versus smaller-sized tumors, and in higher-grade tumors. Higher-grade breast cancers are known to be more aggressive and to be associated with a poor prognosis14. These findings indicate that WTAP may be closely related to the occurrence of breast cancer, to highly invasive breast cancer and a poor prognosis. Our results also revealed that WTAP expression was significantly upregulated in ER- and PR-negative disease. WTAP was closely and negatively correlated with hormone receptor expression. As hormone receptor-negative breast cancer patients are unsuitable for endocrine therapy, further research should examine whether these patients may benefit from WTAP knockdown. Interestingly, we also found that high WTAP expression was negatively associated with axillary lymph node metastasis. Further analysis showed that larger tumor size and TNBC subtype are independently positively correlated with WTAP expression, and that lymph node metastasis is independently negatively correlated with WTAP expression. Paltoglou et al.15 reported that transcription factor Grainyhead-like 2 (GRHL2) plays a multifaceted role in prostate cancer, enhancing the oncogenic androgen receptor (AR) signaling pathway and promoting tumor growth, as well as suppressing metastasis-related phenotypes by suppressing epithelial-mesenchymal transition and cell invasion. These results indicate that WTAP may exert a dual function of promoting breast cancer growth and inhibiting lymph node metastasis.
Previous studies have shown that WTAP overexpression facilitates the tumor growth and progression of hepatocellular carcinoma via the HuR-ETS1 axis5 and β-arrestin2 (ARRB2) promoted the growth and migration of colorectal cancer (CRC) cells by upregulating WTAP expression16. In addition, a study showed that WTAP promotes osteosarcoma tumorigenesis by repressing HMBOX1 expression in an m6A-dependent manner6. Whether WTAP may affect breast tumor growth through the above-mentioned molecular pathways needs further study. Our results showed that the expression of WTAP in TNBC is significantly higher than that in non-TNBC. Studies show that TNBC subtype had lower odds of LVI17,18 and axillary lymph node involvement18,18,19,21 relative to other subtypes. Therefore, we further analyzed the relationship between WTAP and LVI, and the result shows a negative correlation between high WTAP expression and LVI. It may be one of the explanations for the low incidence of lymph node metastasis in breast cancer with high WTAP expression.
Our survival analysis failed to reveal any association between WTAP expression and survival, which we suspect may be due to the oncogenic functions of WTAP being counterbalanced by its ability to suppress lymph node metastasis. Our study analyzed the relationship between WTAP and breast cancer growth and lymph node metastasis at the histological level, but have not explored the molecular mechanism of WTAP affecting breast cancer growth and lymph node metastasis. More research is needed to determine how WTAP exerts oncogenic functions and to clarify its molecular mechanisms that inhibit lymph node metastasis. Such knowledge is of important clinical significance for the future use of WTAP in the inhibition of breast cancer.
Materials and methods
Patients and tissue samples
Breast cancer tissue samples were obtained from 347 Chinese Han women who underwent breast cancer surgery in the Affiliated Dongyang Hospital of Wenzhou Medical University (Dongyang, Zhejiang, China) between 2007 and 2019. Contains 332 cases of invasive ductal carcinoma, 5 cases of mucinous carcinoma, 4 cases of medullary carcinoma, 4 cases of metaplastic carcinoma and 2 cases of invasive micropapillary carcinoma. Twenty-three samples of adjacent normal breast tissue were also obtained following surgical resection. Inclusion criteria: a patient’s surgical specimen was diagnosed with invasive breast cancer by pathological diagnosis; exclusion criteria: antitumor therapy such as targeted therapy, chemotherapy, immunotherapy and radiotherapy before surgery. Breast cancer patients were aged between 24 and 84 years, with a median age of 50 years. A pathohistological diagnosis was made according to breast tumor classification criteria of the World Health Organization22,23. Histological grading was based on the Scarff–Bloom–Richardson system14. According to estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status, tissue samples were classified into triple-negative breast cancer (TNBC) (ER−, PR−, HER2−) or non-TNBC subtype24,24,25,27. Four cases with ER−, PR−, and HER2 2+ equivocal status did not undergo fluorescence in situ hybridization (FISH) were not classified into TNBC or non-TNBC groups. Follow-up information was available for 200 patients with a median follow-up time of 60 months (range 17–60 months). The study was approved by the Ethics Committee of the Affiliated Dongyang Hospital of Wenzhou Medical University (2020-YX-063). Written informed consent was obtained from all the participants. All of the study methodology satisfied the relevant guidelines and regulations issued by the Affiliated Dongyang Hospital of Wenzhou Medical University.
Tissue array preparation and IHC analysis
Tissue Array Preparation: We followed the methods described by Wang et al.28. In brief, the Quick-Ray® UT-06 (Unitma Co., Ltd., Seoul, Korea) tissue microarray system and the Quick-Ray premade recipient block (UB-06) wax model were used to prepare tissue specimens (1 mm in diameter). Two representative sites from each breast cancer tissue sample were selected for sampling. IHC Analysis: IHC staining of paraffin-embedded tissue sections used the Envision System (Dako, Glostrup, Denmark), as described previously25,26. Primary antibodies consisted of anti-WTAP rabbit monoclonal antibody (clone EPR18744, diluted at 1:3200; Abcam, Cambridge, England), ready-to-use anti-ER rabbit monoclonal antibody (clone SP1, Dako), ready-to-use anti-PR mouse monoclonal antibody (clone PgR636, Dako), ready-to-use anti-Podoplanin mouse monoclonal antibody (clone D2-40, Dako) and HercepTest (Dako). The secondary antibody was Dako’s HRP rabbit/mouse universal antibody (Dako, Glostrup, Denmark). The negative control was incubated with vehicle then with secondary antibody, without primary antibody.
Assessment of staining
WTAP staining in breast tissues was practically uniform throughout all tumor cells, so we needed to only evaluate WTAP staining intensity. The intensity of nuclear staining for WTAP was assessed in breast tissue and scored on a 4-point scale from 0 (negative) to 1 (weak), 2 (moderate), or 3 (strong)29,13; high WTAP expression was defined as a nuclear staining intensity of 313. A case was considered to be ER- or PR-positive when the percentage of positive invasive cancer cells (nuclear staining) was ≥ 1%30. HER2 status was determined by the 2018 American Society of Clinical Oncology/College of American Pathologists guidelines for HER2 testing in breast cancer31. Each entire section was scanned and scored independently by two pathologists.
Patient follow-up
Patients were followed-up using previously described methods27,28. In brief, each patient was followed-up postoperatively by telephone call and thereafter at 6-monthly hospital appointments; follow-up was discontinued in the event of the patient’s death. A diagnosis of local breast cancer recurrence was made by clinical or histology results. Relapse-free survival (RFS) was defined as the time from surgery to relapse/metastasis; overall survival (OS) was the time from surgery to death (excluding non-tumor-related deaths); distant metastasis-free survival (DMFS) was the time from surgery to metastasis.
Flow diagram
A flowchart of the study methodology is shown in Fig. 3.
Statistical analysis
Statistical analyses were conducted using SPSS software version 19.0 (SPSS Inc, Chicago, IL, USA). Between-group differences of WTAP expression were compared using a Pearson’s chi-square test for qualitative variables. Independent correlation factors of WTAP expression were assessed by multivariate logistic regression analysis. Relapse-free survival (RFS) and overall survival (OS) rates were estimated by the Kaplan–Meier method and compared using log-rank testing. P < 0.05 was considered to be statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- WTAP:
-
Wilms tumor 1-associated protein
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- RFS:
-
Relapse-free survival
- OS:
-
Overall survival
- DMFS:
-
Distant metastasis-free survival
- IHC:
-
Immunohistochemistry
- TNBC:
-
Triple-negative breast cancer
- m6A:
-
N6-Methyladenosine
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Liu, J. et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95 (2014).
Shi, H., Wei, J. & He, C. Where, when, and how: Context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650 (2019).
Chen, Y. et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol. Cancer 18, 127 (2019).
Chen, S. et al. WTAP promotes osteosarcoma tumorigenesis by repressing HMBOX1 expression in an m(6)A-dependent manner. Cell Death Dis. 11, 659 (2020).
Li, Q., Wang, C., Dong, W., Su, Y. & Ma, Z. WTAP facilitates progression of endometrial cancer via CAV-1/NF-kappaB axis. Cell Biol. Int. 45, 1269–1277 (2021).
Li, H. et al. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J. Cell. Mol. Med. 24, 4452–4465 (2020).
Yu, H. L. et al. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Ther. 12, 6191–6201 (2019).
Chen, L. & Wang, X. Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis. Oncol. Lett. 16, 6966–6970 (2018).
Wu, L. S., Qian, J. Y., Wang, M. & Yang, H. Identifying the role of Wilms tumor 1 associated protein in cancer prediction using integrative genomic analyses. Mol. Med. Rep. 14, 2823–2831 (2016).
Wu, L., Wu, D., Ning, J., Liu, W. & Zhang, D. Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer 19, 326 (2019).
Zeng, Y. et al. Combined high resistin and EGFR expression predicts a poor prognosis in breast cancer. Biomed. Res. Int. 2020, 8835398 (2020).
Elston, C. W. & Ellis, I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 19, 403–410 (1991).
Paltoglou, S. et al. Novel androgen receptor coregulator GRHL2 exerts both oncogenic and antimetastatic functions in prostate cancer. Cancer Res. 77, 3417–3430 (2017).
Liang, H., Lin, Z., Ye, Y., Luo, R. & Zeng, L. ARRB2 promotes colorectal cancer growth through triggering WTAP. Acta Biochim. Biophys. Sin. 53, 85–93 (2021).
Xue, C. et al. Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci. 103, 1679–1687 (2012).
Ugras, S., Stempel, M., Patil, S. & Morrow, M. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann. Surg. Oncol. 21, 3780–3786 (2014).
Mazouni, C. et al. Outcome in breast molecular subtypes according to nodal status and surgical procedures. Am. J. Surg. 205, 662–667 (2013).
Si, C., Jin, Y., Wang, H. & Zou, Q. Association between molecular subtypes and lymph node status in invasive breast cancer. Int. J. Clin. Exp. Pathol. 7, 6800–6806 (2014).
Kim, M. J. et al. Clinicopathologic significance of the basal-like subtype of breast cancer: A comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum. Pathol. 37, 1217–1226 (2006).
Lakhani, S. R. E. I., Schnitt, S. J., Tan, P. H. & van de Vijver, M. J. WHO Classification of Tumours of the Breast 4th edn. (IARC Press, 2012).
Yang, W. T. & Bu, H. Updates in the 5(th) edition of WHO classification of tumours of the breast. Zhonghua Bing Li Xue Za Zhi 49, 400–405 (2020).
Park, S. et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 21, 50–57 (2012).
Wang, C. Q. et al. Fascin-1 as a novel diagnostic marker of triple-negative breast cancer. Cancer Med. 5, 1983–1988 (2016).
Wang, C. Q. et al. EGFR conjunct FSCN1 as a novel therapeutic strategy in triple-negative breast cancer. Sci. Rep. 7, 15654 (2017).
Dong, X. F. et al. Downregulated METTL14 expression correlates with breast cancer tumor grade and molecular classification. Biomed. Res. Int. 2020, 8823270 (2020).
Wang, C. Q. et al. High expression of both resistin and fascin-1 predicts a poor prognosis in patients with colorectal cancer. Biomed. Res. Int. 2020, 8753175 (2020).
Wang, C. Q. et al. Expression of HMGB1 protein in breast cancer and its clinicopathological significance. Zhonghua Bing Li Xue Za Zhi 49, 57–61 (2020).
Nguyen, T. H., Nguyen, V. H., Nguyen, T. L., Qiuyin, C. & Phung, T. H. Evaluations of biomarker status changes between primary and recurrent tumor tissue samples in breast cancer patients. Biomed. Res. Int. 2019, 7391237 (2019).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 36, 2105–2122 (2018).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81802660). We would like to thank Iona J. MacDonald from China Medical University for her English language revision of this manuscript.
Author information
Authors and Affiliations
Contributions
C.Q.W. designed the experiments. Y.W. and Q.W. performed the experiments. C.Q.W. and Y.W. analyzed the data. B.F.H., G.N.H. and J.K.S. were responsible for management of the clinical samples. The manuscript was written by C.Q.W. and revised by C.H.T. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, CQ., Tang, CH., Wang, Y. et al. Upregulated WTAP expression appears to both promote breast cancer growth and inhibit lymph node metastasis. Sci Rep 12, 1023 (2022). https://doi.org/10.1038/s41598-022-05035-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05035-y
- Springer Nature Limited
This article is cited by
-
WTAP Mediates NUPR1 Regulation of LCN2 Through m6A Modification to Influence Ferroptosis, Thereby Promoting Breast Cancer Proliferation, Migration and Invasion
Biochemical Genetics (2024)
-
Lactoferrin suppresses the progression of colon cancer under hyperglycemia by targeting WTAP/m6A/NT5DC3/HKDC1 axis
Journal of Translational Medicine (2023)
-
Wilms tumor 1 associated protein promotes epithelial mesenchymal transition of gastric cancer cells by accelerating TGF-β and enhances chemoradiotherapy resistance
Journal of Cancer Research and Clinical Oncology (2023)
-
Relevance and mechanism of STAT3/miR-221-3p/Fascin-1 axis in EGFR TKI resistance of triple-negative breast cancer
Molecular and Cellular Biochemistry (2023)
-
EXOSC2 Mediates the Pro-tumor Role of WTAP in Breast Cancer Cells via Activating the Wnt/β-Catenin Signal
Molecular Biotechnology (2023)