Abstract
In the present study, we developed a genus-specific rGroEL1-524 IgM-ELISA assay for use in screening diagnosis of suspected leptospirosis among acute undifferentiated febrile illness patients during acute fever. The diagnostic accuracies of the rGroEL1–524 IgM-ELISA, commercial Panbio IgM-ELISA, and Virion-Serion Classic IgG-ELISA were evaluated using 133 Thai leptospirosis sera and 210 controls. Sensitivities were 91.7%, 59.6%, and 17.7% for acute infection, and the specificities were 92.6%, 90.2%, and 88.3% for the non-leptospirosis control, respectively. The rGroEL1-524 IgM-ELISA had high sensitivity, at 92.3% and 91.7%, among culture-positive and MAT-negative cases at 1–3 days post-onset of symptoms (DPO1–3), respectively. Impaired specificity on scrub typhus was found, possibly from antibody cross-reaction to ortholog GroEL. Commercial Panbio IgM-ELISA had sensitivities at DPO1–3 of 30.8% and 41.7% for culture-positive and MAT-negative cases whereas Virion-Serion IgG-ELISA showed sensitivities of 5.9% and 13.3%, respectively. The rGroEL1-524 IgM-ELISA could be useful as a screening test for early diagnosis. The performance of the commercial ELISA suggests the applicability of IgM-ELISA for diagnosis, while IgG-ELISA is useful for seroprevalence surveys. However, confirmation by reference tests is recommended.
Similar content being viewed by others
Introduction
Leptospirosis is recognized as a waterborne zoonosis with high incidence in tropical and sub-tropical areas, particularly rural areas and urban slum communities in developing and industrialized countries. Its epidemiology is undergoing changes due to global warming and migration. The disease continues to be a global public health burden, afflicting 0.1–1 per 100,000/population with a 10% case fatality rate annually; it also has a socio-economic impact1. Epidemic outbreaks occasionally occur and are associated with flooding in epidemiological settings including Thailand, the Philippines, New Caledonia, Hawaii, and Nicaragua. The Thai Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health, reported incidence and case fatality rates in 2019 of 3.26 and 0.04 per 100,000/population, respectively, with demographic shifts in southern Ranong, Phang Nga, Yala, and northeastern Sisaket and Yasothon provinces. It is usually associated with farmers, laborers, students, and government services staff, and is also associated with recreational activities and travelers2,3,4. The disease is seasonal, with a peak incidence in the late rainy season to early winter, and occasionally occurs after high rainfall and flooding, such as in the epidemic outbreak in Loei in 20025,6.
Humans are usually infected by pathogenic bacteria of the genus Leptospira, which are allocated into three distinct phylogenetic clusters by the virulence of the bacteria, comprising 13 pathogenic and 5 intermediately pathogenic species and free-living saprophytes7. The main disease-causing species in humans and other animals are pathogenic Leptospira interrogans, L. borgpetersenii, and L. kirschneri8; the intermediate group members that infect humans and mammals causing mild disease include L. broomii, L. fainei, L. inadai, L. licerasiae, and L. wolffii, whereas non-pathogenic species do not cause disease7,9,10. Based on serology, the Leptospira spp. are classified into more than 300 different serovars and clustered into at least 24 serogroups8,11. Human hosts commonly acquire infection by contact with bacterially contaminated urine, soil, or water through abraded skin or mucous membranes, which causes a wide range of clinical manifestations ranging from asymptomatic to mild or acute undifferentiated febrile illness (AUFI)4 to severe leptospirosis symptoms such as Weil’s disease, severe pulmonary hemorrhage syndrome, and aseptic meningitis, and is a potentially fatal illness12,13. Most patients present with non-specific febrile illness similar to other tropical diseases, such as dengue fever, rickettsioses, malaria, influenza, septicemic melioidosis, viral hemorrhagic fever, and enteric fever, making misdiagnosis possible14,15,16. Patients receiving early diagnosis and appropriate antibiotic therapies within 4–5 days post-onset of symptoms (DPO) have higher rates of recovery17,18. Delayed case diagnosis and late treatment can rapidly lead to severe complications where chemotherapy becomes useless2,16.
The World Health Organization suggests that the gold standard laboratory method for confirming leptospirosis diagnosis should be: (i) isolation of Leptospira by the culture method, (ii) detection of organism DNA by PCR, and (iii) detection of antibodies by microscopic agglutination test (MAT)17. MAT detects both immunoglobulin M (IgM) and IgG agglutinating antibodies. However, MAT provides low sensitivity at the early course of infection, as MAT can detect IgM antibodies after DPO 8 and requires paired-sera testing to confirm diagnosis. The test cannot differentiate current, recent, or past infections. Furthermore, MAT is technically demanding, time-consuming, and requires well-trained personnel for interpretation. Culturing provides definite proof of leptospiral infection and could identify locally pathogenic serovars. However, culture is not useful as a diagnostic tool because by the time a diagnosis is made by culture, antibodies are already detectable by serological techniques and the result is relatively delayed. MAT and culture methods have low diagnostic sensitivities but high specificity. Molecular diagnosis by PCR, quantitative PCR (qPCR), and recently whole genome sequencing are not affordable in primary healthcare and in rural areas with resource-limited settings17,19. Hence, the development of reliable and valid diagnostic tests providing high accuracy is needed for the diagnosis of leptospirosis, so that the disease can be diagnosed and treated early in its course.
Immunodiagnostics using the detection of IgM antibodies during acute illness, such as the enzyme-linked immunosorbent assay (IgM-ELISA), immunofluorescence assay (IFA), and immunochromatography (ICT) formats20,21, have been implemented for the diagnosis of infectious diseases in the tropics including leptospirosis, dengue fever, rickettsioses, and melioidosis22,23,24. IgM-ELISA is recommended by the World Health Organization (WHO) as useful in early diagnosis; the test has shown high sensitivity and specificity and is more sensitive than MAT. Commercially available ELISAs have shown inconsistent performance when evaluated in different epidemiological settings, and their accuracy requires systematic evaluation in Thailand17,18,25. The diagnostic accuracy of the commercial IgG-ELISA has been evaluated in limited studies and has not been evaluated in Thailand. The commercial Panbio IgM-ELISA has been evaluated in different settings, including Malaysia26, Hawaii27, Laos28, Southern Vietnam29, southern Sri Lanka30, and the French West Indies31. The Panbio IgM-ELISA demonstrated limited diagnostic sensitivity and specificity, at 76.1% and 82.6%, respectively, when evaluated in high-prevalence northeast Thailand32.
Leptospira infections cause upregulation of bacterial heat shock protein 60 (GroEL) in response to temperature stress conditions, eliciting long-lasting immune responses with high antibody titers. GroEL has shown to be a genus-specific immunodominant antigen, as revealed by anti-Leptospira immune serum and leptospirosis paired sera33,34,35. This protein has shown less cross-reactivity with sera from patients who have recovered from melioidosis or dengue hemorrhagic fever33,34,35. In this study, we developed an IgM-ELISA screening test using a genus-conserved region of GroEL1-524 (recombinant GroEL1–524) as antigen to detect specific IgM antibody in blood specimens of suspected leptospirosis cases among AUFI patients. We then evaluated its diagnostic performance for early leptospirosis diagnosis using local Thai blood samples as compared to the culture and MAT methods. We also assessed the usefulness of commercial ELISAs, Panbio Leptospira IgM-ELISA and the Virion-Serion classic Leptospira IgG-ELISA, as screening tests for detecting anti-leptospiral antibodies using Thai blood samples in the context of disease outbreaks and compared to Leptospira culture and MAT.
Results
Verification of GroEL1-524 sequence conservation within the genus Leptospira spp. and among orthologous GroEL proteins
The Leptospira GroEL1-524 sequence had a high degree of homology, at 99% (522/524) sequence identity to other leptospiral serovars in the Leptospira spp. To evaluate their protein-sequence conservation across genera, GroEL orthologs among other tropical infectious diseases including leptospirosis, scrub typhus, melioidosis, and malaria were examined. Orthologous GroEL proteins of influenza A and dengue viruses were not found in the NCBI database. The orthologous GroEL proteins of L. interrogans, Burkholderia pseudomallei, Plasmodium vivax, and Orientia tsutsugamushi organisms demonstrated 60.5%, 51.3%, and 51.9% identity, respectively, compared with the cloned Leptospira GroEL1-524 sequence. The GroEL1-524 sequence was highly conserved in the genus Leptospira spp. and shared conserved peptides among orthologous GroEL proteins.
Prediction of GroEL1-524 linear B-epitope peptides
Two B-epitope peptides of 30-LGPKGRN-36 (85.7% identity) and 404-AAVEEGIVPG-413 (100% identity) have been shown to be highly conserved among leptospirosis, scrub typhus, melioidosis, and malaria pathogens. The epitope peptide similarity suggests a degree of immunological cross-reactivity among leptospirosis, scrub typhus, malaria, and melioidosis sera.
Recombinant GroEL1-524 protein preparation
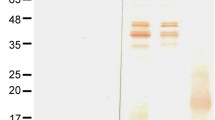
The C-terminal His-Tagged GroEL1-524 protein was produced as a soluble protein of estimated 58.7 kDa. The purified rGroEL1–524 protein was verified for antigenic specificity, which revealed a reactive band at 60 kDa (supplementary Fig. S1). The rGroEL1–524 protein was used as an antigen in the development of a recombinant antigen-based IgM-ELISA.
IgM sera reactivity of leptospirosis and controls to rGroEL1–524 antigen by IgM-ELISA
The IgM antibody reactivities of leptospirosis paired sera in an optimized rGroEL1–524 IgM-ELISA were presented as actual optical density (AOD) values with a range of 0–1.01. Median AOD values were 0.40 and 0.43 for leptospirosis paired sera, and were significantly higher than those of the non-leptospirosis control (P < 0.0001) and other febrile illness (P < 0.001). The IgM reactivities of leptospirosis sera versus scrub typhus sera were not significantly different (P = 0.15, P = 0.09, respectively) (Fig. 1). The median IgM reactivities of culture positive samples and seroconversion were 0.50 and 0.45 for acute sera and 0.25 and 0.34 for convalescent sera, respectively (Fig. 1).
IgM reactivity of confirmed leptospirosis paired sera and controls assessed by rGroEL1-524 IgM-ELISA. The IgM reactivity of acute and convalescent leptospirosis sera (closed circle), non-endemic healthy and AUFI control plasma (open circle), and other febrile illness control samples (open circle), comprising dengue fever, influenza, malaria, melioidosis and scrub typhus subgroups, were assessed rGroEL1-524 IgM-ELISA assay. Individual IgM reactivity is expressed as AOD ELISA. The median AOD ELISA value and standard deviation (SD) of each subgroup are indicated. P < 0.001 is considered significantly different.
Determination of optimal cut-off values
To optimize cut-off values, receiver operating characteristic (ROC) curves were generated from leptospirosis paired sera and controls to define the optimal optical density (OD) of 0.15 to achieve an estimated sensitivity of 91.6% and 95.5% for the paired sera and a specificity of 87.5% for the controls.
The results of IgM detection by Panbio IgM-ELISA and IgG detection by Virion-Serion IgG-ELISA on leptospirosis sera and controls are illustrated with adjusted cut-off values in supplementary Fig. S2. The optimal threshold for Panbio IgM-ELISA cut-off value was ≥ 7 Panbio units, and the optimized cut-off value for Serion IgG-ELISA was ≥ 0.35 OD ELISA to achieve higher sensitivities.
Diagnostic accuracy of rGroEL1–524 IgM-ELISA
Thai blood samples were randomly selected to evaluate rGroEL1–524 IgM-ELISA performance compared with reference methods. The diagnostic sensitivities were 91.7% for acute sera and 95.6% for convalescent sera (Table 1).
The specificity was 92.6% for the non-leptospirosis control (Table 1), with corresponding AUC values of 0.93 (P < 0.001). Of the following febrile illness specificities, the results were 60.0% for dengue fever, 54.5% for scrub typhus, 81.8% for influenza, 70.0% for malaria, 91.7% for melioidosis, and 100% for other febrile illness whole blood samples. The diagnostic sensitivities of MAT-negative leptospirosis samples and culture-positive samples were 91.7% and 92.3% at DPO1–3, and 92.3% and 90.9% at DPO4–10, respectively (Table 2).
Diagnostic performance of commercial ELISA test kits against local Thai sera
The performance of the commercial ELISAs was determined using the same panel samples of leptospirosis and non-leptospirosis sera. The diagnostic sensitivities of the Panbio IgM-ELISA were 59.6% for acute and 71.4% for convalescent sera, whereas the Virion-Serion IgG-ELISA showed 17.7% and 48.9% sensitivities for leptospirosis paired sera, respectively. The specificity was 88.3% for the non-leptospirosis control.
Sensitivities of the commercial Panbio IgM-ELISA were 41.7% for MAT-negative cases and 30.8% for culture-positive samples at DPO1–3 and 30.0% for MAT-negative cases and 18.2% for culture-positive samples at DPO4–10 (Table 2).
Analysis of false negative results among leptospirosis samples from rGroEL1–524 IgM-ELISA
False negative results were found on seronegative (MAT ≤ 1:50) leptospirosis samples on paired-leptospirosis sera and culture positive samples; they had IgM reactivities of 0.06–0.13. Anti-IgM positive result of MAT-negative cases suggested insufficient GroEL-specific IgM antibody or levels under the limit of detection. False negative of culture-positive samples might have arisen from the window of active infection. (supplementary Table S3).
Analysis of false-positive results among controls from rGroEL1–524 IgM-ELISA
IgM positivity among controls by rGroEL1–524 IgM-ELISA was 7.5% (supplementary Table S4). All false-positive samples were negative for anti-Leptospira IgM and IgG detection by commercial ELISA, suggesting that the false positives in the non-leptospirosis control may have arisen from non-specific binding or pre-existing antibodies (background antibody) in those samples.
The analysis of false-positive samples among the other febrile illness controls is summarized in supplementary Table S4. Melioidosis samples had 8.3% (1/12) false-positive results by rGroEL1-524 IgM-ELISA. Serum showed anti-Leptospira IgG positivity using the commercial test, which suggested antibody cross-reactivity from previous exposure. The scrub typhus sera showed IgM reactivity ranging from 0.03 to 1.07; 45.5% (5/11) were deemed false positives. Two of the false positives had anti-Leptospira IgM positivity, which suggested antibody cross-reactivity from recent or current exposure or leptospirosis-scrub typhus coinfection. Three of the false-positive results were likely caused by antigen cross-reactivity to ortholog GroEL antigen or pre-existing antibody from endemic leptospirosis.
Two of three false-positive malaria samples had anti-Leptospira IgG positivity by Virion-Serion IgG-ELISA detection. The false-positive reactions might be related to pre-existing antibodies from previous exposure. One malaria sample had false-positive result by an in-house IgM-ELISA, which suggested antigen cross-reactivity to ortholog GroEL antigen or pre-existing antibody.
Two of ten influenza samples had false-positive results by IgM-ELISA (AOD, 0.16–0.2) at 1:100 dilution, but all were negative for anti-Leptospira IgM and IgG detection by the commercial tests. False positives were likely caused by non-specific reactions. False positives of dengue samples were 40% (4/10) by in-house IgM-ELISA. Three of the false-positive samples tested negative for anti-Leptospira detection, which suggests that false positives may arise from endemic background antibodies. One false-positive dengue sample had anti-Leptospira IgM positivity, which suggested recent or current infection and leptospirosis-dengue coinfection.
Discussion
Genus-specific antigen-based ELISAs using immunodominant outer membranes as antigens, such as LipL32, LipL41, Loa22, LigA, Lsa63, GroEL, and a combination (multiple antigens), have been widely developed for use as a screening test for leptospirosis35,36,37,38. The present study developed a prototype IgM-ELISA using a recombinant GroEL1-524 formatted antigen as an early laboratory screening test for leptospirosis and evaluated its diagnostic accuracy in the context of disease outbreaks in Thailand compared with reference methods. We produced rGroEL1–524 protein (C-terminal deletion of 22 amino acids) and used an ELISA antigen to detect anti-rGroEL1–524 IgM antibody during the course of illness for early diagnosis of suspected cases among AUFI caused by other infections. The heat shock GroEL chaperonin has shown as a diagnostic potential in leptospirosis based on its upregulated expression during infection (temperature upshift). GroEL has been shown to be an immunodominant antigen and has less cross-reactivity with melioidosis and dengue hemorrhagic fever33,34,35. The immunoreactivity of severe leptospirosis, such as pulmonary involvement and renal failure, to recombinant GroEL has been shown to have 90.6% sensitivity and 94.9% specificity39. In addition, the GroEL1-524 sequence is highly conserved within the genus Leptospira and shares lower sequence conservation with the orthologous GroEL. We evaluated the diagnostic performance of the rGroEL1–524 IgM-ELISA using leptospirosis paired-sera derived from northeastern Thailand, i.e., Loei, Nakhon Ratchasima, Sakol Nakhon, and controls from non-endemic Bangkok and other febrile illnesses, compared to MAT and culture methods. Leptospirosis sera from Loei were collected from an outbreak in 2002. Samples were found to be positive by Leptospira isolation (30 culture-positive acute sera) and by seroconversion criteria. A small sample size of 28 single MAT ≤ 1:400 leptospirosis sera was obtained.
In the present study, a single IgM-ELISA was designed as a highly sensitive screening test. The cut-off was determined to be 0.15 AOD for single IgM-ELISA testing to achieve an estimated sensitivity of 91.6% and 95.5% for paired sera and specificity of 92.5% and 76.0% among the non-leptospirosis and febrile controls, respectively. One limitation of acute-phase IgM testing with a single specimen is that people in endemic areas are expected to have pre-existing antibodies causing impaired specificity. ELISA results give no indication of the infecting serovar, and a confirmatory diagnosis of leptospirosis should be performed. IgM antibody usually persists for 5 months40, ELISA can be used as a simple and rapid laboratory screening test for the diagnosis of leptospirosis for several months after the onset of symptoms.
The sensitivities of the rGroEL1-524 IgM-ELISA were 91.7% and 95.6% for leptospirosis paired sera, and the specificity was 92.6% among the non-leptospirosis control. Lessa-Aquino et al.35 reported GroEL IgM-ELISA sensitivities of 90% and 92.0% and specificities of 53.8% and 62.5% in paired sera. A systematic review and meta-analysis of the performance of Leptospira IgM-ELISA averaged 84% sensitivity and 91% specificity for acute infection41. The rGroEL1-524 IgM-ELISA had higher diagnostic performance than previously reported35,41 due to the ability of the refined rGroEL1-524 molecule to encompass more antigenic moieties of the whole genus. The prototype IgM-ELISA had high sensitivities of 95.9% in culture-positive sera, 91.2% in seroconversion samples, and 88.2% in MAT-positive subgroups. With a cut-off of 0.2 AOD, expected test performance was 87.5% and 86.7% for sensitivity in paired sera and 81.5% specificity in the febrile control. The most prevalent serovars infecting patients in the sera used in this study were Bratislava, Autumnalis, Australis, New, Sarmin, and Bangkok5, while Autumnalis, Bratislava, and Pyrogenes were the most common serovars in Thailand in 2003–201242. The in-house IgM-ELISA can detect IgM antibody as early as DPO1. The false negative results in the acute phase by in-house IgM-ELISA might be due to the long window by the dynamics of antibody production. Symptomatic patients may have no or low antibody levels at 1–2 weeks post-exposure, and the antibody titer will rise with time. We found two false negative convalescent sera, which might have been caused by a delayed response, which sometimes occurs over 30 days after infection20.
Leptospirosis infections are often under-reported due to false negatives among mild cases or those who have already received antibiotics, have suppressed immunity, or are in the very early or late phase of the immune response. In coinfection patients, weak or cross-reactions may occur. The false-positive rate among febrile patients is possibly caused by cross-reactivity, anti-GroEL1-524 IgM antibody from leptospirosis co-infections, or pre-existing IgM antibody in patients with recent exposure in endemic areas.
Commercial ELISA tests have been used for the diagnosis of leptospirosis in Thai endemic settings, including the Panbio Leptospira IgM-ELISA and Virion-Serion classic Leptospira IgM/IgG. The performance of commercial ELISA tests varies by geographical setting, with the sensitivity of the Leptospira IgM-ELISA being 35–76% and specificity being 76–98% in different endemic settings27,28,29,30,31,32,41. These ELISA tests use whole-cell lysates from pathogenic L. interrogans, intermediate L. fainei, or saprophytic Leptospira biflexa antigens to detect genus-specific anti-Leptospira IgM/IgG antibodies. Heterogeneous native antigens in ELISA tests may not recognize the local serovars, so their sensitivities are frequently poor and have been limited by the heterogeneity of host immunological responses to native antigens.
Whole cell-based ELISAs (Panbio IgM- and Virion-Serion IgG-ELISA) demonstrated poor sensitivity against local Thai leptospirosis paired sera in the present study (Table 1). Another study found that the Panbio IgM-ELISA showed 90.8% positivity among samples from northeastern Thailand32. IgM antibodies appear earlier than IgG antibodies and remain detectable at low titers for months or even years. An IgG titer of 1:100 can be present due to past infection. Whole cell-based ELISA, which is affected by serogroup-specific antigens or whole-cell antigens, has poor sensitivity and may not recognize local infectious strains in different endemic areas20,32,43. IgM-dominant and IgG-dominant L. biflexa serovar Patoc antigen, i.e., LPS, cytoplasmic, secreted, and envelop membrane proteins, do not encompass local infecting serovars in the genus Leptospira. L. biflexa Patoc I antigen is known to cross-react with several serovars, but usually does not cross-react with animal strains. The most predominant infecting serovars in suspected patients from 2003–2004 in Thailand were Autumnalis, Bataviae, Pyrogenes, Javanica, Hebdomadis and Grippotyphosa44. The most predominant infecting serovars between 2010 and 2015 were serovars (associated reservoir) Shermani (cattle, buffalo, pig), Bratislava (livestock, i.e., cattle, buffalo), Panama, and Sejroe (rodents)45. Another study examined the potential risk of a leptospirosis outbreak in Bangkok and Nakhon Pathom between 2011 and 2012, and found L. wolffii and intermediate L. licerasiae46. Poor sensitivity can be attributed to several factors, such as acute serum being collected too early in the course of illness (less than DPO4–5), inadequate IgM antibody levels in the patient, second or subsequent episode of infection leading to IgG antibody production, and patient receiving antibiotic medication. To improve specificity due to high background antibodies among the seropositive population requires validation and adjustment of the cut-off. In this study, we optimized cut-offs for commercial ELISAs. An adjusted Panbio unit of ≤ 7 showed a sensitivity improvement to 54.8% for the DPO1–3 acute phase and specificity of 86.6%. The Panbio IgM-ELISA provided sensitivity and the ability to detect IgM antibodies as early as DPO1–3. Virion-Serion IgG-ELISA with an adjusted cut-off provided 17.7% and 48.9% sensitivity on leptospirosis paired sera and 81.7% specificity among the controls. False-positive IgG detection was 19% among the controls (10% for non-endemic samples and 25% for each infection, i.e., dengue, malaria, scrub typhus, and melioidosis). IgG seropositivity rates of 17.7% and 48.9% for paired sera suggest that IgG responses should be due to epidemic leptospirosis in Loei rather than background antibody, with 10% seropositivity among healthy and AUFI patients in low-prevalent leptospirosis areas such as Bangkok.
Several studies have reported that ELISA-based assays detect anti-Leptospira IgM antibodies earlier than MAT assay during the early course of disease20,47,48. Nicofa et al.20 suggested that Leptospira-specific IgM antibodies appear 1–2 days earlier than the agglutinating antibodies detected in the MAT assay; therefore, earlier positive results could be expected from our genus-specific IgM detection. We found that the sensitivities in seronegative and culture-positive acute sera were 91.2%, and 95.9% for the prototype IgM-ELISA and 63.2% and 50.0% for commercial IgM-ELISA, respectively. An unvalidated diagnostic test with poor specificity may contribute to overdiagnosis of leptospirosis, because IgM antibodies from past infections are frequently detected among people living in endemic areas20,48.
The cross-reactivity of the rGroEL1-524 IgM-ELISA was evaluated using sera from the local population and a non-leptospirosis febrile control group. The specificity of anti-Leptospira IgM detection is limited in pathogens expressing orthologous GroEL proteins, such as scrub typhus, malaria, and melioidosis, causing IgM cross-reactivity in the rGroEL1-524 IgM-ELISA. High anti-Leptospira IgM levels in sera collected from patients along the Thai-Myanmar border have been reported49,50. However, it should be noted that cross-reactivity with bacterial infections can occur when patients harbor co-infections or have cross-reactive antibodies, especially in the early phase of leptospirosis when the IgM-ELISA lacks full specificity20.
The varied sensitivities likely reflect different case definitions and control groups, timing of collection, local prevalent serovar distribution, and the platform and protocol used in detection. A significant limitation of the rGroEL1-524 IgM-ELISA was poor specificity for leptospirosis-endemic areas. The test specificity was affected by ortholog GroEL antigen cross-reactivity, antibody cross-reactivities caused by previous exposure, and co-infections. Co-infections with leptospirosis were not assessed in samples from other febrile illnesses. The use of the rGroEL1-524 IgM-ELISA as a screening test for leptospiral infection would facilitate the difficult reference and differential tests. However, the test should not be used as the sole criterion for diagnosing leptospirosis. The ELISA results must be confirmed by convalescent serum. MAT is still recommended for disease confirmation and epidemiological study, and Leptospira isolation and molecular characterization should be performed for confirmation of the infecting serovars51.
Conclusion
Our data demonstrated that IgM-ELISA using rGroEL1-524 antigen has sufficiently high sensitivity to screen for anti-GroEL1-524 IgM antibodies in the early leptospirosis diagnosis of suspected cases and among high-risk groups during leptospirosis epidemics. However, diagnostic specificity needs to be improved for implementation in areas with high levels of infectious tropical diseases. The commercial ELISA performance data suggest the applicability of IgM-ELISA for early diagnosis during disease outbreaks in low-prevalence areas for leptospirosis. IgG-ELISA is useful for seroprevalence surveys; however, confirmation by reference tests is recommended.
Methods
Ethics and biosafety
The protocol for using achieved sera and patient data was performed in concordance with the recommendation of the Declaration of Helsinki. Documentary Proof of Exemption Review was obtained from the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (MUTM-EXMPT2017-005). The written informed consents were obtained from participants. Sample anonymity was maintained, and all samples were re-coded without name and hospital ID. Biosafety was approved by the institute’s Biosafety Committee (MU2019-002).
Study design
A retrospective study was carried out to assess the diagnostic performance of rGroEL1–524 IgM-ELISA and to evaluate commercial whole-cell antigen-based ELISA performance using local Thai blood samples compared with culture and MAT methods. The laboratory investigations were conducted at the Faculty of Tropical Medicine, Mahidol University, in Bangkok.
Reference leptospirosis diagnosis
Patients’ specimens were investigated by cultivation or MAT. The reference diagnosis was conducted at Loei Provincial Hospital. Leptospira isolation was performed on the day of patient hospitalization by culturing blood specimens in EMJH (Ellinghausen-McCullough-Jonson-Harris) medium, followed by incubation for 16 weeks5. Sera were tested by MAT assay with 20 reference Leptospira serovars, as described previously5,36. Single leptospirosis sera were confirmed for MAT titers at the Faculty of Tropical Medicine, Mahidol University. MAT-positive criteria were defined as single MAT titer of ≥ 1:400 in a single specimen, sero-conversion from negative to titer ≥ 1:400, or a fourfold rise in MAT titer using paired sera. A MAT-negative sample was defined as MAT titer ≤ 1:5017,21,32,47,52.
Leptospirosis patients and sera
A suspected leptospirosis case was clinically diagnosed based on WHO criteria, i.e., AUFI in patients (fever ≥ 38 °C) with headache and myalgia and a history of exposure to animal reservoirs or flooded environments17. A confirmed leptospirosis case is defined as a clinically diagnosed, suspected leptospirosis case combined with positive laboratory diagnosis by the culture method or MAT assay.
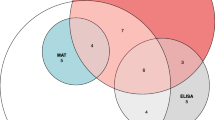
Leptospirosis sera (n = 133) were obtained from patients during an epidemic outbreak at Loei Provincial Hospital (n = 95) and sporadic cases in the Nakhon Ratchasima and Sakhon Nakhon provinces (n = 38)5,36 (Fig. 2). Leptospirosis sera (n = 133) were acute sera (n = 52) and classified as DPO1–3 (n = 34), DPO 4–10 (n = 12), and convalescent sera (n = 51). Among the sera, samples with a single MAT titer of 1:100–1:200 (n = 26) were excluded. The most prevalent serogroups (serovars) among the MAT-positive sera were Autumnalis (Autumnalis, New), Australis (Australis, Bangkok, Bratislava), Icterohaemorrhagiae (Copenhageni), Sarmin (Sarmin), and Sejroe (Sejroe)5.
Flow diagram of the assessment of diagnostic accuracy of rGroEL1-524 IgM-ELISA, and commercial ELISAs. Confirmed leptospirosis sera (n = 133) and control samples (n = 210) were subjected to an assessment of the diagnostic sensitivity and specificity of the following tests: (i) rGroEL1–524 IgM-ELISA, and commercial (ii) Panbio Leptospira IgM-ELISA, and (iii) Serion-Virion classic Leptospira IgG-ELISA. Leptospirosis sera were acute sera collected on the date of admission (n = 52) and convalescent-sera collected in the later 2 weeks (n = 51). Acute-phase sera were classified according to days post-onset of symptoms (DPO) into DPO1-3 (n = 34), and DPO4-10 (n = 12) acute serum. Control samples were 51 non-leptospirosis plasma (seronegative and negative for leptospirosis IgM detection), 88 serum or plasma samples and 50 whole blood of laboratory-confirmed infectious diseases other than leptospirosis. Of 343 sera, 26 leptospirosis sera and 21 control samples were excluded from study. Positive results of the commercial ELISAs were considered using the recommended cut-offs.
Control samples
To assess the specificity of the ELISA tests, a panel of control samples (n = 210) consisting of 60 non-leptospirosis plasma (seronegative and negative for leptospirosis IgM detection) and 150 laboratory-confirmed infectious diseases other than leptospirosis were used (Fig. 2). Non-leptospirosis control were 60 plasma were collected from healthy volunteers and febrile plasma (n = 60) at the Hospital for Tropical Diseases, Bangkok in 2014. Other laboratory-confirmed febrile illnesses included seropositive dengue paired sera (n = 20) collected from classic dengue fever patients at Sisaket Provincial Hospital, Srisaket Province in 2013; scrub typhus samples (n = 20) that were PCR positive and sero-positive acute serum collected at Umphang Hospital, Tak Province in 2018; influenza paired sera (n = 20) collected from HI seroconversion of H1N1-infected patients; malaria plasma (n = 20) collected from malaria vivax-positive patients in Tak Province (n = 20); and melioidosis sera (n = 20) collected from IgM-seropositive melioidosis patients from northeastern Thailand before 2018. Whole blood samples (n = 50) were collected from AUFI patients admitted to the hospital for Tropical Diseases, Bangkok, from 2013 to 2015. The samples included laboratory-confirmed murine typhus (n = 15), dengue (n = 30), and bacterial sepsis (n = 5) caused by E. coli, Streptococcus agalactiae, Salmonella Typhi, and Viridans Streptococci infections (Fig. 2)14. The samples were collected in microtubes and stored at − 70 °C.
Production of a transformed E. coli carrying a recombinant GroEL 1–524–pET23a(+) plasmid
Briefly, a DNA sequence encoding for GroEL1-524 was amplified from genomic DNA extracted from L. interrogans serovar Icterohaemorrhagiae by PCR reaction using specific primers GroEL-NdeI/F (5ʹ-GGCCCATATGGCGAAAGATATTGAATAT-3ʹ) and GroEL-BamHI/R (5ʹ-TTGGATCC ATCTGGTTTGTCTGTGATTGT-3ʹ). Amplification was performed according to the following conditions: one cycle of 94 °C for 5 min, 29 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1.30 min, followed by a final period of 72 °C for 5 min. The PCR product was analyzed under 1% agarose gel electrophoresis and visualized by gel documentation (Bio-Rad, California, USA).
The GroEL1-524 fragment was digested with NdeI and BamHI restriction endonucleases and ligated into a linearized plasmid backbone to produce a recombinant GroEL1–524-pET23a(+) plasmid, which was subsequently introduced into BL21(DE3) E. coli. The GroEL1–524 sequence was verified by standard sequencing (Bioneer, Daejeon, Republic of Korea). The genetic map of the GroEL1–524-pET23a(+) plasmid is illustrated in supplementary Fig. S1.
Production of recombinant GroEL1–524 protein
Recombinant GroEL1–524 protein was produced under an E. coli expression system using a transformed E. coli strain bearing the recombinant GroEL1–524 plasmid. Briefly, E. coli was grown in Luria–Bertani broth containing 100 µg/mL ampicillin at 37 °C with 200 rpm shaking until the culture reached an OD600nm of 0.5. Thereafter, isopropyl β-d-1-thiogalactopyranoside (IPTG, 1 mM) was added to induce rGroEL1-524 protein expression at 37 °C with 200 rpm shaking for 3 h.
A soluble fraction from the IPTG-induced bacteria containing rGroEL1-524 protein was prepared in phosphate-buffered saline (1× PBS, pH 7.4) using a French pressure cell press at 30 kilo-pounds per square inch, repeated four times. The rGroEL1-524 protein was purified from the soluble proteins by native affinity chromatography using Ni2+-sepharose (GE Healthcare, Uppsala, Sweden). The purified rGroEL1-524 protein was concentrated in 1× PBS (pH 7.4) using a 3-kDa cut-off Amicon Ultra filter (Merck Millipore, MA, USA), and the protein concentration was determined using Bradford assay (Thermo Fisher Scientific, MA, USA). Aliquots of the protein (1 mg/mL) were lyophilized using the Labcono Freeze Dry system and then kept at − 70 °C.
SDS-PAGE and Western blotting
Protein was analyzed under 13% SDS-PAGE gel electrophoresis, denaturing conditions, and Coomassie Brilliant Blue G250 stain. Antigenic specificity testing of the rGroEL1-524 protein was performed by probing the blotted membrane with anti-6× His-Tag monoclonal antibody (1:1000) (R&D Systems, MN, USA) for 1 h at 25 °C, followed by HRP-conjugated goat anti-mouse IgG secondary antibody (1:2,000) (Jackson ImmunoResearch, PA, USA) for 1 h at 25 °C (Southern Biotechnology, AL, USA). The reactive band was developed using 3,3-diaminobenzidine (DAB) chromogenic substrate (Thermo Fisher Scientific, MA, USA).
In-house rGroEL1–524 IgM-ELISA
Recombinant GroEL1–524 (1 μg) immobilized ELISA strips (Jet Biofil, Guangzhou, China) were prepared as follows: rGroEL1–524 protein in 100 µL of carbonate–bicarbonate buffer (pH 9.6) was immobilized on ELISA wells at 37 °C for 24 h, and the antigen-coated wells were washed using washing buffer (300 µL/well of PBST; 0.05% Tween 20 in 1× PBS, pH 7.4). Washing was conducted by an automated microplate washer (Tecan Trading AG, Switzerland) three times to remove unbound material. The coated wells were then incubated with blocking reagent (300 µL of 1% BSA in 1× PBS) for 1 h at 37 °C, followed by incubation of the pre-blocked wells with 2% sucrose solution (300 µL) at 25 °C for 1 h. The ELISA wells were washed after each incubation step, as described above, and then air-dried. The pre-blocked rGroEL1–524 ELISA strips were packed with desiccant in press-seal bags and stored at − 20 °C until use.
To detect anti-GroEL1-524 IgM antibody, serum dilution (1:100, 100 µL) in a serum diluent (1× PBS containing 0.2% gelatin, 0.2% BSA), along with an internal positive control (pooled MAT-positive patient sera, where the adjusted AOD exceeded 0.2) and a reagent control (serum diluent) were incubated in pre-blocked antigen-coated wells at 37 °C for 1 h, followed by washing three times with PBST. Thereafter, HRP-conjugated goat anti-human IgM antibody (100 μL, 1:2,000) (Southern Biotechnology, AL, USA) was added to ELISA wells at 37 °C for 1 h incubation. ABTS chromophore diammonium salt (EMD Millipore, Germany) substrate solution (1 mg/mL ABTS tablet in 0.1 M sodium citrate buffer) was added (100 µL), and the plate was incubated for 15 min at 37 °C, after which 1% SDS solution (100 µL) was added to stop the reaction. The OD was measured at a wavelength of 410 nm against the reference at 650 nm (OD410nm/650 nm) using a microplate reader (Bio-Tek Instruments, VT, USA). Sample AOD was calculated by subtracting the OD of the reagent blank. The IgM-ELISA assay is valid when the OD of the reagent blank is < 0.2 and the positive AOD control is ≥ 0.2. A rGroEL1-524 IgM-ELISA protocol was optimized, and the optimal concentration of rGroEL1-524 was 1 µg/well; serum dilution was 1:100 and secondary antibody dilution was 1:1000–1:3000 dilutions.
Panbio Leptospira IgM-ELISA
The diagnostic performance of the commercial Panbio Leptospira IgM-ELISA (Abbott Diagnostics, Illinois, USA) (Lot no. 02P10E001), using Leptospira genus-specific antigen, was assessed in Thai blood samples. The Panbio IgM-ELISA protocol was performed per the manufacturer’s instructions, measuring absorbance at OD450nm/650 nm. An index value was calculated by dividing the sample absorbance by the cut-off value. The result was expressed as Panbio units (index value multiplied by 10). Interpretation of the validity results was as follows: Panbio units (anti-Leptospira IgM) < 9 was a negative result, suggesting no evidence of recent infection, Panbio units ≥ 9 to < 11 was an equivocal result, suggesting possible recent infection, and Panbio units ≥ 11 was positive by IgM detection and interpreted as a recent or current infection. An equivocal result was considered a positive result. The Panbio IgM-ELISA test performance showed 96.5% sensitivity and 98.5% specificity and has been validated to detect Leptospira infections by serovars Pomona, Copenhageni, Australis, Canicola, Grippotyphosa, Tarsassovi, Hardjo, Madanesis, Kremastos, Nokolaevo, Cellodoni, Szwajizak, and Djasiman.
Virion-serion classic Leptospira IgG-ELISA
Institute Virion-Serion ELISA Classic Leptospira IgG (Institut Virion/Serion GmbH, Warburg, Germany) (order no. ESR 125 G) was used to detect anti-Leptospira IgG antibody from serum or plasma using a crude membrane extract of L. biflexa serovar Patoc strain Patoc I, which contains genus-specific epitopes for all Leptospira spp. The Virion-Serion IgG-ELISA procedure was performed per the manufacturer’s instructions, with absorbance measured at OD405nm/650 nm. To interpret the qualitative results, the upper and lower cut-off range was calculated according to parameters provided with the kit. Actual OD (AOD) value (anti-Leptospira IgG) lower than the cut-off was considered a negative result suggesting no evidence of past exposure, an AOD value in the cut-off range was a borderline result suggesting possible past exposure, and an AOD value higher than the upper cut-off was positive by IgG detection, suggesting previous exposure. A borderline value was considered a positive result. The diagnostic performance of the Virion-Serion Leptospira IgG-ELISA was 96.7% sensitivity and 99.8% specificity.
Evaluation of diagnostic accuracy
The Standards for Reporting of Diagnostic Accuracy studies (STARD 2015) checklist for reporting diagnostic accuracy is provided in supplementary Table S6.
Sample size was estimated as a minimum of 35 cases and control samples to achieve 90% sensitivity and specificity at a 95% confidence interval (CI) and 7% precision. All sera were tested as anonymous samples. Leptospirosis sera and controls (Fig. 1) were randomly selected to evaluate the performance of the following tests: (i) rGroEL1–524 IgM-ELISA, (ii) commercial Panbio Leptospira IgM-ELISA, and (iii) Virion-Serion Classic IgG-ELISA. The estimated diagnostic sensitivity and specificity with 95% CI were calculated by a 2 × 2 cross-tabulation table.
Bioinformatics
Conservation of selected GroEL sequences in the genus Leptospira and among GroEL orthologs was determined using Clustal Omega multiple sequence alignment program interface53, and the results were analyzed using the BioEdit sequence alignment editor tool. Linear B-cell epitopes of the L. interrogans serovar Icterohemorrhagiae GroEL sequence were computationally predicted using a Bepipred-1.0 Linear Epitope Prediction tool54
Statistical calculations
Data were collected in Microsoft Excel and analyzed using MedCalc Statistical Software version 19.2.5 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020). Diagnostic parameters were calculated as follows: sensitivity = [(true positive (TP)/(TP + false negative (FN))] × 100; specificity = [(true negative (TN))/(TN + false positive (FP))] × 100. Normal distribution was tested using the Kolmogorov-Smirnov test. The Mann-Whitney test was used in non-normal distributed data. P < 0.05 was considered statistically significant.
Data availability
The datasets used and analyzed from the current study are available from the corresponding author on reasonable request.
References
Abela-Ridder, B., Sikkema, R. & Hartskeerl, R. A. Estimating the burden of human leptospirosis. Int. J. Antimicrob. Agents. 36(Suppl 1), S5-7 (2010).
Bharti, A. R. et al. Leptospirosis: A zoonotic disease of global important. Lancet Infect. Dis. 3(12), 757–771 (2003).
Bandara, M., Ananda, M., Wickramage, K., Berger, E. & Agampodi, S. Globalization of leptospirosis through travel and migration. Glob. Health. 10, 61 (2014).
Niwattayakul, K., Homvijitkul, J., Niwattayakul, S., Khow, O. & Sitprija, V. Hypotention, renal failure, and pulmonary complications on leptospirosis. Ren. Fail. 24(3), 297–305 (2002).
Niwetpathomwat, A., Niwatayakul, K. & Doungchawee, G. Surveillance of leptospirosis after flooding at Loei Province, Thailand by year 2002. Southeast Asian J. Trop. Med. Public Health 36(4), 202–205 (2005).
Suwanpakdee, S. et al. Spatio-temporal patterns of leptospirosis in Thailand: Is flooding a risk factor?. Epidemiol. Infect. 143(10), 2106–2115 (2015).
Vincent, A. T. et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 13(5), e0007270 (2019).
Picardeau, M. Virulence of the zoonotic agent of leptospirosis: Still terra incognita?. Nat. Rev. Microbiol. 15(5), 297–307 (2017).
Adler, B. & de la Pena-Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 140, 287–296 (2010).
Ko, A., Goarant, C. & Picadeau, M. Leptospira: The dawn of the molecular genetics’ era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 7(10), 736–747 (2009).
Thibeaux, R. et al. Deciphering the unexplored Leptospira diversity from soils uncovers genomic evolution to virulence. Microb. Genom. 4(1), e000144 (2018).
Ganoza, C. A. et al. Asymtomatic renal colonization of humans in the Peeruvian Amazon by Leptospira. PLoS Negl. Trop. Dis. 4(2), e612 (2010).
Abidi, K. et al. The clinical picture of severe leptospirosis in critically ill patients. Intensive. Care. Med. 43(11), 1740–1741 (2017).
Luvira, V. et al. Etiologies of acute undifferentiated febrile illness in Bangkok, Thailand. Am. J. Trop. Med. Hyg. 100(3), 622–629 (2019).
Wangdi, K. et al. Diversity of infectious etiologies of acute undifferentiated febrile illnesses in South and Southeast Asia: A systematic review. BMC Infect. Dis. 19(1), 577 (2019).
Toyokawa, T., Ohnishi, M. & Koizumi, N. Diagnosis of acute leptospirosis. Expert. Rev. Anti. Infect. Ther. 9(1), 111–121 (2011).
World Health Organization WHO, International Leptospirosis Society ILS. Human Leptospirosis: guidance for diagnosis, surveillance, and control. Preprint at https://apps.who.int/iris/handle/10665/42667 (2003).
Pappas, G. & Cascio, A. Optimal treatment of leptospirosis querires and projections. Int. Antimicrob. Agents. 28(6), 491–496 (2006).
Smyth, L. D. et al. The microscopic agglutination test (MAT) is an unreliable predictor of infecting Leptospira serovart in Thailand. Am. J. Trop. Med. Hyg. 81(4), 695–697 (2009).
Niloofa, R. et al. Diagnosis of leptospirosis: Comparison between Microscopic Agglutination Test, IgM-ELISA and IgM rapid immunochromatography test. PLoS ONE 10(6), e0129236 (2015).
Doungchawee, G. et al. Development and evaluation of an immunochromatographic assay to detect serum anti-leptospiral lipopolysaccharide IgM in acute leptospirosis. Sci. Rep. 7(1), 2309 (2017).
Hunsperger, E. A. et al. Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 antigen and anti-Dengue virus IgM antibody. PLoS Negl. Trop. Dis. 8(10), e3171 (2014).
Gupta, N. et al. Scrub Typhus and Leptospirosis: The fallacy of diagnosing with IgM enzyme linked immunosorbant assay. J. Microb. Biochem. Technol. 8, 071–075 (2016).
Suttisunhakul, V. et al. Development of rapid enzyme-linked immunosorbent assays for detection of antibodies to Burkholderia pseudomallei. J. Clin. Microbiol. 54(5), 1259–1268 (2016).
Goris, M. G. et al. Towards the burden of human leptospirosis: Duration of acute illness and occurrence of post-leptospirosis symptoms of patients in the Netherlands. PLoS ONE 8(10), e76549 (2013).
Esa, H. A. H., Amran, F. & Halim, N. A. N. Evaluation of a commercial enzyme-linked immunosorbent assay kit for detection of IgM antibody against Leptospira in human sera for patients admitted in Malaysia hospitals. Clin. Microbiol. 7, 306 (2018).
Effler, P. V. et al. Evaluation of eight rapid screening tests for acute leptospirosis in Hawaii. J. Clin. Microbiol. 40(4), 1464–1469 (2002).
Blacksell, S. D. et al. Limited diagnostic capacities of two commercial assays for the detection of Leptospira immunoglobulin M antibodies in Laos. Clin. Vaccine. Immunol. 13(10), 1166–1169 (2006).
Wagenaar, J. F. et al. Rapid serological assays for leptospirosis are of limited value in southern Vietnam. Ann. Trop. Med. Parasitol. 98(8), 843–850 (2004).
Reller, M. E. et al. Leptospirosis as frequent cause of acute febrile illness in Southern Sri Lanka. Emerg. Infect. Dis. 17(9), 1678–1684 (2011).
Courdurie, C. et al. Evaluation of different serological assays for early diagnosis of leptospirosis in Martinique (French West Indies). PLoS Negl. Trop. Dis. 11(6), e0005678 (2017).
Desakorn, V. et al. Accuracy of commercial IgM ELISA for the diagnosis of human leptospirosis in Thailand. Am. J. Trop. Med. Hyg. 86(3), 524–527 (2012).
Sakolvaree, Y. et al. Proteome and immunome of pathogenic Leptospira spp. revealed by 2DE and 2DE-immunoblotting with immune serum. Asian. Pac. J. Allergy. Immunol. 25(1), 53–73 (2007).
Kositanont, U. et al. Application of immunoproteomic to leptospirosis: Towards clinical diagnostics and vaccine discovery. Proteomics. Clin. Appl. 1(4), 400–409 (2007).
Lessa-Aquino, C. et al. Identification of seroreactive proteins of Leptospira interrogans serovar Copenhageni using a high-density protein microarray approach. PLoS Negl. Trop. Dis. 7(10), e2499 (2013).
Chalayon, P. et al. Leptospirosis serodiagnosis by ELISA based on recombinant outer membrane protein. Trans. R. Soc. Trop. Med. Hyg. 105(5), 289–297 (2011).
Srimanote, P. et al. Recombinant ligA for leptospirosis diagnosis and ligA among the Leptospira spp. clinical isolates. J. Microbiol. Method. 72(1), 73–81 (2008).
Alizadeh, S. A. et al. Diagnostic efficacy of Lsa63 antigen for human leptospirosis. Iran. Red. Crescent. Med. J. 16(3), e14753 (2014).
Natarajaseenivasan, K. et al. Surface-associated Hsp60 chaperonin of Leptospira interrogans serovar Autumnalis N2 strain as an immunoreactive protein. Clin. Microbiol. Infect. Dis. 30(11), 1383–1389 (2011).
Silva, M. V. et al. Behaviour of specific IgM, IgG and IgA class antibodies in human leptospirosis during the acute phase of the disease and during convalescence. J. Trop. Med. Hyg. 98(4), 268–272 (1995).
Rosa, M. I. et al. IgM ELISA for leptospirosis diagnosis: A systematic review and meta-analysis. Cien. Saude. Colet. 22(12), 4001–4012 (2017).
Hinjoy, S. et al. Environmental and behavioral risk factors for severe leptospirosis in Thailand. Trop. Med. Infect. Dis. 4(2), 79 (2019).
Schreier, S., Doungchawee, G., Chadsuthi, S., Triampo, D. & Triampo, W. Leptospirosis: Current situation and trends of specific laboratory tests. Expert. Rev. Clin. Immunol. 9(3), 263–280 (2013).
Wuthiekanun, V. et al. Optimization of culture of Leptospira from humans with leptospirosis. J. Clin. Microbiol. 45(4), 1363–1365 (2007).
Chadsuthi, S. et al. Investigation on predominant Leptospira serovars and its distribution in humans and livestock in Thailand, 2010–2015. PLoS Negl. Trop. Dis. 11(2), e0005228 (2017).
Thaipadungpanit, J. et al. Leptospira species in floodwater during the 2011 floods in the Bangkok metropolitan region, Thailand. Am. J. Trop. Med. Hyg. 89(4), 794–796 (2013).
Doungchawee, G. et al. Early diagnosis of leptospirosis by immunoglobulin M immunoblot testing. Clin. Vaccine. Immunol. 15(3), 492–498 (2008).
Cumberland, P. C., Everard, C. O. R. & Levett, P. N. Assessment of the efficacy of the IgM enzyme-linked immunosorbent assay (ELISA) and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am. J. Trop. Med. Hyg. 61(5), 731–734 (1996).
Sonthayanon, P. et al. Rapid diagnosis of scrub typhus in rural Thailand using polymerase chain reaction. Am. J. Trop. Med. Hyg. 75(6), 1099–1102 (2006).
Wongsrichanalai, C. et al. Co-infection with malaria and leptospirosis. Am. J. Trop. Med. Hyg. 68(5), 583–585 (2003).
Chirathaworn, C., Inwattana, R., Pooworawan, Y. & Suwancharoen, D. Interpretation of microscopic agglutination test for leptospirosis diagnosis and seroprevalence. Asian. Pac. J. Trop. Biomed. 4(Suppl 1), S162-164 (2014).
Maneewatchararangsri, S. et al. Development of enzyme-linked immunosorbent assay for human leptospirosis serodiagnosis using Leptospira secretome antigen. Southeast Asian. J. Trop Med. Public Health 48(3), 576–584 (2017).
Madeira, F. et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47(W1), W636–W641 (2019).
Larsen, J. E., Lund, O. & Neilsen, M. Improved method for predicting linear B-cell epitopes. Immunome. Res. 2, 2 (2006).
Acknowledgements
We greatly appreciate Prof. Dr. Pilaipan Puthavatana of the Faculty of Medical Technology, Mahidol University, who provided influenza sera; Assoc. Prof. Dr. Yupaporn Wattanagoon, Faculty of Tropical Medicine, Mahidol University, who provided scrub typhus sera; Assoc. Prof. Dr. Narisara Chantratita, Faculty of Tropical Medicine, who provided melioidosis sera; Assoc. Prof. Dr. Pongrama Ramasoota, Centre of Excellence for Antibody Research, and Assoc. Prof. Dr. Kriengsak Limkittikul, Faculty of Tropical Medicine, who provided dengue sera; Prof. Dr. Jetsumon Prachumsri, Mahidol Vivax Research Unit, Faculty of Tropical Medicine, who provided vivax malaria sera; and Dr. Rapatbhorn Patrapuvich, Drug Research Unit for Malaria, Faculty of Tropical Medicine, Mahidol University who provided healthy control sera and AUFI sera. We thank Enago Group for English editing a revised manuscript.
Funding
This work was supported by the Center of Excellence in Medical biotechnology, Thailand (Grant No. SD-60-003-16) and the Faculty of Tropical Medicine, Mahidol University.
Author information
Authors and Affiliations
Contributions
S.M. contributed to the study conception and design. Material preparation, laboratory work and data collection were performed by all authors. Laboratory and statistical analysis were performed by S.M., P.V., and N.S. The manuscript was mainly written by S.M., and all authors commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maneewatchararangsri, S., Doungchawee, G., Kalambaheti, T. et al. Evaluation of a genus-specific rGroEL1-524 IgM-ELISA and commercial ELISA kits during the course of leptospirosis in Thailand. Sci Rep 11, 19785 (2021). https://doi.org/10.1038/s41598-021-99377-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99377-8
- Springer Nature Limited