Abstract
Tumor multifocality and location are prognostic factors for upper tract urothelial carcinoma (UTUC). However, confounding effects can appear when these two factors are analyzed together. Therefore, we aimed to investigate the impact of tumor distribution on the outcomes of multifocal UTUC after radical nephroureterectomy. From the 2780 UTUC patients in the Taiwan UTUC Collaboration Group, 685 UTUC cases with multifocal tumors (defined as more than one tumor lesion in unilateral upper urinary tract) were retrospectively included and divided into three groups: multiple renal pelvic tumors, multiple ureteral tumors, and synchronous renal pelvic and ureteral tumors included 164, 152, and 369 patients, respectively. We found the prevalence of carcinoma in situ was the highest in the synchronous group. In multivariate survival analyses, tumor distribution showed no difference in cancer-specific and disease-free survival, but there was a significant difference in bladder recurrence-free survival. The synchronous group had the highest bladder recurrence rate. In summary, tumor distribution did not influence the cancer-specific outcomes of multifocal UTUC, but synchronous lesions led to a higher rate of bladder recurrence than multiple renal pelvic tumors. We believe that the distribution of tumors reflects the degree of malignant involvement within the urinary tract, but has little significance for survival or disease progression.
Similar content being viewed by others
Introduction
Upper tract urothelial carcinoma (UTUC) comprises renal pelvic tumors (RPTs) and ureteral tumors (UTs). It is a relatively rare but aggressive urological cancer with a poor prognosis1,2. The current standard treatment for high-risk lesions is radical nephroureterectomy (RNU). However, disease recurrence or metastasis occurs in approximately 20–30% of patients3,4,5. Hence, to provide patients with better management options, a more precise and comprehensive UTUC assessment is needed.
The fact that UTUC spans two neighboring localities has led to the comparison of RPTs and UTs. The incidence of RPTs seem to be higher3,6,7 than that of UTs and RPTs often present a more advanced disease stage at the time of diagnosis6,7. Conversely, UTs are more likely to be organ-confined and have a greater association with bladder tumor history or recurrence5,7. Whether the prognosis of the two tumors is different has therefore been a matter of debate, but recent studies agree that RPTs are associated with better outcomes3,5,6,8,9. A possible explanation for this includes the difference in the anatomical nature of RPTs vs UTs. The renal pelvis has tougher physical surroundings that can facilitate a more complete dissection and prevent tumor extrusion, whereas the adventitial layer of the ureter is too thin to be a solid barrier10. The differences between RPTs and UTs have been validated, and tumor location is considered a profound factor for the evaluation of UTUC.
In addition to the differences in the disease features of RPTs and UTs, the existence of two localities within a single cancer entity results in heterogeneity of tumor multifocality. Multifocality is not uncommon in UTUC, presenting in 7–42% of patients among different study populations3,6,11,12,13,14,15,16,17,18,19, and leads to worse disease outcomes than single lesions3,6,8,11,12,13,14,15,16,17,18,19,20. However, the classification of multifocal tumors has failed to reach consistency among various studies3,6,11,12,14,15,16,20. Some definitions of multifocal tumors require the involvement of both the renal pelvis and ureter3,6,11,16,20, and some only consider the number of tumors12,14,15. Inappropriate grouping may mask the true impact of tumor location and multifocality on prognosis.
With this in mind, we aimed to develop a more explicit classification for multifocal tumors based on tumor distribution. We conducted a retrospective study using the nationwide multi-institution UTUC database to elucidate whether tumor location influences the disease outcomes of patients with multifocal lesions.
Methods
This nationwide study was conducted by the Taiwan UTUC Collaboration Group and was approved by the Kaohsiung Medical University Hospital Institutional Review Board [KMUHIRB-E(I)-20180214], which waived the need for formal informed consent due to the retrospective nature of the study. All personally identifiable information was removed. The study was performed according to the 1964 Helsinki declaration and strictly followed institutional guidelines and regulations.
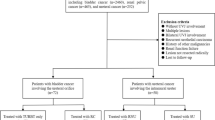
A total of 16 participating Taiwanese hospitals contributed to the comprehensive database, which contained data on 2780 patients entered between 1988 and 2020. Exclusion criteria included those who did not receive RNU, those with only a single tumor lesion, and those without complete clinicopathological information. The remaining 685 patients all had multifocal tumors (defined as more than one tumor, regardless of location) and were divided into three groups according to tumor distribution as follows: multiple (defined as more than one tumor in a single anatomical site) RPTs, multiple UTs, and synchronous tumors (RPT + UT). Each group consisted of 164, 152 and 369 patients, respectively.
The database included the following parameters: age, gender, comorbidities [coronary artery disease, end-stage renal disease, hypertension, diabetes mellitus, gout, and non-urothelial carcinoma (UC) malignancy], tumor distribution (multiple or synchronous), history of bladder tumor, pathological tumor grade, pathological tumor stage, lymph node status, histologic variant, carcinoma in situ (CIS), lymphovascular invasion (LVI), tumor necrosis, mortality from UTUC, disease recurrence, and bladder cancer recurrence. Pathological examinations were performed by genitourinary pathologists at each institution based on the same criteria. Tumor staging and grading strictly followed the 2010 American Joint Committee on Cancer TNM classification and the 2004 World Health Organization/International Society of Urologic Pathology consensus classification, respectively.
Regular follow-up appointments were arranged by urologists after RNU. Physical, laboratory and instrumental examinations were all performed according to standard guidelines. Patients with disease recurrence suffered from local relapse in the tumor bed or metastasis to the regional lymph nodes or distant organs; bladder recurrence was regarded as a separate clinical event. The cause of death was determined by attending physicians or death certificates.
For comparison of the demographic and clinicopathological factors of the three groups, Pearson’s chi-square test and Student’s t test were used for categorical and continuous variables, respectively. The Kaplan–Meier method was used to estimate the incidence of disease outcomes, including cancer-specific survival (CSS), disease-free survival (DFS) and bladder-recurrence free survival (BRFS) rates, and the Cox proportional hazards model was selected to evaluate the adjusted survival and recurrence rates. The association of each clinicopathological characteristic with prognosis was examined in the univariate analysis, and those characteristics that showed statistical significance were included in the multivariate analysis. All statistical assessments were two-sided, and p < 0.05 was considered statistically significant. SPSS software, version 26 (IBM; Armonk, NY, USA), was used to perform all analyses.
Results
Table 1 shows the demographic and clinicopathological features of the three groups based on tumor distribution. There were significant differences in history of bladder tumor (p < 0.001), tumor grade (p = 0.002), CIS (p = 0.028), LVI (p = 0.016), and tumor necrosis (p = 0.006) among the three groups.
Cancer-specific outcomes
Cancer-specific outcomes include CSS and DFS. Focusing on patients with multifocal tumors, Table 2 summarizes the univariate and multivariate analyses of the CSS and DFS rates. Regarding the CSS rate, tumor grade (p = 0.017), histologic variant (p < 0.001), and tumor necrosis (p < 0.001) were significant in the univariate analysis but not in the multivariate analysis. Non-UC malignancy (p = 0.002; p = 0.003), pathological T stage (both p < 0.001) and LVI (p < 0.001; p = 0.002) were significantly associated with CSS in both analyses. The overall 5-year CSS rate in this population was 75%. Figure 1 show the unadjusted and adjusted survival curves of the three groups.
For the DFS rate, tumor grade (p < 0.001), pathological N stage (p = 0.001) and histologic variant (p < 0.001) were only significant in the univariate analysis, while end-stage renal disease on dialysis (p = 0.003; p = 0.046), pathological T stage (both p < 0.001), LVI (p < 0.001; p = 0.001), and tumor necrosis (p < 0.001; p < 0.04) were demonstrated to be independent prognostic factors for the DFS rate in both analyses (Table 2). Overall, the 5-year DFS rate was 60%, and the corresponding DFS rate curve is shown in Fig. 2.
Bladder recurrence
The overall 5-year BRFS rate in this cohort was 57%. In the univariate analysis, age (p = 0.014), gender (p = 0.001), gout (p < 0.001), history of bladder tumor (p = 0.002), non-UC malignancy (p = 0.022), and tumor distribution (p = 0.046) were significantly correlated with the BRFS rate (Table 3). Additionally, age (p = 0.014), gender (p = 0.020), gout (p < 0.001), history of bladder tumor (p = 0.002), and tumor distribution (p = 0.020) showed significant associations with the BRFS rate in the multivariate analysis (Table 3). Figure 3 demonstrates the cumulative BRFS of the three groups, and the adjusted curve shows that the recurrence rate of bladder cancer in the synchronous group is higher than that in the multiple RPT group (Fig. 3b, p = 0.018).
Discussion
Previous studies showed that multifocal tumors had a worse prognosis and higher progression rate than single lesions12,13,14,19. A possible explanation included the correlation of multifocal tumors with a larger volume of disease20. Moreover, 61.8% of the patients with multifocal tumors in this population presented with pT2 stage disease or higher at the time of diagnosis. This result was similar to that of the study by Chromecki et al.12, in which multifocal tumors were found to be more advanced than single lesions (65.1% vs 50.6% of tumors at stage pT2 or higher). Such extensive involvement and aggressive tumor behavior are likely to worsen patient prognosis. In addition, a correlation between the total tumor load and tendency of metastasis has been observed in other cancers21. We hypothesize that a similar trend might exist in UTUC, and this requires further research.
However, UTUC arises from two anatomical sites that present different biological contextures, and tumor location has been shown to have prognostic significance in recent studies3,6,8,9. It is unknown whether the distribution of lesions has an impact on the disease outcomes of multifocal UTUC. In previous analyses, the definitions of tumor multifocality were dissimilar. We reviewed the literature and found that tumor multifocality, tumor multiplicity, and synchronous RPT and UT were individually reported to be correlated with poor prognosis3,4,5,6,11,12,13,14,15,16,17,18,19,20. However, comparisons between multiple and synchronous tumors are lacking. Therefore, we divided patients with multifocal tumors into three groups according to tumor distribution for outcome analysis.
Under strict patient selection, we first sought to clarify whether the cancer-specific outcomes of multifocal tumors were affected by tumor distribution. We compared multiple RPTs, multiple UTs and synchronous tumors and found no difference in the CSS and DFS rates between the groups. Thus, we considered that tumor multifocality as a whole may exert a dominant impact on cancer-specific outcomes regardless of how the tumors are distributed. Moreover, the development of multiple lesions might also blur the distinction of localities, since multiple lesions in one location may encroach on the other location. Therefore, differences in the CSS and DFS rates among the three groups may have been obscured.
Although tumor distribution seemed to have little impact on cancer progression and prognosis in patients with multifocal tumors, our study did find differences in bladder recurrence rates based on tumor distribution in the multivariate analysis. Previous studies appeared to indicate that the bladder recurrence rate was higher in multifocal tumors compared to single tumors14,15,18,19. Through the subdivision of multifocal tumors, our analysis showed that the bladder recurrence rate of synchronous tumors was significantly higher than that of multiple RPTs. The results of Fradet et al.17 and our previous study5 partially supported our findings by demonstrating no significant difference in the BRFS rate between patients with multiple lesions and those with single lesions in multivariate analyses. In summary, we believe that within multifocal tumors, synchronous lesions have a more profound impact on intravesical recurrence compared with multiple RPTs.
There are two main theories for the development of multifocal UTUC. The first is field cancerization, in which carcinogens cause independent genetic alterations in different sites of the urothelium22. Although all included patients had multifocal lesions, the urinary tract involvement in patients with synchronous tumors appeared to be more extensive than in patients with multiple RPTs because they had skip lesions affecting the renal pelvis and ureter. The hypothesis is also supported by the higher incidence of urothelial CIS in synchronous tumors (Table 1, p = 0.028), because CIS is usually multifocal and can be a manifestation of extensive filed cancerization. Therefore, we consider that patients with synchronous tumors may have a wider range of cancerous changes, which are more likely to further develop bladder recurrence.
Tumor seeding is another hypothesis of multifocal UTUC, indicating that monoclonal cancer cells from the first tumor flow antegrade and redeposit in the urinary tract to grow new lesions23,24. We assumed that patients with multiple RPTs may be less likely to experience remote seeding compared to patients with synchronous tumors characterized by long-distance spread (renal pelvis to ureter). Therefore, patients with synchronous tumors had a higher risk of recurrence at the distal end (bladder).
While comprehensive molecular or genetic evidence is still needed, the findings of this study have clinical relevance. A single dose of intravesical chemotherapy after RNU has been suggested for high-risk patients by the authors of several studies and in the latest guideline to prevent bladder cancer development2,25,26. Our results indicated that those with synchronous tumors had the highest risk of bladder recurrence and may benefit from multiple intravesical instillations with stricter follow-up regimens.
Another finding of our study was the association of gout with bladder cancer recurrence in patients with UTUC. Previous studies have shown a higher risk of cancer development in patients with gout, especially the development of bladder cancer27,28. Based on our analysis, patients with gout were three times more likely to experience intravesical recurrence after RNU. Therefore, we suggest that these patients should also be closely monitored after RNU and prophylactic intravesical therapy.
Several limitations existed in this study because of its retrospective nature. First, among the multifocal tumors, only the pathological features of the dominant lesion were collected, preventing detailed analyses of individual lesions. Additionally, a centralized pathological review was difficult to conduct because this was a multi-institutional study. Furthermore, surgical practice may have changed over time throughout the three decades and, therefore, could have biased the disease outcomes. Nonetheless, this study provided insight into tumor multifocality and identified that tumor distribution had limited association with cancer-specific outcomes; however, the specific population with synchronous tumors had an increased risk for bladder recurrence.
In conclusion, multifocal UTUC represents a heterogeneous population in which tumor distribution affects disease outcomes to varying degrees. We demonstrated no difference in the CSS and DFS rates among patients with multiple RPTs, multiple UTs and synchronous tumors. However, synchronous tumors portended a higher bladder recurrence rate than multiple RPTs, which should be considered in clinical assessment. We believe that synchronous tumors may be a manifestation of widespread malignant involvement of the entire urinary tract, but have limited significance in the cancer-specific outcomes of multifocal UTUC.
Data availability
The authors declare that all data analyzed during the current study are available from the corresponding author upon reasonable request.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33. https://doi.org/10.3322/caac.21654 (2021).
Rouprêt, M. et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 79, 62–79. https://doi.org/10.1016/j.eururo.2020.05.042 (2020).
Ouzzane, A. et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur. Urol. 60, 1258–1265. https://doi.org/10.1016/j.eururo.2011.05.049 (2011).
Raman, J. D. et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur. Urol. 57, 1072–1079. https://doi.org/10.1016/j.eururo.2009.07.002 (2010).
Yu, L. C. et al. Prognostic significance of primary tumor location in upper tract urothelial carcinoma treated with nephroureterectomy: A retrospective, multi-center cohort study in Taiwan. J. Clin. Med. 9(12), 3866. https://doi.org/10.3390/jcm9123866 (2020).
Yafi, F. A. et al. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: A homogeneous series without perioperative chemotherapy. BJU Int. 110, E7-13. https://doi.org/10.1111/j.1464-410X.2011.10792.x (2012).
Wang, Q. et al. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China. BMC Urol. 19, 24. https://doi.org/10.1186/s12894-019-0457-5 (2019).
Wu, Y., Dong, Q., Liu, L., Han, P. & Wei, Q. The impact of tumor location and multifocality on prognosis for patients with upper tract urothelial carcinoma: A meta-analysis. Sci. Rep. 4, 6361. https://doi.org/10.1038/srep06361 (2014).
Lwin, A. A., Hsu, C. H. & Chipollini, J. Urothelial carcinoma of the renal pelvis and ureter: Does location make a difference?. Clin. Genitourin. Cancer 18, 45-49.e1. https://doi.org/10.1016/j.clgc.2019.10.023 (2020).
Yoo, S. et al. Impact of tumor location on local recurrence after nephroureterectomy for upper tract urothelial carcinoma: Implications for adjuvant radiotherapy. Clin. Genitourin. Cancer 15(2), e199–e204. https://doi.org/10.1016/j.clgc.2016.07.010 (2017).
Hurel, S. et al. Influence of preoperative factors on the oncologic outcome for upper urinary tract urothelial carcinoma after radical nephroureterectomy. World J. Urol. 33, 335–341. https://doi.org/10.1007/s00345-014-1311-8 (2015).
Chromecki, T. F. et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur. Urol. 61, 245–253. https://doi.org/10.1016/j.eururo.2011.09.017 (2012).
Novara, G. et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: Multi-institutional dataset from 3 European centers. Cancer 110, 1715–1722. https://doi.org/10.1002/cncr.22970 (2007).
Zou, L., Zhang, L., Zhang, H., Jiang, H. & Ding, Q. Comparison of post-operative intravesical recurrence and oncological outcomes after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. World J. Urol. 32, 565–570. https://doi.org/10.1007/s00345-013-1160-x (2014).
Fang, D. et al. Pattern and risk factors of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: A large Chinese center experience. J. Formos. Med. Assoc. 113, 820–827. https://doi.org/10.1016/j.jfma.2013.11.004 (2014).
Tanaka, N. et al. The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: A multi-institutional study. Urol. Oncol. 32(48), e19-26. https://doi.org/10.1016/j.urolonc.2013.07.003 (2014).
Fradet, V. et al. Risk factors for bladder cancer recurrence after nephroureterectomy for upper tract urothelial tumors: Results from the Canadian Upper Tract Collaboration. Urol. Oncol. 32, 839–845. https://doi.org/10.1016/j.urolonc.2014.04.006 (2014).
Otsuka, M. et al. Lower ureteral lesion is an independent predictor of intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinoma. Urol. Oncol. 34(59), e9-13. https://doi.org/10.1016/j.urolonc.2015.08.015 (2016).
Wu, Y. T. et al. Gender effect on the oncologic outcomes of upper urinary tract urothelial carcinoma in Taiwan. Int. Urol. Nephrol. 52, 1043–1048. https://doi.org/10.1007/s11255-020-02396-z (2020).
Williams, A. K. et al. Multifocality rather than tumor location is a prognostic factor in upper tract urothelial carcinoma. Urol. Oncol. 31, 1161–1165. https://doi.org/10.1016/j.urolonc.2011.12.004 (2013).
Coombs, N. J. & Boyages, J. Multifocal and multicentric breast cancer: Does each focus matter?. J. Clin. Oncol. 23, 7497–7502. https://doi.org/10.1200/jco.2005.02.1147 (2005).
Jones, T. D. et al. Molecular evidence supporting field effect in urothelial carcinogenesis. Clin. Cancer. Res. 11, 6512–6519. https://doi.org/10.1158/1078-0432.ccr-05-0891 (2005).
Habuchi, T. et al. Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet 342, 1087–1088. https://doi.org/10.1016/0140-6736(93)92066-3 (1993).
Hafner, C., Knuechel, R., Stoehr, R. & Hartmann, A. Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies. Int. J. Cancer 101, 1–6. https://doi.org/10.1002/ijc.10544 (2002).
O’Brien, T., Ray, E., Singh, R., Coker, B. & Beard, R. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: A prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C trial). Eur. Urol. 60, 703–710. https://doi.org/10.1016/j.eururo.2011.05.064 (2011).
Ito, A. et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: The THP monotherapy study group trial. J. Clin. Oncol. 31, 1422–1427. https://doi.org/10.1200/jco.2012.45.2128 (2013).
Chen, C. J., Yen, J. H. & Chang, S. J. Gout patients have an increased risk of developing most cancers, especially urological cancers. Scand. J. Rheumatol. 43, 385–390. https://doi.org/10.3109/03009742.2013.878387 (2014).
Oh, Y. J. et al. Cancer risk in Korean patients with gout [published online ahead of print, 2021 Mar 23]. Korean J. Intern. Med. 1, 1. https://doi.org/10.3904/kjim.2020.259 (2021).
Acknowledgements
We thank Dr. Yu-Tsai Li for statistical assistance and all members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration Group. This study was supported by Kaohsiung Municipal Ta-Tung Hospital (Grant Number kmtth-109-R003) and supported partially by the Ministry of Science and Technology (Grant Number MOST 109-2314-B-037-095), Kaohsiung Medical University Hospital (Grant Number KMUH-DK(C)-110006), and Kaohsiung Medical University (Grant Numbers KMU-TC109A02, KMU-TC109B05). All members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration Group: Allen W. Chiu, Bing-Juin Chiang, Chao-Hsiang Chang, Chao-Yuan Huang, Cheng-Huang Shen, Cheng-Kuang Yang, Cheng-Ling Lee, Chen-Hsun Ho, Che-Wei Chang, Chia-Chang Wu, Chieh-Chun Liao, Chien-Hui Ou, Chih-Chen Hsu, Chih-Chin Yu, Chih-Hung Lin, Chih-Ming Lu, Chih-Yin Yeh, Ching-Chia Li, Chi-Ping Huang, Chi-Rei Yang, Chi-Wen Lo, Chuan-Shu Chen, Chung-Hsin Chen, Chung-You Tsai, Chung-Yu Lin, Chun-Hou Liao, Chun-Kai Hsu, Fang-Yu Ku, Hann-Chorng Kuo, Han-Yu Weng, Hao-Han Chang, Hong-Chiang Chang, Hsiao-Jen Chung, Hsin-Chih Yeh, Hsu-Che Huang, Ian-Seng Cheong, I-Hsuan Alan Chen, Jen-Kai Fang, Jen-Shu Tseng, Jen-Tai Lin, Jian-Hua Hong, Jih-Sheng Chen, Jungle Chi-Hsiang Wu, Kai-Jie Yu, Keng-Kok Tan, Kuan-Hsun Huang, Kun-Lin Hsieh, Lian-Ching Yu, Lun-Hsiang Yuan, Hao-Lun Luo, Marcelo Chen, Min-Hsin Yang, Pai-Yu Cheng, Po-Hung Lin, Richard Chen-Yu Wu, See-Tong Pang, Shin-Hong Chen, Shin-Mei Wong, Shiu-Dong Chung, Shi-Wei Huang, Shuo-Meng Wang, Shu-Yu,Wu, Steven Kuan-Hua Huang, Ta-Yao Tai, Thomas Y. Hsueh, Ting-En Tai, Victor Chia-Hsiang Lin, Wei-Chieh Chen, Wei-Ming Li, Wei-Yu Lin, Wen-Hsin Tseng, Wen-Jeng Wu, Wun-Rong Lin, Yao-Chou Tsai, Yen-Chuan Ou, Yeong-Chin Jou, Yeong-Shiau Pu, Yi-Chia Lin, Yi-Hsuan Wu, Yi-Huei Chang , Yi-sheng Lin, Yi-Sheng Tai, Yu-Khun Lee, Yuan-Hong Jiang, Yu-Che Hsieh, Yu-Chi Chen, Yu-Ching Wen, Yung-Tai Chen, Zhe-Rui Yang.
Author information
Authors and Affiliations
Contributions
H.C.Y. conceived the project, all authors collected the data, H.C.Y. analyzed the results, Z.L.S. drafted the manuscript, H.C.Y. and W.J.W. edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheu, ZL., Huang, CP., Chang, CH. et al. Tumor distribution affects bladder recurrence but not survival outcome of multifocal upper tract urothelial carcinoma treated with radical nephroureterectomy. Sci Rep 11, 19059 (2021). https://doi.org/10.1038/s41598-021-98696-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98696-0
- Springer Nature Limited