Abstract
This study investigated the role of dietary prebiotic mannan-oligosaccharides (MOS), and probiotic Bifidobacterium bifidum (BFD) in lipid metabolism, deposition, and consequent health indices in broiler chicken. The supplementation of 0.2% MOS along with either 106 or 107 CFU BFD/g feed resulted in downregulation of Acetyl-CoA carboxylase, fatty acid synthase, sterolregulatory element binding protein-1, and apolipoprotein B100; and up-regulation of peroxisome proliferator activated receptor-α AMP-activated protein kinase α-1, and stearoyl CoA (∆9) desaturase-1 hepatic expression in broiler chicken. The birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed depicted lower body fat percentage, palmitic acid, stearic acid, and saturated fatty acid contents, whereas, higher palmitoleic acid, oleic acid, and MUFA contents were observed. The ∆9-desaturase indices of chicken meat have shown higher values; and elongase index (only thigh) and thioesterase index have shown lower values in birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed. The meat health indices such as Polyunsaturated fatty acids (PUFA)/Saturated fatty acids (SFA) ratio, Mono-saturated fatty acids (MUFA)/SFA ratio, unsaturated fatty acids (UFA)/SFA ratio, hypocholesterolemic/hypercholesterolemic fatty acid ratio, saturation index, atherogenic index, thrombogenic index, and hypercholesterolemic fatty acid content were positively improved in birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed. Similarly, the birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed have shown lower serum triglyceride and total cholesterol levels along with higher high density levels and improved serum health indices cardiac risk ratio, atherogenic coefficient, and, atherogenic index of plasma.
Similar content being viewed by others
Introduction
The major organ of intermediary metabolism of lipids in broiler chicken is liver1,2. A balance between lipogenesis and lipolysis determines the rate of lipid deposition in broiler chicken3,4. A coordinated action of a series of genes involved in lipogenesis, lipolysis, transport, and deposition of lipids, such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), sterol regulatory element binding protein-1c (SREBP-1c), malic enzyme (ME), stearoyl CoA (Δ9) desaturase 1 (SCD-1), peroxisome proliferator activated receptor-α (PPAR-α), AMP-activated protein kinase (AMPK), and apolipoprotein B100 (apoB100) result in a net fat accretion in the body of broiler chicken3,5,6. Furthermore, the development of adipose in broiler chicken is determined by the serum triglyceride levels and the major substrates of lipid metabolism are triglycerides along with cholesterol fractions6. All these genes involved in lipogenesis, lipolysis, and transport of lipids are responsive to nutritional interventions directly or indirectly which results in alteration of lipid metabolism and the consequent lipid deposition in chicken3,6,7.
One of the prominent nutritional interventions in broiler chicken is the supplementation of dietary prebiotics and probiotics for desired growth, immunity, and meat quality of birds8,9; and recent studies have focussed on the importance of prebiotics and probiotics in the regulation of lipid metabolism in broiler chicken3,10,11,12. The supplementation of prebiotics in broiler chicken foster the population of lactic acid producing bacteria of Bifidobacterium and Lactobacillus genera4,13. The probiotic strains have the potential to regulate lipid metabolism in chicken by altering the expression of genes involved in lipogenesis, lipolysis, and transport of lipids which elicit hypolipidemic effects and enhance the health value of chicken meat10,12,13,14,15,16.
Furthermore, modern broiler chicken strains are hyperphagic in nature and tend to deposit more fat in body3. But, the excess fat deposition in chicken is a double facet problem—firstly, it is an economic burden on poultry producers which is discarded while processingand causes waste management problems, secondly, it is highly associated with cardiovascular diseases in humans17,18. However, the supplementation of synbiotics—prebiotic and probiotic combinations, have shown hypolipidemic and hypocholesterolemic effects3,13,19,20,21. Therefore, this study investigated the role of dietary prebiotic mannan-oligosaccharides (MOS), and probiotic Bifidobacterium bifidum (BFD) in lipid metabolism, deposition, and consequent health indices in broiler chicken.
Results
Hepatic gene expression
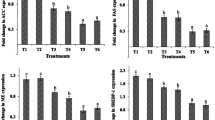
The relative expression pattern of genes involved in lipid metabolism of broiler chicken under the influence of BFD and MOS are given in Figs. 1, 2, 3, and 4. The expression pattern of ACC, FAS, ME, SERBP-1 (21 days only), and apoB100 depicted a progressive (P < 0.05) decrease from T1 (control) to T6. There were no significant differences between T1 and T2; and T5 and T6. Also, the expression pattern of SERBP-1 in broiler chicken at 42 days of age did not show any significant dietary effects. However, the expression pattern of PPAR-α, AMPKα-1 (21 days only), and SCD-1 revealed an increasing trend from T1 (control) to T6. Again, no significant differences were observed between T1 and T2; and T5 and T6. The expression of AMPKα-1 at 42 days of was not affected by dietary treatments.
Effect of dietary B. Bifidum (BFD) and mannan-oligosaccharides (MOS) on hepatic ACC, FAS, ME, and SREBP-1 gene expression in 21 days old broiler chicken. T1 (no BFD/MOS/BMD), T2 (20 mg BMD/kg diet), T3 (0.1% MOS + 106 cfu BFD/g feed), T4 (0.1% MOS + 107 cfu BFD/g feed), T5 (0.2% MOS + 106 cfu BFD/g feed), T6 (0.2% MOS + 107 cfu BFD/g feed). Results are presented as means ± SEM with six birds per treatment. Means values with different superscripts letters differ significantly (P < 0.05).
Effect of dietary B. Bifidum (BFD) and mannan-oligosaccharides (MOS) on hepatic apoB100, PPAR-α, AMPKα-1, and SCD-1 gene expression in 21 days old broiler chicken. T1 (no BFD/MOS/BMD), T2 (20 mg BMD/kg diet), T3 (0.1% MOS + 106 cfu BFD/g feed), T4 (0.1% MOS + 107 cfu BFD/g feed), T5 (0.2% MOS + 106 cfu BFD/g feed), T6 (0.2% MOS + 107 cfu BFD/g feed). Results are presented as means ± SEM with six birds per treatment. Means values with different superscripts letters differ significantly (P < 0.05).
Effect of dietary B. Bifidum (BFD) and mannan-oligosaccharides (MOS) on hepatic ACC, FAS, ME, and SREBP-1 gene expression in 42 days old broiler chicken. T1 (no BFD/MOS/BMD), T2 (20 mg BMD/kg diet), T3 (0.1% MOS + 106 cfu BFD/g feed), T4 (0.1% MOS + 107 cfu BFD/g feed), T5 (0.2% MOS + 106 cfu BFD/g feed), T6 (0.2% MOS + 107 cfu BFD/g feed). Results are presented as means ± SEM with six birds per treatment. Means values with different superscripts letters differ significantly (P < 0.05).
Effect of dietary B. Bifidum (BFD) and mannan-oligosaccharides (MOS) on hepatic apoB100, PPAR-α, AMPKα-1, and SCD-1 gene expression in 42 days old broiler chicken. T1 (no BFD/MOS/BMD), T2 (20 mg BMD/kg diet), T3 (0.1% MOS + 106 cfu BFD/g feed), T4 (0.1% MOS + 107 cfu BFD/g feed), T5 (0.2% MOS + 106 cfu BFD/g feed), T6 (0.2% MOS + 107 cfu BFD/g feed). Results are presented as means ± SEM with six birds per treatment. Means values with different superscripts letters differ significantly (P < 0.05).
Fat percentage and fatty acid profile of chicken meat
The results given in Tables 1 and 2 reveal significant effects of BFD and MOS supplementation on fat percentage and some constituent fatty acids of broiler chicken breast and thigh at 42 days of age, respectively. However, it is noteworthy to point out that CARIBRO Vishal tends to deposit more fat compared to other commercial chicken. From T1 (control) to T6 a significant decreasing trend was observed in percentages of fat (P < 0.01), palmitic acid (P < 0.01), stearic acid (P < 0.05), and SFA (P < 0.05). However, increasing trend was observed in percentage of palmitoleic acid (P < 0.01), oleic acid (P < 0.05), and MUFA (P < 0.01) in chicken breast and thigh meat from treatment T1 to T6. But, no significant differences were observed between T1 and T2; and T5 and T6. The other constituent fatty acids and PUFA were not affected by dietary treatments. Similar pattern was observed at 21 days of age (data not shown).
Lipid metabolism and health related indices
The product: precursor ratios were used to assess the activities of various enzymes involved in lipid metabolism. The enzyme activity indices at 42 days of age have revealed a progressive increase (P < 0.01) in ∆9-DI (18), ∆9-DI (16), and total DI; and a progressive decrease (P < 0.05) was observed in thioesterase index and elongase index (thigh only) from treatment T1 (control) to T6 in chicken meat (Table 3). The ∆5 + ∆6 Desaturase index and elongase index (breast only) did not reveal any significant treatment effect and no significant differences were observed between T1 and T2; and T5 and T6.
The health related indices measured in chicken breast and thigh meat were significantly influenced by BFD and MOS supplementation except ω-6: ω-3 PUFA ratio (Table 4). The PUFA:SFA ratio, MUFA:SFA ratio, UFA:SFA ratio (P < 0.01), DFA content, and h/H ratio (P < 0.05) exhibited a progressive increase; and S/P, AI, TI (P < 0.01), and HFA content (P < 0.05) revealed a progressive decrease from treatment T1 (control) to T6. But, no significant differences were observed between treatment group T1 and T2; and T5 and T6. Similar trend in lipid metabolism and health indices pattern was observed at 21 days of age (data not shown).
Serum lipid chemistry and health related indices
All the serum lipid parameters of broiler chicken measured in this study were significantly (P < 0.01) affected by BFD and MOS supplementation (Table 5). The serum TG and TC concentrations revealed a decreasing trend and serum HDL C an increasing trend from T1 (control) to T6. Similarly, the serum health indices—CRR, AC, and AIP exhibited a decreasing trend from T1 (control) to T6. However, for all the serum indices measure no significant differences were observed between treatment group T1 and T2; and T5 and T6.
Discussion
In this study, a nutrigenomic approach was adopted to understand the molecular mechanisms behind the effects of dietary MOS and BFD supplementation on lipid metabolism in broiler chicken. Since modern day broiler chickens are hyperphagic and thus, more prone to obesity they are considered as models of choice to study lipid metabolism and their consequent effects in response to dietary synbiotic supplementation3. Liver was chosen for the transcriptome analysis because it is a major metabolic organ of the body involved in lipid metabolism22. Lipid deposition is the function of balance between the lipolysis and lipogenesis in the body14 and alteration in the expression pattern of genes involved in lipid metabolism in response to dietary synbiotics affect the lipid deposition in broiler chicken3. The key enzymes involved in lipogenesis are ACC, FAS, ME, and SCD-1 which are regulated by SERBP-1 and the key enzymes involved in lipolysis are PPAR-α and AMPKα-12,3,23.
The present study revealed lower fat percentage of chicken meat from birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed. The possible determinants of lower fat percentage of chicken meat in the present study could be decline in the synthesis of lipids due to the down regulation of ACC, FAS, ME, and SERBP-1, lesser transport and deposition of synthesised lipids due to down regulation of apoB100, and enhanced oxidation or inhibition of lipogenesis due to upregulation of PPAR-α and AMPKα-1 expression in chicken liver. The process of lipogenesis is initiated by ACC by catalysing the rate-limiting step of carboxylation of acetyl-CoA to malonyl-CoA followed by a series of repetitive reaction by FAS3,24. During this process of lipogenesis FAS requires a continuous supply of reducing agent NADPH which is provided via oxidative decarboxylation of malic acid to pyruvic acid and CO2by ME25,26. In chicken, synbiotic supplementation down regulates the hepatic expression of SERBP-1c, a lipogenic nuclear transcriptional regulator that directly regulates the expressions of ACC, FAS, and ME3,25. On the other hand increased hepatic expression of PPAR-α was observed in broiler chicken due to dietary synbiotic supplementation3,27 which upregulates the expression of fatty acid catabolism genes, such as Carnitine palmitoyl transferase-1 and Acyl CoA oxidase-1, resulting in enhanced fatty acid β-oxidation in mitochondria28. Another mechanism put forward by earlier researchers as a cause of reduced body fat deposition is stimulation of farnesoid X receptor–PPAR-α–acyl CoA oxidase pathway in laying hens14 and mice29. Furthermore, the upregulation of AMPKα has been reported to decrease FAS expression by negatively regulating the expression of SERBP-1 in avian species30. Therefore, the consequent effect of SERBP-1c down regulation and upregulation of PPAR-α and AMPKα under dietary synbiotic supplementation is lower deposition of fat content in broiler chicken2,3 as observed in the present study. Another protein involved in lipid metabolism and investigated in this study was apoB100 protein. It is directly involved in transport and deposition of lipids in tissues31 and upregulation in its expression results in greater fat accumulation in broiler chicken32. Similar to the results of present study, synbiotic supplementation has been reported to down regulate the hepatic gene expression of apoB100 protein with the corresponding decline in muscle fat percentage3. Therefore, down regulation of hepatic apoB100 expression by synbiotic supplementation can be another possible mechanism of fat reduction in broiler chicken. However, it is noteworthy to state that lipid synthesis and its consequent deposition also depends on the age of birds and older chicken tend to deposit more fat in their body33. Corresponding to this age dependence of lipid deposition in chicken present study revealed the up regulation of PPAR-α and AMPKα-1 expression, and down regulation of SERBP-1 expression at 21 days of age but not at 42 days of age. It indicates that in young chicken rate of lipogenesis and lipid deposition is lower compared to adult birds3,34.
In the present study, ∆9-DI (18), ∆9-DI (16), and total DI increased significantly in birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed by mediating the up regulation of SCD-1 which catalyses the biosynthesis of MUFA from corresponding SFA3,35. Therefore, decrease in palmitic acid, stearic acid, and total SFA content; and increase in palmitoleic acid, oleic acid, and total MUFA content of chicken meat can be associated with up-regulation of SCD-1 expression. Lower thioesterase and elongase indices of chicken meat observed in the present study could be because of increased conversion of palmitic and stearic acids to their unsaturated counterparts under the influence of SCD-1 upregulation. The ∆5 + ∆6 desaturase indexof chicken meat, associated with catalysis of PUFAs synthesis, did not reveal any significant effect of synbiotic supplementation. This non-significant effect of synbiotic supplementation on ∆5 + ∆6 desaturase index and in turn on the PUFA content of chicken meat was also reported by Dev et al.3. On the similar pattern, the health indices of chicken meat detailed in this study were maximally improved in birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed because of increased MUFA content at the cost of SFA content of chicken meat which happened under the influence of SCD-1 up regulation. Other genes involved in lipid metabolism and studied in this experiment did not reveal any significant effect on health indices of chicken meat because of their non-significant effect on the fatty acid profile3.
Serum lipid metabolites such as triglycerides, total cholesterol, and other lipoproteins are sensitive indicators of lipid metabolism rate in chicken3,36. In the present study, serum TG, and TC decreased, whereas, serum HDL C increased in birds supplemented with 0.2% MOS along with either 106 or 107 CFU BFD/g feed. This hypocholesterolemic and hypolipidemic effect of synbiotic supplementation in broiler chicken resulted in improved serum health indices – CRR, AC, and AIP which was also reported by3 in broiler chicken in response to synbiotic supplementation. Improved serum lipid profile has been reported earlier in broiler chicken due to synbiotic supplementation2,3,37,38. Various mechanism of lipid lowering effects due to probiotics have been put forward, such as enhancement of deconjugation of bile acids39 which diverts more cholesterol towards synthesis of bile acids, intestinal conversion of cholesterol to coprostanol40, and subsequent excretion via faeces3. Also, the inhibition of 3-hydroxyl-3-methylglutaryl-coenzyme (HMG-CoA) reductase has been linked to cholesterol lowering effect of probiotics9. On the other hand, the lipid lowering effects of prebiotics have been ascribed to their ability to increase gut viscosity and mucus layer thickness which in turn inhibits cholesterol uptake from intestines; and further has been reported to enhance cholesterol breakdown3,13.
In conclusion, this study establishes that reduction in fat content of broiler chicken meat under the influence of synbiotic supplementation in chicken feed occurs by three possible mechanisms—reduction in rate of lipogenesis by down regulation of ACC, FAS, ME, and SERBP-1 expression, reduced transport and deposition of lipids in tissues by down regulation of apoB100 expression, and enhancement of β-oxidation of body lipids by upregulation of PPAR-α and AMPKα-1 expression in chicken liver. The ∆9-desaturation of chicken meat increased significantly under the influence of synbiotic supplementation by mediating the upregulation of SCD-1 expression which consequently resulted in increase of MUFA content at the cost of SFA content and in turn improved certain health indices of chicken meat. Further, the synbiotic supplementation in chicken feed produced hypocholesterolemic and hypolipidemic effect which improved the serum health indices of broiler chicken.
Materials and methods
Animal ethics compliance
This study was approved and carried out according to the guidelines of Institutional Animal Ethics Committee (IEAC) of Central Avian Research Institute, Izatnagar. The study was carried out in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Experimental design
In this study a total of 288 one-day-old CARIBRO Vishal with considerable uniformity in body weight were obtained from the hatchery of Central Avian Research Institute, Izatnagar. The antibiotic bacitracin methylene disalicylate (BMD), prebiotic mannan-oligosaccharides (MOS), and probiotic Bifidobacterium bifidum (BFD) were added to corn-soybean meal basal diet of chicken to form six treatment groups—T1 (negative control diet), T2 (positive control, 20 mg BMD/kg diet), T3 (0.1% MOS + 106 cfu BFD/g feed), T4 (0.1% MOS + 107 cfu BFD/g feed), T5 (0.2% MOS + 106 cfu BFD/g feed), and T6 (0.2% MOS + 107 cfu BFD/g feed). The details of ingredients and nutrient composition of basal diet is shown in Supplementary 1. Each treatment group was allotted 48 birds in six replicates with eight birds in each. The diets in all the treatment groups were similar in energy, protein, and fatty acid profile to avoid potential confounding effects on the results of the study. BMD, with a certified 44% bacitracin activity, was purchased from ALPHARMA Animal Health Division New Jersey- USA and MOS was purchased from Kothari Fermentation & Biochem Ltd. India. BFD (UBBB-55), with strain number MTCC 5398, was purchased from Unique Biotech Ltd. India. It is of healthy human fecal origin in the form of cream to brown coloured powder with characteristic odour and certified stable at room temperature. The E. coli and Salmonella species per 10 g powder; and Staphylococcus aureus and Pseudomonas aeruginosa per g powder are certified to be absent with yeast and mould count not more than 100 cfu/g. To ensure the exact dose of probiotic, the concentration of live bacteria in BFD powder was verified by culture-based counting. Bifidobacterium Selective Count Agar Base (BSC Propionate Agar Base) was used for enumeration of BFD by making a serial dilution of BFD powder in sterile phosphate buffer saline. The dilution showing most visible and countable colony forming units on the agar was replicated six times and an average concentration was calculated at this dilution. The birds were fed ad libitum with free access to clean drinking water for an experimental period of 42 days.
For the analysis of lipid deposition and the consequent effects in broiler chicken in response to MOS and BFD supplementation sampling was done at 21 and 42 days of age. Six birds were sacrificed (one bird from each replicate with equal number of males and females) from each treatment group after 12 h of fasting and fresh breast and thigh meat samples (5 g) without skin (from same place) were collected in duplicate for fatty acid profile analysis. From the same birds liver samples (1 g) from similar areas were also collected and stored in RNAlater to analyze the expression profile of genes involved in lipid deposition in broiler chicken. Further, blood samples (2 ml) were collected from the same birds in duplicate in test tubes without anticoagulant. The serum was extracted from the blood samples and stored at − 20 °C till lipid profile analysis.
Hepatic RNA extraction and expression of genes involved in lipid metabolism
From the liver samples RNA extraction was done by using Trizol reagent (INVITROGEN, Carlsbad, CA, USA) following the instructions of manufacturer. Nanodrop (NANO DROP 1000, thermo-scientific, Singapore) was used to check RNA sample concentration at 260 nm, ethidium bromide staining was used to check RNA integrity by agarose gel-electrophoresis, and RNA sample purity was checked by UV spectrophotometry (OD260/OD280). The reverse transcription of extracted RNA samples (5 µg) was carried out along with a negative control by using ‘Revert Aid First strand cDNA synthesis kit’ (MBI Fermentas, Hanover, MD, USA) for cDNA synthesis. Again, the concentration of synthesized cDNA samples was determined by using Nanodrop at 260 nm (NANO DROP 1000, thermo-scientific, Singapore) and thereafter, stored frozen at − 20 °C till further use.
The expression analysis of major enzymes involved in lipid metabolism such as acetyl carboxylase (ACC), fatty acid synthase (FAS), malic enzyme (ME), apolipoprotein B100 (apoB100), sterolregulatory element binding protein-1 (SREBP-1), Stearoyl-CoA (Δ9) desaturase-1 (SCD-1), Peroxisome proliferator activated receptor- α (PPAR-α), and AMP-activated protein kinase α-1 (AMPKα-1) was carried out by using their specific primers against β-actin as housekeeping gene. Oligonucleotide primer pairs used for lipid metabolism related gene expression study shown in Table 6. The primers were synthesized commercially by Integrated DNA technologies (New Delhi). The ideal conditions for each gene specific primer pair was worked out through gradient PCR in Gradient thermal cycler (BIO-RAD, USA). Real time qPCR analysis was performed by IQ5 Cycler system (BIO-RAD, Hercules, CA, USA) with SYBER Premix Ex Taq reagent Kit (TAKARA Biotech, Japan). The qRT-PCR conditions were as follows: initial denaturation at 95 °C for 15 min; followed by 40 cycles of subsequent denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s. All the samples were run in triplicate in nuclease-free 8 tube-strips with optically clear flat caps (AXYGEN Scientific, Inc. USA). The gene expression levels were normalized to β-actin and the results were analyzed by 2–ΔΔCT method41.
Chromatography and fatty acid profile
Fatty acid methyl esters (FAMEs) were prepared directly from meat samples as described by O’Fallon et al.42 and C13:0 ME was used as internal standard. The fatty acid composition of FAMEs was analysed by capillary gas chromatograph by following the standardised laboratory protocol43. Further, fat percentage of meat samples was estimated with the help of Soxhlet extraction apparatus by refluxing of meat samples (2 g) in petroleum ether for 5–6 h44.
Lipid metabolism indices
The lipid metabolism indices were calculated from the data of fatty acid profile of meat samples. The measurement of enzyme activity indices determine the extent of desaturation activities in tissues and the conversion of fatty acids to relatively longer chain fatty acids. The activity of steroyl-CoA desaturases convert saturated fatty acids (SFA) to monounsaturated fatty acids (MUFA) which were calculated by relating the percentage of product to the correspondingsubstrate45:
The conversion of essential fatty acids (EFA)—linoleic acid (LA) and ALA was measure in terms of Δ5+ Δ6 desaturase index and the conversion of myristic acid (C14:0) to palmitic acid (C16:0) and further to steric acid (C18:0) was measured by thioesterase and elongase indices, respectively46:
Health indices of meat
Various health indices, useful for evaluating the nutritional value and healthiness of the chicken meat, were also calculated from the data of fatty acid profile. The saturated fatty acids (SFA) are considered as proatherogenic and prothrombogenic fatty acids, whereas, unsaturated fatty acids (UFA) are considered as antiatherogenic and antithrombogenic fatty acids. The common health indicators of chicken meat ω-6 to ω-3 PUFA, PUFA to SFA, MUFA to SFA, and UFA to SFA ratios were calculated. The other indicators of health value of chicken meat calculated in this study were saturation index (S/P), atherogenic index (AI), thrombogenic index (TI)46, desirable fatty acid (DFA) content, hypercholesterolemic fatty acids (HFA), and hypocholesterolemic to hypercholesterolemic fatty acid ratio (h/H)47.
Serum lipid chemistry and health related indices
The serum samples collected were used for the estimation of serum triglyceride (TG), total cholesterol (TC), and HDL cholesterol by using Span Diagnostic kits as per manufacturer’s instructions. Further, the atherogenic indices of serum Cardiac Risk Ratio (CRR), Atherogenic Coefficient (AC), and Atherogenic Index of Plasma (AIP) were calculated based on the serum lipid profile48.
Statistical analysis
Data are presented as mean ± SEM and each sampled bird was taken as an experimental unit. Following the General Linear Model procedure, one-way ANOVA (SPSS software-20) was conducted to determine the dietary effects on the parameters measured above. The significant mean differences were separated by Tukey post-hoc analysis and significance level was set at P < 0.05.
Ethical approval
All applicable institutional guidelines for the care and use of animals were followed. The experimental procedures carried out in this study were approved by the Institutional Animal Ethics Committee (IEAC) following the guidelines of ‘Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) 2012’established under the “Prevention of Cruelty of Animals Act 1960” of Indian Penal Code (18 September 2017/Project No. 11). The study was carried out in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Huang, J. et al. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 61, 8565–8572 (2013).
Zhou, M. et al. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids Health Dis. 15, 1–10 (2016).
Dev, K. et al. Dietary lactobacillus acidophilus and mannan-oligosaccharides alter the lipid metabolism and health indices in broiler chickens. Probiot. Antimicrob. Proteins 1, 1–14. https://doi.org/10.1007/s12602-020-09717-9 (2020).
Al-Khalaifa, H. et al. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 98, 4465–4479 (2019).
Tu, T., Su, Y., Li, G., Zhang, X. & Tong, H. Expression of lipid metabolism-associated genes in male and female white feather chicken. J. Poult. Sci. 53, 118–123 (2016).
Zhang, B., Yang, X., Guo, Y. & Long, F. Effects of dietary lipids and Clostridium butyricum on serum lipids and lipid-related gene expression in broiler chickens. Animal 5, 1909–1915 (2011).
Yang, X., Zhang, B., Guo, Y., Jiao, P. & Long, F. Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult. Sci. 89, 254–260 (2010).
Begum, J., Buyamayum, B., Lingaraju, M. C. & Dev, K. Probiotics: Role in immunomodulation and consequent effects. Lett. Anim. Biol. 01(01), 01–07 (2021).
Saleh, A. A., Paray, B. A. & Dawood, M. A. O. Olive cake meal and Bacillus licheniformis impacted the growth performance, muscle fatty acid content, and health status of broiler chickens. Animals 10, 695. https://doi.org/10.3390/ani10040695 (2020).
Saleh, A. A., Hayashi, K., Ijiri, D. & Ohtsuka, A. Beneficial effects of Aspergillus awamori in broiler nutrition. World Poult. Sci. J. 70, 857–864 (2014).
Park, Y. et al. Application of probiotics for the production of safe and high-quality poultry meat. Korean J. Food Sci. 36, 567–576 (2016).
Maiorano, G. et al. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 96, 511–518 (2017).
Dev, K. et al. Dietary Mannan-oligosaccharides potentiate the beneficial effects of Bifidobacterium bifidum in broiler chicken. Lett. Appl. Microbiol. 71(5), 520–530 (2020).
Wang, W. W., Wang, J., Zhang, H. J., Wu, S. G. & Qi, G. H. Supplemental Clostridium butyricum modulates lipid metabolism through shaping gut microbiota and bile acid profile of aged laying hens. Front. Microbiol. 11, 600 (2020).
Yoo, S. R. et al. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 21, 2571–2578 (2013).
Saleh, A. A., Shukry, M., Farrag, F., Soliman, M. M. & Abdel-Moneim, A. M. E. Effect of feeding wet feed or wet feed fermented by bacillus licheniformis on growth performance, histopathology and growth and lipid metabolism marker genes in broiler chickens. Animals 11(1), 83. https://doi.org/10.3390/ani11010083 (2021).
Bera, I. et al. Soapnut shell powder as immunomodulatory and welfare friendly feed additive in broiler chicken. Indian J. Anim. Sci. 89, 1135–1139 (2019).
Kannan, M., Karunakaran, R., Balakrishnan, V. & Prabhakar, T. G. Influence of prebiotics supplementation on lipid profile of broilers. Int. J. Poult. Sci. 4, 994–997 (2005).
Zhang, J. et al. Preventive effect of Lactobacillus plantarum CQPC10 on activated carbon induced constipation in Institute of Cancer Research (ICR) mice. Appl. Sci. 8, 1498 (2018).
Rather, S. A. et al. Anti-obesity effect of feeding probiotic dahi containing Lactobacillus casei NCDC 19 in high fat diet-induced obese mice. Int. J. Dairy Technol. 67, 509 (2014).
Ooi, L. G. & Liong, M. T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 11, 2499–2522 (2010).
Sevane, N. et al. Dietary inulin supplementation modifies significantly the liver transcriptomic profile of broiler chickens. PLoS ONE 9, e98942 (2014).
Lu, M. W., Cao, Y., Xiao, J., Song, M. Y. & Ho, C. T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 9, 4569–4581 (2018).
Nelson, D. L. & Cox, M. M. (eds) Lehninger Principles of Biochemistry (Worth Publishers, 2011).
Richards, M. P. et al. Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J. Nutr. 133, 707–715 (2003).
Zhao, S., Ma, H., Zou, S., Chen, W. & Zhao, R. Hepatic lipogenesis in broiler chickens with different fat deposition during embryonic development. J. Vet. Med. A 54, 1–6 (2007).
Royan, M., Meng, G. Y., Othman, F., Sazili, A. Q. & Navidshad, B. Effects of conjugated linoleic acid, fish oil and soybean oil on PPARs (α & γ) mRNA expression in broiler chickens and their relation to body fat deposits. Int. J. Mol. Sci. 12, 8581–8595 (2011).
Han, S., Vaziri, N. D., Gollapudi, P., Kwok, V. & Moradi, H. Hepatic fatty acid and cholesterol metabolism in nephrotic syndrome. Am. J. Transl. Res. 5, 246 (2013).
Weng, H., Endo, K., Li, J., Kito, N. & Iwai, N. Induction of peroxisomes by butyrate-producing probiotics. PLoS ONE 10, e0117851 (2015).
Proszkowiec-Weglarz, M. & Richards, M. P. Expression and activity of the 5’-adenosine monophosphate-activated protein kinase pathway in selected tissues during chicken embryonic development. Poult. Sci. 88, 178 (2009).
Basiricò, L. et al. Down-regulation of hepatic ApoB100 expression during hot season in transition dairy cows. Livest. Sci. 137, 49–57 (2011).
Zhang, W. et al. FoxO1 regulates multiple metabolic pathways in the liver: Effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281, 10105–10117 (2006).
Zerehdaran, S., Vereijken, A. L., Arendonk, J. V. & Van der Waaij, E. H. Effect of age and housing system on genetic parameters for broiler carcass traits. Poult. Sci. 84, 833–838 (2005).
de Souza Khatlab, A., Del Vesco, A. P., Gasparino, E. & de Oliveira Neto, A. R. Gender and age effects on the expression of genes related to lipid metabolism in broiler’s liver. Czech J. Anim. Sci. 63, 103–109 (2018).
Huang, J. B., Zhang, Y., Zhou, Y. B., Wan, X. C. & Zhang, J. S. Effects of epigallocatechin gallate on lipid metabolism and its underlying molecular mechanism in broiler chickens. J. Anim. Physiol. Anim. Nutr. 99, 719–727 (2015).
Bogusławska-Tryk, M., Piotrowska, A., Szymeczko, R., Burlikowska, K. & Głowińska, B. Lipid metabolism indices and fatty acids profile in the blood serum of broiler chickens fed a diet with lignocellulose. Braz. J. Poult. Sci. 18, 451–456 (2016).
Yeon, S. J., Kim, S. K., Kim, J. M., Lee, S. K. & Lee, C. H. Effects of fermented pepper powder on body fat accumulation in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 77, 2294–2297 (2013).
Naghi, S. A., Ghasemi, H. A. & Taherpour, K. Evaluation of Aloe vera and symbiotic as antibiotic growth promoter substitutions on performance, gut morphology, immune responses and blood constitutes of broiler chickens. Anim. Sci. J. 88, 306–313 (2016).
Begley, M., Hill, C. & Gahan, C. G. M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 72, 1729–1738 (2006).
Lye, H. S., Rusul, G. & Liong, M. T. Removal of cholesterol by Lactobacilli via incorporation of and conversion to coprostanol. J. Dairy Sci. 93, 1383–1392 (2010).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 3, 1101 (2008).
O’Fallon, J. V., Busboom, J. R., Nelson, M. L. & Gaskins, C. T. A direct method for fatty acid methyl ester (FAME) synthesis: Application to wet meat tissues, oils and feedstuffs. J. Anim. Sci. 85, 1511–1521 (2007).
Mir, N. A. et al. Inclusion of flaxseed, broken rice, and distillers dried grains with solubles (DDGS) in broiler chicken ration alters the fatty acid profile, oxidative stability, and other functional properties of meat. Eur. J. Lipid Sci. Technol. 120, 1700470 (2018).
Cunniff, P. Official Methods of Analysis of AOAC International (Association of Official Analytical Chemists, Washington, DC, 1995).
Dal Bosco, A., Mugnai, C., Ruggeri, S., Mattioli, S. & Castellini, C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poult. Sci. 91, 2039–2045 (2012).
Kumar, F. et al. Role of flaxseed meal feeding for different durations in the lipid deposition and meat quality in broiler chickens. J. Am. Oil Chem. Soc. 96, 261–271 (2019).
Pilarczyk, R., Woojcik, J., Sablik, P. & Czerniak, P. Fatty acid profile and health lipid indices in the raw milk of Simmental and Holstein-Friesian cows from an organic farm. S. Afr. J. Anim. Sci. 45, 30–38 (2015).
Frohlich, J. & Dobiášová, M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin. Chem. 49, 1873–1880 (2003).
Funding
This study was funded by the Department of Biotechnology, Ministry of Science and Technology, Government of India (Grant No. BT/PR9724/AAQ/1/571/2013).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.B. and R.K., Methodology: K.D., N.A.M. and A.B.; Formal analysis and investigation: K.D., N.A.M., J.S., R.P. and K.B.; Writing—original draft preparation: A.B., K.D., N.A.M., J.B., K.B. and J.S.; Writing—review and editing: A.B., R.K., S.T., N.A.; Funding acquisition: A.B.; Resources: S.T., J.B., J.S. and R.K.; Supervision: A.B. and R.K. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dev, K., Begum, J., Biswas, A. et al. Hepatic transcriptome analysis reveals altered lipid metabolism and consequent health indices in chicken supplemented with dietary Bifidobacterium bifidum and mannan-oligosaccharides. Sci Rep 11, 17895 (2021). https://doi.org/10.1038/s41598-021-97467-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97467-1
- Springer Nature Limited
This article is cited by
-
Dietary Supplementation of Compound Probiotics Improves Intestinal Health by Modulated Microbiota and Its SCFA Products as Alternatives to In-Feed Antibiotics
Probiotics and Antimicrobial Proteins (2024)