Abstract
Nocturnal enuresis (NE) is a common problem among 10% school-aged children. The etiologies underlying childhood NE is complex and not fully understood nowadays. Nevertheless, increasing evidence suggests a potential link between neurobehavioral disorders and enuresis in children. In this study, we aimed to explore novel metabolomic insights into the pathophysiology of NE and also, its association with pediatric psychiatric problems. Urine collected from 41 bedwetting children and 27 healthy control children was analyzed by using 1H-nuclear magnetic resonance spectroscopy from August 2017 to December 2018. At regular follow-up, there were 14 children with refractory NE having a diagnosis of attention deficient hyperactivity disorder (ADHD) or anxiety. Eventually, we identified eight significantly differential urinary metabolites and particularly increased urinary excretion of betaine, creatine and guanidinoacetate linked to glycine, serine and threonine metabolism were associated with a comorbidity of neurobehavioral disorders in refractory bedwetting children. Notably, based on physiological functions of betaine acting as a renal osmolyte and methyl group donor, we speculated its potential role in modulation of renal and/or central circadian clock systems, becoming a useful urinary metabolic marker in diagnosis of treatment-resistant NE in children affected by these two disorders.
Similar content being viewed by others
Introduction
Nocturnal enuresis (NE), also known as bedwetting, is defined as involuntary urination while asleep in children above 5 years of age by the International Children’s Continence Society1. Based on the association with daytime voiding dysfunction, it can be further categorized into mono-symptomatic nocturnal enuresis (MNE) and non-monosymptomatic nocturnal enuresis (NMNE), respectively2. The literatures reported the prevalence of childhood NE is highly variable among different countries, ranging from 2.3 to 25%3,4,5. In Taiwan, there was 6.8% of elementary-school children reported to have the bedwetting problem5. Overall, the global prevalence of NE is around 10% among school-aged children, particularly predominance in boys6. Notably, if bedwetting children and adolescents are not treated properly or ignored without treatment, there is 2% of the population persistent into adulthood7.
Until the present, the exact pathophysiology of childhood NE remains unclarified and is likely to be multifactorial, involving biological, developmental, genetic, psychosocial, and environmental aspects8. Theoretically, nocturnal polyuria caused by vasopressin deficiency, detrusor overactivity and high arousal thresholds have been regarded as the three major pathogenetic mechanisms for MNE. In this context, pharmacological therapy consisting of desmopressin, anticholinergics (e.g., oxybutynin and tolterodine), or tricyclic antidepressants (e.g., imipramine, amitriptyline and desipramine) and bedwetting alarms become the mainstay of clinical management and treatment in the present days1. However, 40% of bedwetting children and adolescents still have insufficient responses to the standard treatment or experience a relapse after stopping treatment, particularly for those with NMNE1,9. This reflects beyond the three-system model, there are other factors involved in enuresis pathogenesis.
In the early era, bedwetting was viewed as a primarily pediatric psychiatric disorder. Since the end of twentieth century, however, the bio-behavioral perspective on childhood NE has considerably grown and indicates enuresis is more likely the result of developmental delays than psychiatric illness8. Nevertheless, numerous clinical studies and population-based studies have uncovered the strong association between enuresis and attention deficit hyperactivity disorder (ADHD)10,11,12,13,14,15. In this modern world, ADHD is the most common neurodevelopmental disorder affecting 7.2–15.5% of school-aged children and adolescents, particularly in boys16. Furthermore, it is evident that ADHD children are vulnerable to NE and other voiding disturbances, such as daytime incontinence, urgency and frequency16. The prevalence estimates of enuresis in children with ADHD have been reported in 22–32%12,13. On the other hand, 30–40% of bedwetting children ages six to 12 years had a diagnosis of different types of ADHD11,14. Although psychiatric problems and NE are still a “chicken-and-egg” situation, this bidirectional relationship between the two disorders is potentially explained by deficits in arousal and/or developmental delays in central nervous system17,18.
Among contemporary research, metabolomic approaches have been widely applied in diverse clinical conditions, allowing assessment of metabolic pathways and networks liked with genetic and environmental factors. In this study, we attempted to characterize urine metabolic phenotyping in relation to childhood NE using 1H-nuclear magnetic resonance (NMR) spectroscopy. Furthermore, we also aimed to explore potentially useful metabolic markers and relevant pathways associated with a comorbidity of neurobehavioral disorders such as ADHD in children particularly with treatment-resistant NE.
Results
Participant characteristics
A total of 68 participants consisting of 41 children with NE (age: 8.9 ± 2.4 years; male/female:22/19) and 27 healthy controls (age:7.4 ± 0.5 years; male/female:10/17) were enrolled in this study. During 3- to 32-month follow-up of drug treatment, 14 bedwetting children had a diagnosis of neurobehavioral or emotional disorders (age: 8.9 ± 2.5 years; male/female: 7/7), including 10 cases of ADHD, two cases of ADHD and coexisting autism spectrum disorder, and two cases of anxiety disorder, based on the Diagnostic and Statistical Manual of Mental Disorders, fourth edition19. Of those 14 children with both NE and neuropsychiatric problems, there were 12 cases of MNE and two cases of NMNE. Table 1 showed demographic data of all participants. The baseline characteristics (i.e., gender, height, weight and body mass index) were no different between the three groups, bedwetting children with and without ADHD or anxiety, and health controls. Regarding frequent or severe bedwetting (> three episodes per week), there was male predominance of 61.9% (13/21) in the subgroup of childhood NE without ADHD or anxiety. On the other hand, males and females with childhood NE were at equal risk for a comorbidity of ADHD or anxiety. During the follow-up period, more than half of children with both NE and neurobehavioral disorders (57%) were resistant to the combination medications of Minirin® and oxybutynin or imipramine, whereas 52% of the bedwetting children without comorbid ADHD or anxiety had adequate response to the monotherapy with Minirin®.
Urinary metabolic phenotyping differentiates children with NE and the population subgroups of the affected children with/without concomitant neurobehavioral disorders from health controls
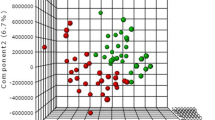
In Fig. 1A, PLS-DA illustrated the recognition of the two groups, children with NE and healthy controls, according to global metabolites discovered in urine. Based on the VIP score (≥ 1.0) and fold change of the identified metabolic species, 17 urinary metabolites significantly differentially expressed in the group of childhood NE compared with healthy controls (p < 0.05), shown in Table 2. These 17 characteristic urinary metabolites were N-isovaleroylglycine, isoleucine, glycine, alanine, N, N-dimethylglycine (DMG), tyrosine, creatine phosphate, lysine, histidine, 3-hydroxyisovalerate, tiglylglycine, N-acetylglutamate, 4-hydroxyphenylacetate, methanol, hypoxanthine, N-acetylglutamine and acetylsalicylate. Accordingly, a clustering analysis of the differential urinary metabolites was illustrated in the heatmap (Fig. 1B), displaying the degree of individual metabolite of interest contributed to the group distinction.
(A) The PLS-DA of 1H NMR urine spectra is measured from 41 children with NE (red colored dots) and 27 healthy controls (green colored dots). The score plot shows the model discrimination between the two groups based on the global metabolites identified in urine. 29.3% and 10.2% are the scores of components 1 and 2, respectively. (B) In the heat map, it illustrates the degree of the 17 differential urinary metabolites discovered contributes the group separation.
Furthermore, in comparison with non-bedwetting healthy children, the increased concentrations of 23 urinary metabolites were significant in children with NE and comorbid ADHD or anxiety disorder (p < 0.05) (Table 2). These 23 differentially expressed urinary metabolites were N-isovaleroylglycine, isoleucine, glycine, alanine, DMG, tyrosine, creatine phosphate, lysine, histidine, 3-aminoisobutyrate, 3-hydroxyisovalerate, tiglylglycine, N-acetylglutamate, 4-hydroxyphenylacetate, methanol, leucine, valine, 3-hydroxyisobutyrate, formate, guanidoacetate, betaine, creatine and taurine. Among these metabolites of interest, there were some overlapping metabolites of interest identified in the population subgroup of childhood NE but without ADHD (Table 2). Furthermore, there were three urinary metabolites, including valine, 3-aminoisobutyrate and dimethylamine, significantly higher expressed in children affected by the two disorders compared with bedwetting children without comorbid ADHD or anxiety (p < 0.05) (Table 2). All three are known physiological metabolites involved in brain functions and disease20,21,22.
Identification of characteristic urinary metabolites and the relevant functional pathway in bedwetting children affected by the presence of ADHD or anxiety
Among the three data sets consisting of NE vs. health controls, NE with comorbid ADHD or anxiety versus health controls, and NE without ADHD or anxiety vs. health controls, Venn diagram (Fig. 2) demonstrated the number of overlapping urinary metabolites from each data set. Apparently, there were nine characteristic urinary metabolites (i.e., alanine, creatine phosphate, glycine, histidine, isoleucine, lysine, DMG, N-isovaleroylglycine, and tyrosine) associated with childhood NE. Furthermore, eight characteristic urinary metabolites (i.e., 3-aminoisobutyrate, 3-hydroxyisobutyrate, betaine, creatine, formate, guanidoacetate, taurine and valine) linked to children with NE and comorbid ADHD or anxiety disorder. Tenfold internal cross-validation as well as permutation tests were performed to assess the quality of the resulting statistical models. Quality parameters Q2 (quality of prediction) and R2 (explained variance) were measured to judge the predictive power of PLS-DA among these three data sets (Supplemental Table S1). Subsequently, the KEGG pathway analysis revealed the changes of urinary betaine, creatine and guanidinoacetate concentrations involved in glycine, serine and threonine metabolism appeared to have statistically significant relations to a comorbidity of ADHD or anxiety in persistent bedwetting children. And, more than half of children affected by the two disorders were resistant to the standard medications and presented frequent NE (> three episodes per week).
The three-cycle Venn diagram (the upper of the diagram) identifies several overlapping urinary metabolites from each data set, children with both NE and ADHD or anxiety versus health controls (HC), bedwetting children without ADHD or anxiety versus HC, and children with NE versus HC, respectively. As a result, nine urinary metabolites of interest are associated with childhood NE. Furthermore, eight characteristic urinary metabolites statistically link to co-occurrence of neurobehavioral problems in children with NE. The KEGG database is applied for functional metabolic pathway analysis (the bottom of the diagram), demonstrating urinary betaine, creatine and guanidinoacetate involved in glycine, serine and threonine metabolism (p = 0.0005 and FDR = 0.039) may have an influential role to play in children affected by these two disorders.
Discussion
In this study, 1H-NMR spectroscopy was applied to investigate global metabolomic profiling of urine from children with NE, particularly interest in biomarker discovery of children with both NE and neurobehavioral disorders. However, distinct clinical entities with overlapping manifestations, and untargeted metabolomics approach could be an obstacle to identification of specific metabolites. Nevertheless, through the integrated analysis of urine metabolomic data, the result uncovered the critical metabolic pathway involved in treatment- resistant NE in children affected by the presence of ADHD or anxiety. Also, several potential and characteristic urinary metabolic markers were identified in children affected by these two disorders, particularly urinary betaine is one of the most likely candidates in the pathogenesis.
Betaine (N, N, N-trimethylglycine) is a dietary nutrient essential for health. It is mostly obtained either from foods (e.g., wheat, spinach, beets and shellfish), or synthesized by mitochondrial oxidation of choline23. Betaine accumulates in many organs of the human body, including liver, kidneys and brain24. In the kidney, betaine is freely filtered through the glomerulus and nearly completely reabsorbed by the renal tubules. Physiologically, betaine functions as an organic osmolyte to protect cells against hyperosmotic stress (e.g., hypernatremia and hyperglycemia), and maintain cell volume without disrupting cell function. Besides, it is also a direct methyl-group donor via transmethylation for use in many biochemical pathways25. For example, betaine donates a methyl group to guanidinoacetate via methionine to produce creatine, which is beneficial for athletic performance and muscle strength26. Because of its dual roles, betaine deficiency or abnormalities in betaine metabolism is increasingly linked to various human diseases such as obesity, diabetes mellitus, cardiovascular and neuropsychiatric disorders27,28,29.
In the present study, we found children with NE and comorbid ADHD or anxiety presented excessive urinary betaine loss. Besides, increased urinary DMG excretion was also observed in bedwetting children compared with healthy controls. DMG is a metabolite of betaine, and normally excreted in urine or metabolized to sarcosine. It exerts an osmoregulatory influence on sodium excretion by the kidney, which is similar to betaine. Taken together, our result speculated the potential role of imbalances in renal osmolyte regulation in enuresis pathogenesis. Nocturnal polyuria caused by circadian rhythm disturbance of the antidiuretic pituitary hormone vasopressin has been considered as the important pathogenesis of childhood NE30. However, beyond the rhythm driven by hypothalamus (central clocks), many literatures reported disturbed renal circadian rhythm (local clocks) played an influential role in nocturnal polyuria31,32. For instance, increased nocturnal sodium excretion, disturbances in hormones responsive for water and sodium handling (e.g. angiotensin II, aldosterone and atrial natriuretic factor), higher urinary excretion of renal autacoid prostaglandin E2, higher nocturnal blood pressure and decreased glomerular filtration rate (GFR) overnight were reported33,34.
Recently, Gil and his colleagues applied NMR-based method to analyze urine samples from 277 patients with chronic kidney disease (CKD) at the stage 1–535. They discovered several urinary metabolites significantly linked to severity and the progression of CKD. Particularly urinary betaine and myo-inositol concentrations were negatively correlated with annual declines in GFR. This finding was further validated by kidney transcriptome analysis, demonstrating decreased renal expressions of betaine and myo-inositol transporters in CKD mice. Besides, abnormally increased urinary excretion of betaine was also significant in diabetic patients, which was associated with hyperglycemia and proximal tubular dysfunction27. Taken together, these suggested disturbed renal osmolyte regulation could lead to renal cell damage and deterioration of renal function. Increased urinary excretion of betaine and DMG in childhood NE was first reported in our study. We hypothesized that betaine and/or DMG might attribute to disturbed renal circadian rhythm, caused by impaired urinary concentration ability via osmolyte (tonicity)-dependent pathway.
Betaine has gained great attention as its anti-inflammatory or anti-oxidative functions on human disease in the recent days36. Considerable neuroscience re-search has shown the deficiencies of betaine and its precursor, choline, are associated with a variety of neurocognitive disorders (e.g., epilepsy, autism spectrum disorder, depression, schizophrenia and Alzheimer’s disease)29,37,38. And, systemic betaine supplementation was shown to benefit cognitive and memory functions in both animal and human studies39. The growing knowledge of betaine and other methyl group donors such as folate and vitamin B12 indicates their role as powerful epigenetic modulators essential for normal biological function and development. The deficiencies had a close linkage of modifications in histone and DNA methylation in brain38,39. Furthermore, the influence of osmolyte homeostasis on neural communication has been studied in many literatures40,41,42. Knight and his colleagues demonstrated a prominent role of brain betaine in neurotransmission43. They found betaine could provide neuroprotection via inhibitory neurotransmitter production and/or recycling, particularly involved in modulation of hippocampal functions. As NE and ADHD in children are widely accepted as the disorders of delayed brain development, our finding might speculate the role of betaine metabolism to play directly or indirectly in these two disorders.
Conclusively, in this pilot study, our results provided metabolomics insights into pathophysiological of childhood NE and its association with neurobehavioral disorders such as ADHD. We also suggested the likelihood of betaine in modulation of renal and central circadian clock systems in children with both NE and ADHD.
The study limitations
There are several limitations and unclarified issues in our study. Firstly, the sufficient number of patient samples and the longer duration of follow-up will be required for better characterization and categorization of participants into different population subgroups. Although higher urinary expression of betaine was observed in the persistent bedwetting children with concomitant ADHD or anxiety, we do not analyze betaine concentration in blood and its interaction with other osmolytes or neuro-transmitters. Also, children diagnosed of neuropsychiatric disorders but without the problem of enuresis are not included in this study. Nearly half of the participants from the group of childhood NE collected their urine for metabolomics analysis before initiation of Minirin® treatment. Although our analysis showed very few metabolites significantly differentially expressed between NE children with and without receiving Minirin®, the potential of drug interference cannot be completely ignored. Therefore, further studies are needed for investigating the usefulness of urinary betaine as a non-invasive biomarker, and non-pharmacological approach for children with treatment-resistant enuresis affected by the presence of neurobehavioral disorders.
Methods
Participants and study design
This was a cross-sectional study consisting of 68 children while visits in outpatient clinics of Lin-Kou Chang Gung Memorial Hospital in Northern Taiwan from August 2017 to December 2018. Among the participants, 41 children were taken to see a pediatric nephrologist or urologist for bedwetting problems with and without daytime lower urinary symptoms such as incontinence, frequency or urgency. 27 children undergoing a well-child check were enrolled as health controls. A spot morning urine sample from individuals was collected during a morning visit. All of the participants had normal urinalysis and normal findings in renal ultrasonography. For 41 children with NE, furthermore, urine osmolality and the ratio of urine calcium to urine creatinine of spot morning urine samples were also measured, showing the values were within a normal range. At the time of urine collection, 21 bedwetting children did not start taking desmopressin acetate (Minirin®), but another 20 cases were already treated with Minirin® for one to three months. Nevertheless, desmopressin medication appeared not to be a factor for subsequent metabolomics analysis (Supplementary Table S2).
During the period of follow-up, bedwetting children who did not respond to the combination treatment consisting of Minirin® and oxybutynin or imipramine, and presented frequent NE (≥ three episodes per week) were referred to the psychiatric outpatient clinic in our hospital for evaluation. This study was approved by the local institutional review board (No. 201601179A3) of Lin-Kou CGMH, Taiwan. Written informed was obtained from children and/or their parent or legal guardian. All methods were carried out in accordance with the approved guidelines and regulations for medical research involving human subjects.
1H-NMR spectroscopy: urine sample preparation, data processing and analysis
Urine samples required for spectrum acquisition were prepared as described previously44. Firstly, 900 μL of urine was mixed with 100 μL of 1.5 M phosphate buffer in deuterium water containing 0.04% 3-(trimethylsilyl)-propionic-2,2,3,3-d4 ac-id sodium salt (TSP) as an internal chemical shift reference standard. The samples were vortexed for 20 s and centrifuged at 12000 g at 4 °C for 30 min. 600 μL supernatant was then transferred to a 5-mm NMR tube analysis. Sample spectra were measured by Bruker Avance 600 MHz NMR spectrometer (Bruker-Biospin GmbH, Karlsruhe, Germany). A total of 64 scans were collected for NMR spectra into 64 K computer data points with a spectral width of 10, 000 Hz (10 ppm). 1D NMR spectra were preprocessed by zerofills and exponential multiplication (0.3 Hz line broadening factor) prior to Fourier transformation. The acquired 1H-NMR spectra were then manually phased, baseline-corrected, and referenced the chemical shift to TSP (δ 0.0 ppm) using TopSpin 3.2 software (Bruker BioSpin, Rheinstetten, Germany).
Subsequently, the 1H-NMR spectra were imported into NMRProcFlow software, providing comprehensive tools for spectra processing, ppm calibration, baseline correction, alignment, spectra bucketing and data normalization45. Least-squares algorithm and parametric time warping were used to correct the misalignment spectra. Spectrum bucketing was performed using the method of intelligent bucketing and variable size bucketing46. Metabolites were identified using the Chenomx NMR Suite 8.1 software (Chenomx Inc., Edmonton AB, Canada). Urine spectra were specifically normalized to the integral of creatinine peak at δ 3.045 ppm to compensate the differences in urinary concentration. As established NMR data analysis in the previous experiment44, the normalized 1H-NMR spectra data were transformed using generalized log transformation (glog) and uploaded to MetaboAnalyst 4.0 (http://www.metaboanalyst.ca) to identify metabolites used for discrimination be-tween the groups using partial least squares-discriminant analysis (PLS-DA). Spectral variables were mean-centered and scaled using Pareto scaling. A further tenfold in-ternal cross-validation was performed to assess the quality of statistical models using the diagnostic measures R2 and Q247. Metabolites with a variable importance in projection (VIP) score ≥ 1.0 or p-value < 0.05 were selected. The Kyoto Encyclopedia of Genes and Genomes database (KEGG) was employed to analyze the functional metabolic pathways.
Statistical analysis
The baseline characteristics between bedwetting children with and without ADHD or anxiety disorder, and healthy controls were compared using Kruskai-Wallis test (GraphPad Prism 8.4.2). A p-value < 0.05 was considered statistically significance. The differences in metabolites between two groups were assessed using the Mann–Whitney test with the MetaboAnalyst web server. A false discovery rate (FDR) of 5% was applied to correct for multiple sets.
References
Neveus, T. et al. Evaluation of and treatment for monosymptomatic enuresis: A standardization document from the International Children’s Continence Society. J Urol. 183, 441–447 (2010).
Austin, P. F. et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the Standardization Committee of the International Children’s Continence Society. Neurourol. Urodyn. 35, 471–481 (2016).
Yeung, C. K., Sreedhar, B., Sihoe, J. D., Sit, F. K. & Lau, J. Differences in characteristics of nocturnal enuresis between children and adolescents: A critical appraisal from a large epidemiological study. BJU Int. 97, 1069–1073 (2006).
Butler, R. J. & Heron, J. The prevalence of infrequent Bedwetting and nocturnal enuresis in childhood. A large British cohort. Scand. J. Urol. Nephrol. 42, 257–264 (2008).
Tai, H. L. et al. The epidemiology and factors associated with nocturnal enuresis and its severity in primary school children in Taiwan. Acta Paediatr. 96, 242–245 (2007).
Bower, W. F., Moore, K. H., Shepherd, R. B. & Adams, R. D. The epidemiology of childhood enuresis in Australia. Br. J. Urol. 78, 602–606 (1996).
Yeung, C. K. et al. Characteristics of primary nocturnal enuresis in adults: An epidemiological study. BJU Int. 93, 341–345 (2004).
Nevéus, T. Pathogenesis of enuresis: Towards a new understanding. Int. J. Urol. 24, 174–182 (2017).
Kamperis, K., Rittig, S., Jørgensen, K. A. & Djurhuus, J. C. Nocturnal polyuria in monosymptomatic nocturnal enuresis refractory to desmopressin treatment. Am. J. Physiol. Renal. Physiol. 291, F1232-1240 (2006).
Biederman, J. et al. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: Patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch. Gen. Psychiatry. 49, 728–738 (1992).
Shreeram, S., He, J. P., Kalaydjian, A., Brothers, S. & Ries Merikangas, K. Prevalence of enuresis and its association with attention deficit/hyperactivity disorders among U.S. children: Results from a nationally representative study. J. Am. Acad. Child Adolesc. Psychiatry. 48, 35–41 (2009).
Elia, J. et al. Nocturnal enuresis: A suggestive endophenotype marker for a subgroup of inattentive attention-deficit/ hyperactivity disorder. J. Pediatr. 155, 239–244 (2009).
Tsai, J. D. et al. Trend of nocturnal enuresis in children with attention deficit/ hyperactivity disorder: A nationwide population-based study in Taiwan. J. Invest. Med. 65, 370–375 (2017).
Baeyens, D. et al. The prevalence of ADHD in children with enuresis: Comparison between a tertiary and non-tertiary care sample. Acta Paediatr. 95, 347–352 (2006).
Baeyens, D. et al. Attention deficit/hyperactivity disorder in children with nocturnal enuresis. J. Urol. 171, 2576–2579 (2004).
Wolraich, M. L. et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 144, e20192528 (2019).
Järvelin, M. R. Developmental history and neurological findings in enuretic children. Dev. Med. Child. Neurol. 31, 728–736 (1989).
Fehlow, P. EEG findings in 130 enuretics with special reference to spike potentials. Psychiatr. Neurol. Med. Psychol. (Leipz). 37, 221–227 (1985).
Guze, S.B. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), by American Psychiatric Association (Washington, D.C., 1994).
Sperringer, J. E., Addington, A. & Hutson, S. M. Branched-chain amino acids and brain metabolism. Neurochem. Res. 42, 1697–1709 (2017).
Fleszar, M. G. et al. Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: Association with pathology, severity, and structural brain changes. Sci. Rep. 9, 13764 (2019).
Park, B. S. et al. Beta-aminoisobutyric acid inhibits hypothalamic inflammation by reversing microglia activation. Cells 8, 1609 (2019).
Craig, S. A. Betaine in human nutrition. Am. J. Clin. Nutr. 80, 539–549 (2004).
Kempson, S. A., Zhou, Y. & Danbolt, N. C. The betaine/GABA transporter and betaine: Roles in brain, kidney and liver. Front. Physiol. 5, 159 (2014).
du Vigneaud, V., Simmonds, S., Chandler, J. P. & Cohn, M. A further investigation of the role of betaine in transmethylation reactions in vivo. J. Biol. Chem. 65, 639–648 (1946).
Borsook, H. & Borsook, M. E. The biochemical basis of betaine-glycocyamine therapy. Ann. West Med. Surg. 5, 825–829 (1951).
Lever, M. et al. Extreme urinary betaine losses in type 2 diabetes combined with bezafibrate treatment are associated with losses of dimethylglycine and choline but not with increased losses of other osmolytes. Cardiovasc. Drugs Ther. 28, 459–468 (2014).
Veskovic, M. et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 848, 39–48 (2019).
Ohnishi, T. et al. Investigation of betaine as a novel psychotherapeutic for schizophrenia. EBioMedicine 45, 432–446 (2019).
Rittig, S., Schaumburg, H. L., Siggaard, C., Schmidt, F. & Djurhuus, J. C. The circadian defect in plasma vasopressin and urine output is related to desmopressin response and enuresis status in children with nocturnal enuresis. J. Urol. 179, 2389–2395 (2008).
Tokonami, N. et al. Local renal circadian clocks control fluid-electrolyte homeostatis and BP. J. Am. Soc. Nephrol. 25, 1430–1439 (2014).
Dossche, L., Vande Walle, J. & Van Herzeele, C. The pathophysiology of monosymptomatic nocturnal enuresis with special emphasis on the circadian rhythm of renal physiology. Eur. J. Pediatr. 175, 747–754 (2016).
Dossche, L., Raes, A., Hoebeke, P., De Bruyne, P. & Vande Walle, J. Circadian rhythm of glomerular filtration and solute handling related to nocturnal enuresis. J. Urol. 195, 162–167 (2016).
Aydin Kahraman, A. et al. Non-dipping phenomenon in children with monosymptomatic nocturnal enuresis. Pediatr. Nephrol. 28, 1099–1103 (2013).
Gil, R. B. et al. Increased urinary osmolyte excretion indicates chronic kidney disease severity and progression rate. Nephrol. Dial. Transplant. 33, 2156–2164 (2018).
Zhao, G. et al. Betaine in inflammation: Mechanistic aspects and applications. Front. lmmunol. 9, 1070 (2018).
Ornoy, A., Weinstein-Fudim, L. & Ergaz, Z. Prenatal factors associated with autism spectrum disorder (ASD). Reprod. Toxicol. 56, 155–169 (2015).
Szyf, M. Prospects for the development of epigenetic drugs for CNS conditions. Nat. Rev. Drug Discov. 14, 461–474 (2015).
Blusztajn, J. K., Slack, B. E. & Mellott, T. J. Neuroprotective actions of dietary choline. Nutrients 9, 815 (2017).
Fisher, S. K., Heacock, A. M., Keep, R. F. & Foster, D. J. Receptor regulation of osmolyte homeostasis in neural cells. J. Physiol. 588, 3355–3364 (2010).
Ando, D. et al. Function and regulation of taurine transport in Müller cells under osmotic stress. Neurochem. Int. 60, 597–604 (2012).
Mueed, Z., Mehta, D., Rai, P. K., Kamal, M. A. & Poddar, N. K. Cross-interplay between osmolytes and mTOR in Alzheimer’s disease pathogenesis. Curr. Pharm. Des. 26, 4699–4711 (2020).
Knight, L. S., Piibe, Q., Lambie, I., Perkins, C. & Yancey, P. H. Betaine in the brain: Characterization of betaine uptake, its influence on other osmolytes and its potential role in neuroprotection from osmotic stress. Neurochem. Res. 42, 3490–3503 (2017).
Chiu, C. Y. et al. Metabolomic profiling of infectious parapneumonic effusions reveals biomarkers for guiding management of children with streptococcus pneumoniae pneumonia. Sci. Rep. 6, 24930 (2016).
Jacob, D., Deborde, C., Lefebvre, M., Maucourt, M. & Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 13, 36 (2017).
De Meyer, T. et al. NMR-based characterization of metabolic alterations in hypertension using an adaptive, intelligent binning algorithm. Anal. Chem. 80, 3783–3790 (2008).
Westerhuis, J. A. et al. Assessment of PLSDA cross validation. Metabolomics 4, 81–89 (2008).
Funding
This research was funded by the Ministry of Science and Technology, Taiwan (NRRPG3H0021) and Lin-Kou Chang Gung Memorial Hospital, Taiwan (CMRPG3K0131).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.Y., T.W, and Y.C.; methodology and experimental statistical analysis, M.Y. and C.C.; resources, M.Y., T.W, and Y.C.; data curation, M.Y., M.Y. and C.L.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y.; supervision, C.C.; project administration, M.Y; funding acquisition, M.Y. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, MC., Wang, TM., Chiou, YH. et al. Urine metabolic phenotyping in children with nocturnal enuresis and comorbid neurobehavioral disorders. Sci Rep 11, 16592 (2021). https://doi.org/10.1038/s41598-021-96104-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96104-1
- Springer Nature Limited