Abstract
Data on the relationship between antimicrobial resistance and mortality remain scarce, and this relationship needs to be investigated in intensive care units (ICUs). The aim of this study was to compare the ICU mortality rates between patients with ICU-acquired pneumonia due to highly antimicrobial-resistant (HAMR) bacteria and those with ICU-acquired pneumonia due to non-HAMR bacteria. We conducted a multicenter, retrospective cohort study using the French National Surveillance Network for Healthcare Associated Infection in ICUs (“REA-Raisin”) database, gathering data from 200 ICUs from January 2007 to December 2016. We assessed all adult patients who were hospitalized for at least 48 h and presented with ICU-acquired pneumonia caused by S. aureus, Enterobacteriaceae, P. aeruginosa, or A. baumannii. The association between pneumonia caused by HAMR bacteria and ICU mortality was analyzed using the whole sample and using a 1:2 matched sample. Among the 18,497 patients with at least one documented case of ICU-acquired pneumonia caused by S. aureus, Enterobacteriaceae, P. aeruginosa, or A. baumannii, 3081 (16.4%) had HAMR bacteria. The HAMR group was associated with increased ICU mortality (40.3% vs. 30%, odds ratio (OR) 95%, CI 1.57 [1.45–1.70], P < 0.001). This association was confirmed in the matched sample (3006 HAMR and 5640 non-HAMR, OR 95%, CI 1.39 [1.27–1.52], P < 0.001) and after adjusting for confounding factors (OR ranged from 1.34 to 1.39, all P < 0.001). Our findings suggest that ICU-acquired pneumonia due to HAMR bacteria is associated with an increased ICU mortality rate, ICU length of stay, and mechanical ventilation duration.
Similar content being viewed by others
Introduction
Hospital-acquired pneumonia (HAP) is a common condition that is responsible for a large proportion of hospital-acquired infections, reaching 22% of cases in the United States and 15.6% of cases in France1,2. In intensive care units (ICUs), HAP refers to both healthcare-associated pneumonia and ventilator-associated pneumonia3. The attributable mortality of ventilator-associated pneumonia has been extensively evaluated in recent studies4,5,6, although this has led to conflicting results because of confounding biases7. Likewise, the attributable mortality of ICU-acquired pneumonia—that is, healthcare-associated pneumonia diagnosed after a 48 h stay in the ICU—remains difficult to accurately assess. However, these infections most likely have a detrimental effect on the outcomes of patients in the ICU1,8,9,10.
In ICU-acquired pneumonia, the resistance level of the causative microorganism may also affect outcome11,12,13. Investigating the relationship between antibiotic resistance and clinical outcome is challenging, as it is difficult to discriminate the confounders and determinants of this relationship. In addition, patients at the highest risk of death are also likely to be those at the highest risk of infection by highly antimicrobial-resistant (HAMR) bacteria14. Therefore, this study aimed to compare ICU mortality rates between patients who developed ICU-acquired pneumonia caused by HAMR bacteria and those who developed ICU-acquired pneumonia caused by non-HAMR bacteria among the following causative pathogens: S. aureus, Enterobacteriaceae, P. aeruginosa, and A. baumannii.
The first aim of our study was to compare the ICU mortality rates in patients who developed ICU-acquired pneumonia due to HAMR and non-HAMR bacteria. The secondary aims were to compare the durations of ICU stay and mechanical ventilation between these two groups.
Material and Methods
We performed a retrospective, observational 9-year study using the REA-RAISIN database (from January 2007 to December 2016), a French national surveillance network for healthcare-associated infections in ICUs (the surveillance period was of 6 months from 2007 to 2014 and then surveillance became continuous as of 2015)15. The number of ICUs contributing to the database increased between 2007 and 2016, varying from 165 to 200. All the patients or their relatives were informed that their data would be used anonymously unless they disagreed with being included. This study was approved by the French Commission Nationale Informatique et Liberté (CNIL No. 588909) and Institutional Review Board (IRB No. 00009118). All included patients were followed up until death or discharge from the ICU.
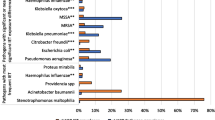
The inclusion criteria were admission to an ICU for at least 48 h and diagnosis of ICU-acquired pneumonia. The diagnosis criteria for pneumonia, following the European Centre for Disease Prevention and Control (ECDC) definition, were one (if the patient had no past medical history of cardiac or pulmonary disease) or two chest radiographs showing pulmonary infiltrates and (1) at least one of the following clinical signs: hyperthermia (> 38 °C) or a leukocyte count less than 4,000 cells/mm3 or greater than 12,000 cells/mm3; (2) at least one of the following clinical criteria: onset of purulent secretions or changes in characteristics; suggestive auscultation, cough, dyspnea, or tachypnea; low oxyhemoglobin saturation; or increased pulmonary oxygen consumption; and (3) microbiological confirmation by a positive culture from directed bronchoalveolar lavage or from tracheal secretions or by an alternative method16. Probable cases of ICU-acquired pneumonia defined as positive according to the radiological, biological, and clinical criteria but with no positive microbiology were excluded. For each patient, we considered only the first episode of ICU-acquired pneumonia. The minimum delay between ICU admission and the onset of ICU-acquired pneumonia was 48 h. The REA-Raisin database allowed the registration of up to two causative pathogens per infection, and resistance profiles were reported only if the causative pathogens were one of the following: S. aureus, Enterobacteriaceae, P. aeruginosa, or A. baumannii.
Data collection
Demographic and clinical data, including clinical and microbiological assessments from the electronic medical charts were analyzed. We extracted age, gender, Simplified Acute Physiology Score (SAPS) 2 at ICU admission, administration of antibiotic treatment within 48 h before or after ICU admission, length of ICU stay, patient’s provenance before ICU admission (in-hospital patient or out-hospital patient, hospitalizations occurring before the stay of interest were not recorded), immunodeficiency according to Acute Physiology and Chronic Health Evaluation II score, medical or surgical origin of patients, use of mechanical ventilation, and trauma diagnosis.
Definition of HAMR status
Each episode of ICU-acquired pneumonia was microbiologically confirmed to identify the causal pathogens. ICU-acquired pneumonia due to HAMR bacteria was defined as the identification of at least one antimicrobial-resistant bacterium in a clinical sample, as reported in Table 115.
Statistical methods
To assess the association between HAMR bacteria and ICU mortality, analyses were conducted in two steps: (1) on the whole sample and (2) on a 1:2 matched sample (at least one control). Matching was based on seven factors: sex, age (within 5 years), SAPS 2 (within 10 points), antibiotic treatment at admission (yes–no), category of patient (medical vs. surgical), mechanical ventilation and type of pathogen. The last factor was determined as follows: when pneumonia was caused by a single pathogen (n = 15,717) or two identical pathogens (n = 991), patients were matched based on the same causative pathogen; for pneumonia caused by two different pathogens (n = 2096), a Delphi review was performed by the authors and a panel of experts to determine on which pathogens the matching should be based. The results of the Delphi review are provided in the Supplementary Material. Patients were matched using the %match SAS macro17, which implements an optimal matching algorithm18. The optimal algorithm sorts cases and controls, identifies all pairs that satisfy the specified distance measures, and then selects the set of pairs that minimizes the total distance between all pairs.
For each sample, patients with pneumonia due to HAMR bacteria were compared to patients with pneumonia due to non-HAMR according to the main characteristics. To assess the link between HAMR status and ICU mortality, comparisons based on socio-demographic, clinical, and hospital data between survivors and non-survivors were performed (1) on the whole sample using chi2 tests or Student’s t tests according to the nature of the variable and (2) on the matched sample using conditional logistic regression19, taking into account the matched procedure. Odds ratios (OR) with a 95% confidence interval (CI) were estimated. Multivariate models were assessed to confirm the effect of HAMR status on ICU mortality after adjusting for the main confounding factors (1) with the whole sample using logistic regression (adjustment for sex, age, SAPS 2, antibiotic at admission, immunodeficiency status, mechanical ventilation, traumatic situation, provenance, category of patients, delay of pneumonia) and (2) with the matched sample using a generalized linear model, PROC GLIMMIX SAS (adjustment for provenance, immunodeficiency, trauma, delay of pneumonia). The link between HAMR status and ICU mortality was also assessed in predefined subgroups: men and women, younger (< 65 years) and older (≥ 65 years) patients, medical and surgical patients, mechanical ventilation and no mechanical ventilation, and patients with or without antibiotics at ICU admission. A two-sided p-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were conducted using IBM SPSS Statistics for Windows (Version 21.0. Armonk, NY: IBM Corp) and SAS 9.4 (SAS Institute).
Results
For the study period of 9 years, the database contained 355,116 patients, of which 30,561 (8.6%) developed at least one episode of ICU-acquired pneumonia. A total of 25,096 patients had a documented infection, and for 18,529, a bacteria profile of the isolated strains corresponding to S. aureus, Enterobacteriaceae, P. aeruginosa, or A. baumannii was available. Of these 18,529 patients, the vital status of 18,497 was available at the time of discharge from the ICU. A flowchart of the study is displayed in Fig. 1, and the patient features are presented in Table 2.
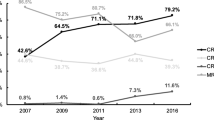
Of the 18,497 included cases of infection, 3081 (17%) were infected with HAMR bacteria and 15,416 (83%) with non-HAMR bacteria (details about the pathogens are provided in the Supplemental Material). The ICU mortality rate was 32%, representing 5872 patients aged 68 ± 13 years with an average SAPS 2 of 55 ± 18. The average ICU length of stay was 33 ± 26 days. Invasive mechanical ventilation was required in 18,109 (98%) patients for a duration of 28 ± 25 days. Of note, 11,512 (62%) patients received antibiotics within 48 h of admission. The reasons for ICU admission were medical (67%) and surgical (33%).
For several sociodemographic and clinical variables, there were significant differences between patients with ICU-acquired pneumonia due to HAMR bacteria and those with ICU-acquired pneumonia due to non-HAMR bacteria (Table 2). Therefore, 5640 non-HAMR ICU-acquired pneumonia cases were matched with 3006 ICU-acquired cases of pneumonia caused by HAMR bacteria. Details are provided in Table 2.
In the whole sample, HAMR group and non-HAMR group were associated with 40.3% and 30.0% ICU mortality rates, respectively (differential 10, odds ratio (OR) and 95% confidence interval (CI) 1.57[1.45–1.70], P < 0.001). Age, sex, provenance, immunosuppression, ICU length of stay, and HAMR status were associated with ICU mortality (Table 3). HAMR status was still associated with ICU mortality (1) in the matched sample (OR 95%, CI 1.39 [1.27–1.52], P < 0.001) (Table 3); (2) after adjusting for the main confounding factors (ORs ranged from 1.34 to 1.39, all p-values < 0.001) (Table 4); and (3) in prespecified subgroups: females versus males, age below 65 years versus above (or equal) 65 years, antibiotic at ICU admission versus no-antibiotic at ICU admission, medical patient versus surgical patient, mechanical ventilation versus no mechanical ventilation, and in-hospital patient versus out-hospital patient (Fig. 2).
The mean durations of ICU length of stay (37 ± 26 days versus 33 ± 26 days, P < 0.001) and mechanical ventilation (31 ± 26 days versus 27 ± 24 days, P < 0.001) were higher in the HAMR group than in the non-HAMR group. The delay between ICU admission and the pneumonia onset differed between HAMR group and non-HAMR group from 16.0 days ± 12.4 to 14.1 days ± 14.4, respectively (P < 0.001).
Discussion
According to our results, developing ICU-acquired pneumonia due to HAMR bacteria was an independent risk factor for ICU mortality. To our knowledge, our study included one of the largest cohorts to assess the association between infection due to HAMR bacteria and ICU mortality.
Lambert et al. published the largest prospective European study (n = 119,699 patients, of whom 8525 were diagnosed with HAP)20. They concluded that the effect of antimicrobial resistance on mortality was modest. In the same line, Paramythiotou et al. concluded that a direct association between infections caused by Gram-negative resistant bacteria and ICU mortality was not confirmed21. However, most studies were performed in single centers and included small numbers of patients. In addition, most were characterized by a high degree of heterogeneity that prevented definitive conclusions from being made21. Three studies have suggested that antibiotic resistance led to an increase in crude mortality, even after adjusting for two of them12,14,22. Here, we found an association between ICU mortality and the occurrence of an infection due to HAMR bacteria.
The effect of bacterial resistance on patient outcomes can be explained by three determinants. First, patients infected by HAMR bacteria are more likely to receive an inadequate empirical antimicrobial therapy23. As there is an association between the adequateness of the empirical antimicrobial therapy and survival, this hypothesis may explain the increased ICU mortality that we reported here24. Second, an increased virulence has been suspected in some resistant bacteria25, as suggested by a murine model of infection due to P. aeruginosa26. The authors found that the acquisition of antibiotic resistance improved the fitness of the bacteria and thus promoted its survival and virulence. However, the higher virulence of HAMR bacteria remains unlikely as conversely, other studies described a loss of potency and virulence in specific bacteria-antibiotic pairs27,28. The third determinant relates to host factors and co-morbidities. A frail patient has a higher risk of recurrent hospitalizations, exposure to antibiotics, and thus colonization and infection by HAMR bacteria29. Moreover, the effects of antibiotics themselves could be deleterious, as suggested previously30.
Our study has several limitations. First, this was a retrospective analysis of a large database. Hence, our choices for the statistical approach could be a matter of debate. However, our results were confirmed using several statistical approaches. Second, the definition of ICU-acquired pneumonia relies on each on-site physician, with different sampling techniques, without external confirmation, while the diagnosis of HAP remains challenging31. Third, only S. aureus, Enterobacteriaceae, P. aeruginosa, and A. baumannii pneumonia were included in the analysis, thus excluding, notably, streptococci and other gram-negative bacteria. Finally, there was no mention in the database of the antimicrobial therapy, specifically the adequacy and delay of the empirical treatment. As discussed above, this is a major determinant of mortality in these patients23,24. However, in our study, the ICU-acquired pneumonia due to HAMR bacteria occurred later than those due to non-HAMR bacteria, suggesting an increased number of late-onset pneumonia in the HAMR group. Following international guidelines, the patients with late-onset pneumonia are more prone to receive broad-spectrum antibiotics than those with early-onset pneumonia31. Notably, 62.2% of the patients included in our study received antibiotics at admission in the ICU. This finding is in line with the rates recently reported in an international observational 24-h point prevalence among 15,202 patients32. The selection of our population was based on voluntary participation in the network and a duration of ICU stay of at least 48 h (for a reduced surveillance workload). Thus, our findings may not be reflective of the entire ICU patient population, as they may pertain specifically to patients exposed to infections acquired in the ICU. Finally, the definition of antimicrobial-resistant bacteria evolved during the study period, which could have affected our findings, despite efforts to ensure comparability across the nine years. As ecology and therapies have evolved over the years with the arrival of new molecules in our therapeutic arsenal, the classification of resistances, established prospectively by the designers of the database, has also evolved towards a more recent and precise definition in 2016 which is more consistent with recent guidelines1,31.
Conclusion
In conclusion, the findings of our study suggest that ICU-acquired pneumonia due to HAMR bacteria was associated with an increased ICU mortality rate, duration of ICU stay, and mechanical ventilation duration. However, the reasons behind this association remain to be elucidated.
Data availability
This study was approved by the French Commission Nationale Informatique et Liberté (CNIL No. 588909) and Institutional Review Board (IRB No. 00009118). Our study has no attached data.
References
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 63, e61–e111 (2016).
Daniau, C. et al. Infections associées aux soins en établissement de santé: résultats de l’enquête nationale de prévalence 2017, france/healthcare-associated infections in healthcare facilities: results of french national point prevalence survey. Science 16, 412–423 (2017).
Timsit, J.-F., Esaied, W., Neuville, M., Bouadma, L. & Mourvillier, B. Update on ventilator-associated pneumonia. F1000Res 6, 2061 (2017).
Timsit, J.-F., Zahar, J.-R. & Chevret, S. Attributable mortality of ventilator-associated pneumonia. Curr. Opin. Crit. Care 17, 464–471 (2011).
Bekaert, M. et al. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am. J. Respir. Crit. Care Med. 184, 1133–1139 (2011).
Nguile-Makao, M. et al. Attributable mortality of ventilator-associated pneumonia: respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med. 36, 781–789 (2010).
Papazian, L., Klompas, M. & Luyt, C.-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. https://doi.org/10.1007/s00134-020-05980-0 (2020).
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 50, 1–26 (2017).
Ibn, S. W. et al. A comparison of the mortality risk associated with ventilator-acquired bacterial pneumonia and nonventilator ICU-acquired bacterial pneumonia. Crit. Care Med. 47, 345–352 (2019).
Melsen, W. G. et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet. Infect. Dis 13, 665–671 (2013).
Damas, P. et al. Severity of ICU-acquired pneumonia according to infectious microorganisms. Intensive Care Med. 37, 1128–1135 (2011).
Razazi, K. et al. Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum β-lactamase-producing Enterobacteriaceae. Ann. Intensive Care 7, 1–7 (2017).
Zahar, J.-R. et al. Is Methicillin resistance associated with a worse prognosis in Staphylococcus aureus ventilator-associated pneumonia?. Clin. Infect. Dis. 41, 1224–1231 (2005).
Bottazzi, A. et al. Mortality attributable to different Klebsiella susceptibility patterns and to the coverage of empirical antibiotic therapy: a cohort study on patients admitted to the ICU with infection. Intensive Care Med. 44, 1709–1719 (2018).
Lepape, A., MacHut, A. & Savey, A. National network REA-RAISIN of adult intensive care acquired infections methods and main results. Med. Intensive Reanim. 27, 197–203 (2018).
Plachouras, D., Lepape, A. & Suetens, C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 44, 2216–2218 (2018).
Bergstralh, E. J. & Kosanke, J. L. Computerized matching of cases to controls. Tech. Rep. 56, 1–28 (1995).
Rosenbaum, P. R. Optimal matching for observational studies. J. Am. Stat. Assoc. 84, 1024–1032 (1989).
Hosmer, D. W., Lemeshow, S. & Sturdivant, R. X. Applied Logistic Regression (Wiley, 2013).
Lambert, M. L. et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet. Infect. Dis 11, 30–38 (2011).
Paramythiotou, E. & Routsi, C. Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World J. Crit. Care Med. 5, 111 (2016).
Barbier, F. et al. Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: What impact on outcomes and carbapenem exposure?. J. Antimicrob. Chemother. 71, 1088–1097 (2016).
Rottier, W. C., Ammerlaan, H. S. M. & Bonten, M. J. M. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing enterobacteriaceae and patient outcome: a meta-analysis. J. Antimicrob. Chemother. 67, 1311–1320 (2012).
Rhodes, A. et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43, 304–377 (2017).
Guillard, T., Pons, S., Roux, D., Pier, G. B. & Skurnik, D. Antibiotic resistance and virulence: understanding the link and its consequences for prophylaxis and therapy. BioEssays News Rev. Mol. Cell. Dev. Biol. 38, 682–693 (2016).
Roux, D. et al. Fitness cost of antibiotic susceptibility during bacterial infection. Sci. Transl. Med. 7, 29 (2015).
Hraiech, S. et al. Impaired virulence and fitness of a colistin-resistant clinical isolate of Acinetobacter baumannii in a rat model of pneumonia. Antimicrob. Agents Chemother. 57, 5120–5121 (2013).
Andersson, D. I. & Hughes, D. Antibiotic resistance and its cost: is it possible to reverse resistance?. Nat. Rev. Microbiol. 8, 260–271 (2010).
Giarratano, A., Green, S. E. & Nicolau, D. P. Review of antimicrobial use and considerations in the elderly population. Clin. Interv. Aging 13, 657–667 (2018).
Jensen, J. U. et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit. Care Med. 39, 2048–2058 (2011).
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 50, 64 (2017).
Vincent, J.-L. et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 323, 1478 (2020).
Acknowledgements
The authors are indebted toward the physicians and nurses who took care of patients and provided the individual data in each center.
Funding
Department of anesthesiology and intensive care unit of Nord Hospital, Marseille, France.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: I.L., S.M., R.R., A.L., M.L. Data curation: I.L., K.B., M.B., L.D. Formal analysis: K.B., M.B. Supervision: L.Z., M.L. Writing – original draft: I.L., S.M., M.L. Writing – review & editing: N.C., E.H., A.L., A.L., A.M., A.S., M.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
IL, SM, RR, LD, EH, AL, LZ, MB, NC, AS, AM and KB have no conflict of interest to disclose. ML served as speaker for MSD, Pfizer and as consultant for Amomed, Aguettant and Gilead AL served as consultant for Fresenius.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lakbar, I., Medam, S., Ronflé, R. et al. Association between mortality and highly antimicrobial-resistant bacteria in intensive care unit-acquired pneumonia. Sci Rep 11, 16497 (2021). https://doi.org/10.1038/s41598-021-95852-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95852-4
- Springer Nature Limited
This article is cited by
-
Automated surveillance of antimicrobial consumption in intensive care, northern Sweden: an observational case study
Antimicrobial Resistance & Infection Control (2024)
-
Association Between Multidrug-Resistant Bacteria and Mortality in Critically Ill Patients
Advances in Therapy (2023)