Abstract
Egg consumption is very high throughout the world and with it comes enormous amount of waste eggshells. To reduce and utilize these wastes, eggshell wastes were simply transformed to low- or high-purity calcium carbonate grades by washing, crushing, and drying to use as raw materials for producing highly valuable calcium phosphate products. Low-purity calcium carbonate grade was used to prepare triple superphosphate for using in fertilizer industry, whereas high-purity calcium carbonate grade was used to produce dicalcium phosphate dihydrate, monocalcium phosphate monohydrate, and tricalcium phosphate for using in mineral feed and food additive industries. All calcium phosphate samples obtained by simple, rapid, cheap, and environmentally safe method using eggshells and phosphoric acid were identified and their structural phases and impurities were determined by XRF, XRD and FTIR techniques. Thermal behaviors of raw materials and the prepared calcium phosphates excepted tricalcium phosphate were investigated by TG/DTG techniques. The methodologies described here will be useful to manage eggshells by converting them to highly valuable products, which can solve eggshell wastes problem from industries and communities. This finding supports the viewpoint of zero waste operation to produce value-added products for obtaining sustainable development, which may be selected as an alternative way for material recycling and waste management in the future.
Similar content being viewed by others
Agro-food from fields of industries and communities produce a substantial amount of pollution every day, so it is becoming urgent to manage this problem1. As the restriction connected to environmental issues is becoming quite rigorous, it is necessary to search and develop treating systems for agro-food wastes1,2,3,4,5,6. Globally, approximately 83 million tons of eggs were produced in 2018, which of course would produce a lot of waste eggshells7. As food, eggs (chicken, duck, ostrich, turkeys, goose, and quail eggs) are used in many food menus for consumption each day and egg products in the industrialized food production (e.g., powder, liquid, and frozen forms) offer economic benefits1. In the case of agro industry, the farming of different types of animal species has a number of waste eggshells after eggs are hatched. In Thailand, egg consumption has used significant amounts of egg that account for more than 15,000 million eggs a year8. From previous research, average weight of an egg is about 60 g, generating shell about 11%; thus, the produced wastes can be estimated as being around 90,000 tons per year1. Every day thousands of eggshells are being discarded, causing serious environmental problem including unpleasant smell, noise of insects and abrasiveness of the eggshells. These wastes could be recycled and converted to valuable calcium compounds by different methods in an environmentally sound nature, which is a good choice for zero waste management.
Fundamentally, eggshells are composed of a network of protein fibers, connected with crystals of calcium carbonate (> 96% of shell weight), magnesium carbonate (< 1%), calcium phosphate (< 1%), magnesium phosphate (< 1%), silicon oxide (< 1%), and also of organic matters and water. The major composition of the shell, calcium carbonate (CaCO3), is a crystal that occurs in the natural form of calcite phase1. Therefore, the use of these eggshells, collected greatly every day, as an alternative source of CaCO3, will help reduce the risk of microbiological and toxic problems, and may reduce the impact on the natural reserves of limestone, non-renewable natural sources. There are several extensive reviews of the applications of eggshells9,10,11,12,13. Several ways have been reported to manage eggshells waste typically destined for landfill and some others such as soil conditioner, fertilizer, animal feed, additive food, adsorbent, calcium supplement, paper manufacture, and material re-used through recycling, all having the potential to be more sustainable methods3,14,15. Recently, researches have studied on using eggshells for removing dyes16,17 and heavy metals18,19,20,21,22,23,24 from wastewater, adsorbing of phosphorus or phosphate25,26, recycling of polyvinyl chloride27, synthesis of PbS/CaCO3 nanocomposites for degradation of tetracycline28, and preparing bio-adsorbent29 and composites as catalyst for some reactions30,31. Eggshell membrane has also been used to produce biochar-supported Cu2+/Cu+ composite for ractopamine detection32, CuO–ZnO nanocomposites for water purification33, and nano-silver-decorated microfibrous eggshell membrane for wound healing and preventing bacterial infection34. In previous researches, eggshell wastes can be used to prepare different calcium carbonate purifications by many methods and then are used to synthesize advanced calcium salts such as calcium oxide, calcium hydroxide, various calcium phosphates, calcium citrate, calcium acetate, and calcium lactate1,35,36,37,38,39. Powdered eggshells have two purified calcium carbonate grades as low and high purity, which were obtained by different preparation approaches such as drying, washing, grinding, and calcining1. Low-purity calcium carbonate was used as animal feed, fertilizers, and heavy metal removal as described by Ok et al.40, whereas high-purity calcium carbonate was used in cosmetic and medical industry, as a base material for bio-ceramics, bone and dental implants, and anti-tartar toothpastes reported by many researchers1,14,41,42,43,44,45,46,47,48,49. Many researches have converted eggshells to calcium oxide (CaO)15,50,51,52,53,54,55,56,57,58 or CaO/Au nanocatalyst59 for use as catalyst in biodiesel productions. Other uses of eggshells are as calcium source to synthesize different calcium salts such as calcium citrate, lactate, gluconate and phosphate1, which are used in food additive and medicine14. For calcium phosphates obtained from eggshells, many researches have studied on synthesis of different tricalcium phosphate forms (α, β, or γ-Ca3(PO4)2) and hydroxyapatite (Ca10(PO4)6(OH)2) to use as a base material for bio-ceramics, bone and dental implants35,36,43,46. However, calcium phosphates produced by the commonly used synthesis routes such as precipitation, sol–gel, microwave or hydrothermal route have several drawbacks, including having the obtained materials in the form of powders. Furthermore, eggshells have still not arrived sufficient attention with regard to converting them from waste to calcium phosphate materials to be used at a large scale. Therefore, there are still a large number of eggshell wastes each day.
The main interest of proposing new routes is to produce four calcium phosphates (triple superphosphate (Ca(H2PO4)2·H2O), dicalcium phosphate dihydrate (CaHPO4·2H2O), monocalclium phosphate monohydrate ((Ca(H2PO4)2·H2O), and tricalcium phosphate (Ca3(PO4)2), used in fertilizer, animal feed, and food additive with enormous use in Thailand, by simple, rapid, cheap, and environmentally benign methods. The main objective of the work is to propose a new and highly competent method in terms of environmental friendliness, cost, quality, and zero-waste management. These synthesis processes offer fast reaction, easy reproducibility, high yield and without controlling various conditions to get four calcium phosphate products. The calcium raw materials and the prepared products were identified by X-ray fluorescence (XRF), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and Thermogravimetric analysis (TGA).
Materials and methods
Preparation of raw materials
Eggshell wastes were obtained from commercial food, bakery, and cake shop (Chachoengsao Province, Thailand), which were normally stored 2 tons a day. These wastes were turned into 2 levels of calcium carbonate purity, low- and high-purity grades, as raw materials for producing fertilizer and feed mineral, respectively. Figure 1 shows a general flowchart of the preparation process of the two calcium carbonate grades from eggshells.
Low-purity calcium carbonate (LPC) was prepared by crushing eggshells followed by sieving through a 50 mesh with grinder machine. Thus, coarse powders with purity levels of 94–97% in calcium carbonate that would be used to produce phosphate fertilizers were obtained.
High-purity calcium carbonate (HPC) was prepared by treating eggshells as follow: washed with household detergent, crushed and sieved through a 50 mesh with grinder machine and then calcined at 500 °C for 2 h with a furnace. Subsequently, coarse powders with purity levels of 97–99% in calcium carbonate that will be used to produce animal feed and food additive are obtained.
Commercial-grade phosphoric acid (85% w/w H3PO4) was used without prior purification. Two concentrations of phosphoric acid prepared by dilution with water are 50 and 70% w/w H3PO4. In a typical process, 10 kg of 85% w/w H3PO4 were added to 7 and 2.15 kg of water for preparing 50 and 70% H3PO4, respectively. The dilution reactions were strong exothermic processes which occurred at temperature range of 50–70 °C.
Production of calcium phosphate compounds
Triple superphosphate (Ca(H2PO4)2·H2O), fertilizer containing more than 54.62% of available phosphoric acid (P2O5) was prepared as follow. At beginning procedure, 3 kg of LPC was placed in a plastic container (20 L). Then 5 L of 70% w/w H3PO4 were slowly added and followed by stirring at 100 rpm for about 15 min. The resulting reaction was strong exothermic and was aged in a plastic container for about 60–120 min to dry spontaneously and then was ground to fine powder. The white-yellow product (Ca(H2PO4)2·H2O) (Fig. 1), triple super phosphate is obtained by a simple, rapid, and low-cost production process according to Eq. (1):
Dicalcium phosphate dihydrate (CaHPO4·2H2O), feed and food grade containing 18% of phosphorus and 24% of calcium, was produced as follows: in the starting process, 5 kg of HPC was placed in a plastic container (20 L), followed by the immediate addition of 5 L of 50% w/w H3PO4 and then stirring at 100 rpm for about 20 min. Finally, the resulting reaction which was moderately exothermic was aged in a plastic container for about 50–70 min to get dried product and then was ground to fine powders and the white product of dicalcium phosphate (CaHPO4·2H2O) is obtained (Fig. 1). This reaction is simple, quick, and cheap and is shown in Eq. (2).
Monocalcium phosphate monohydrate (Ca(H2PO4)2·H2O), feed and food grade containing 21% of phosphorus and 15% of calcium, was prepared similarly to triple superphosphate but using HPC as starting agent and the final white powder product was obtained (Fig. 1).
Tricalcium phosphate (Ca3(PO4)2) (Fig. 1), food grade containing 44% of phosphorus and 38% of calcium, was synthesized as follows: in a typical route, 2.52 kg of monocalcium phosphate monohydrate (Ca(H2PO4)2·H2O) and 1.0 kg of HPC were thoroughly mixed by ball milling. Then the resulting mixture was transferred into a ceramic container and then was calcined at 900 °C for 2 h in a furnace. The calcined product was obtained as white powder corresponding to Eq. (3).
Characterization
The starting materials and as-prepared products were obtained and identified. Four analyses were carried out in this study: X-ray Fluorescence (XRF, Bruker S4 Pioneer), X-Ray Diffraction (XRD, Bruker D8 Advance), Fourier-Transform Infrared Spectrophotometer (FTIR, Perkin Elmer Spectrum GX) and Thermogravimetric Analyzer (TGA, Perkin Elmer Pyris I). The crystal structures of samples were estimated using XRD in 2θ range of 1°–60° with a step size of 0.05 and a step time of 1 s. The room temperature FTIR spectra were recorded in the range of 4000–400 cm−1 with eight scans on a Perkin-Elmer Spectrum GX spectrometer with the resolution of 4 cm−1. Thermal analysis was used to characterize water content in the as-prepared samples as dicalcium phosphate dihydrate, triple superphosphate, and monocalcium phosphate monohydrate.
Results and discussion
Production results
Percentage yields of productions were found to be 98, 97, 98 and 96 for triple superphosphate, dicalcium phosphate dihydrate, monocalcium phosphate monohydrate, and tri calcium phosphate, respectively. Chemical contents and purity of starting agents and all as-prepared products are checked by XRF method and tabulated in Table 1. These results confirm chemical formula to be CaCO3 (Ca:O ratio of 1.10:3.31 for LPC and 1.09:3.38 for HPC), CaHPO4·2H2O (Ca:P ratio of 1.00:1.02), Ca(H2PO4)2·H2O (Ca:P ratio of 1.00:2.02 for triple superphosphate and 1.00:2.05 for monocalcium phosphate dihydrate) and Ca3(PO4)2 (Ca:P ratio of 1.47:1.00), which are well consistent with theoretical information. These obtained data are important for their applications such as fertilizer, feed and food because the standard of materials is indicated.
Characterization results
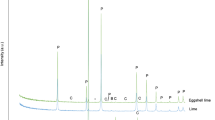
The XRD patterns from powder samples of the starting agents and the as-prepared products are shown in Figs. 2 and 3, respectively. Based on XRD spectra, the starting agents (LPC and HPC) contained calcite (CaCO3, PDF no. 71-937)3. However, the peak intensities of calcite in LPC sample are lower than those of HPC sample because of the impurity effect. The XRD pattern of as- prepared dicalcium phosphate dihydrate (CaHPO4·2H2O) (Fig. 3b) belongs to brushite (CaHPO4·2H2O, PDF no. 01-0653)41. The XRD patterns of as-prepared triple superphosphate (Fig. 3a) and monocalcium phosphate monohydrate (Fig. 3c) are very similar but lower and higher peak intensities are observed because the products were obtained from different purities of calcium carbonate grades36. The lower peak intensities were observed in the XRD pattern of as-prepared triple superphosphate due to the LPC was used as the starting agent. For as-prepared tricalcium phosphate, diffraction lines attribute mainly to beta-tricalcium phosphate (Ca3(PO4)2, PDF no. 70-2065)42. Based on the XRD observation, it can be concluded that no impurity appeared in as-prepared HPC, dicalcium phosphate dihydrate, monocalcium phosphate monohydrate, and tricalcium phosphate powders, and some impurities appeared in as-prepared LPC and triple superphosphate samples.
Figures 4 and 5 show FTIR spectra of the starting agents (LPC and HPC) and the as-prepared products. The FTIR spectra of as-prepared LPC (Fig. 4a) and HPC (Fig. 4b) are very similar and assigned according to vibrational modes of CO32− and Ca–O, which are block units of calcium carbonate (CaCO3). The appeared prominent absorption peak of CO32− at 1478 cm−1 corresponds to the asymmetric stretching mode of C–O bond. The appeared vibrational peaks at 1067, 853, and 710 cm−1 are assigned to the symmetric stretching mode of C–O bond, out of plane and in plane bending of CO32− anion, respectively3. The FTIR spectra of triple superphosphate (Fig. 5a) and monocalcium phosphate monohydrate (Fig. 5b) are very similar and assigned based on the fundamental vibration of Ca(H2PO4)2·H2O. For triple superphosphate (Fig. 5a), the strong bands in the region of 1150–950 and 450–600 cm−1 are attributed to the P–O stretching vibrations and the bending OPO vibrations, respectively. The couple bands at 1221 and 865 cm−1 are assigned to the in-plane P–O–H bending (A2), and the out of plane bending (A1) vibration, respectively. A weak band occurred at approximately 669 cm−1 is assigned to rocking mode involving water molecules. In the O–H stretching mode region of the H2PO4−, which are characteristic for this compound, they appear as the A, B, C trio bands at about 3100–3200 cm−1 (A band), 2300–2400 cm−1 (B band), and 1600–1800 cm−1 (C band). The vibrational bands of water molecule appear in three modes at 1646 cm−1 (ν2), 3192 cm−1 (ν1), and 3459 cm−1 (ν3) and are assigned to bending, asymmetric stretching (O–H), and antisymmetric stretching (O–H) modes, respectively60. Vibrational modes of as-prepared dicalcium phosphate dihydrate (Fig. 5b) are assigned according to the units of HPO42− ion, H2O, and CaO. The typical intense bands at about 1370 cm−1 is due to the in-plane P–O–H bending, while the out of plane bending vibration is observed at about 893 cm−1. The strong bands at about 1120, 1060, and 984 cm−1 are assigned to PO3 asymmetric stretching modes. The weak and broader band at about 569 cm−1 is corresponding to PO4 and P–OH bending modes. The band at 499 cm−1 is assigned to PO3 symmetric bending or Ca–O stretching61. The FTIR spectrum shown in Fig. 5c is very similar to the FTIR spectrum as shown in Fig. 5a because of fundamental vibrational block units (H2PO4−, CaO, and H2O) that come from the same formula (Ca(H2PO4)2·H2O), resulting in closely similar vibrational frequencies60. Finally, the PO43− and CaO subunits of Ca3(PO4)2 structure are assigned (Fig. 5d). Vibrational bands of PO43− anion of the product are observed in the regions of 450–610 and 900–1000 cm−1. These bands are assigned to the ν4(PO43–) and ν3(PO43–) vibrations, respectively. Additionally, the observation of a strong νs(POP) bands (725 cm−1) is known to be the most striking feature of polyphosphate spectrum. The FTIR results are consistent with X-ray diffraction data.
Thermal behaviors of the starting agents (LPC and HPC) and only three as-prepared products (triple superphosphate, dicalcium phosphate dihydrate and monocalcium phosphate monohydrate) investigated by TG/DTG technique are shown in Fig. 6. Tricalcium phosphate is an anhydrous compound, which is not necessary to analyze thermal decomposition. TG/DTG curves of the LPC (Fig. 6a) and HPC (Fig. 6b) samples are quite similar. TG curves of each sample show the mass loss in the range of 600–800 °C, which corresponds to a strong single peak of DTG curves at 770 and 753 °C for the LPC and HPC samples, respectively. Many DTG peaks observed at 297, 512, 540 and 623 °C indicate clearly impurity of the LPC sample. The quantities of mass loss (remaining mass) are found to be 48% (52%) and 44% (56%) for LPC and HPC samples, respectively. The thermal behavior obtained confirms that the LPC sample has a lower purity than the HPC sample. However, the thermal results obtained were well consistent with those of the reference and theory information of CaCO48,49. The thermal results indicate that LPC and HPC powders can be transformed to CaO by calcination at above 800 °C, which may be useful for the production of this compound used in specific applications, revealed by:
TG curves of triple superphosphate and monocalcium phosphate monohydrate are observed in the range of 30–900 °C. The mass remains occurred at above 700 °C, which are found to be 82.50 and 81.00% for triple superphosphate (Fig. 6c) and monocalcium phosphate monohydrate(Fig. 6e), respectively. The mass losses found to be around 18% (2.57H2O) for each sample are related to the loss of three water molecules in Ca(H2PO4)2·H2O structure. The masses of loss and remain observed from each sample are close to that of theoretical values at 21 (3.0H2O) and 79%, respectively53. The relative with TG data, five DTG peaks of both samples are observed the same but peak positions are slightly different. Fiver DTG peaks of triple superphosphate and monocalcium phosphate monohydrate are observed at 125, 184, 224, 282, 331 °C and 130, 175, 230, 261, 321 °C, respectively. Mechanism reactions of thermal transformation for both compounds could be:
where n = o + p + q ≅ 1.
A large amount of intermediate compounds, such as Ca(H2PO4)2·mH2O, Ca(H2PO4)2·pH2O, acid polyphosphate Ca(H2PO4)2, acid condensed phosphate CaH2P2O7 and mixtures of intermediate have been registered. Calcium polyphosphate (Ca(PO3)2) was found to be the product of the thermal decomposition of both prepared compounds over 800 °C as revealed by the TG curve. This result may be not in agreement with literature53, which reported in the range of 500–700 °C of this transition phase. TG curve of dicalcium phosphate dihydrate (CaHPO4·2H2O) (Fig. 6d) show the mass loss in the range of 100–800 °C. The total mass loss is 26%, which is closely to the theoretical value (26.16%, 2.5H2O). The relative with TG data, three intense DTG peaks are observed at 109, 199 and 288 °C, respectively. The formally reactions of thermal transformations could be:
The intermediate compounds including CaHPO4·xH2O, CaHPO4(s) and mixtures of intermediate have been registered. Calcium pyrophosphate (Ca2P2O7) was found to be its final decomposition product 800 °C as revealed by the TG curve. This result may be not in agreement with literature2,4,33,35, which reported lower temperature (< 700 °C) of this transition phase. Thermal results of each compound indicate clearly that the property dependent on various conditions (preparation technique, synthesis condition, raw material, and so on).
The novelty of this work is the production methods that are simple, rapid, cost-saving and environmentally benign for the obtained calcium carbonate (raw material) and four calcium phosphate compounds, shown in Fig. 1. The eggshell wastes were accumulated about 60 tons/month from only one bakery shop in Chachoengsao Province, Thailand. Transportation of these wastes (60 tons) in the distance of 500–800 km is about 1242 USD, which indicates the logistic cost of eggshell no more than 0.021 USD/kg. The eggshells were prepared by two ways to get particle size of 50 mesh. In the first method, they were crushed only to become low-purity calcium carbonate grade, which was in the cost about 0.0031 USD/kg. In the second method, they were washed with detergent and crushed to become high calcium carbonate grade, which had the cost of about 0.009 baht/kg. The ways of the prepared eggshells turn into low- or high-purity calcium carbonates in this work are similar with previous reports1,3. Calcium carbonate (CaCO3) has three crystal forms as calcite, aragonite, and vaterite and is occurred from two sources: living and non-living sources. Non-living source is found in limestone mineral such as calcite (CaCO3) and dolomite (CaxMgyCO3, x + y = 1), which may have impurities of other heavy metals such as lead (Pb), cadmium (Cd), arsenic (As), etc. and has limited quantity, which may run out in the future. The living source is found as the main component of pearls and the shells of marine organisms, snails, and eggs. It happens every day and is collected as large volume of wastes and without heavy toxic metal according to theory of natural selection. The low- or high-purity calcium carbonates prepared from eggshells reported in this work were investigated by XRF, XRD and FTIR techniques for confirmation of nontoxic impurities, phases, and structures. Two calcium carbonate grades obtained in this work were used as calcium sources to react with specific phosphoric acid concentration at very short time (< 30 min) and spontaneously dry solid products were obtained as superphosphate for phosphorus fertilizer (56% P2O5), monocalcium phosphate, dicalcium phosphate, and tricalcium phosphate for feed animal and food additive. In the production of some calcium phosphates, carbon dioxide (CO2) gas was evolved in the process and was tapped by quicklime to turn into calcium carbonate, which is a similar method to previous reports62,63. The production methods of four calcium phosphates reported in this work were simple, rapid, cheap, and safe methods when compared with methods reported in literatures2,4,25,36,41 and the resulting costs were 16, 11, 18 and 24 baht/kg for triple superphosphate, dicalcium phosphate dihydrate, monocalcium phosphate monohydrate, and tricalcium phosphate, respectively. The characterizations of four calcium phosphates were performed using XRF, XRD and FTIR techniques. Impurities, phases and structures were used to confirm that the obtained products were “Green Products” (no metal toxic impurities and according to standard material product) to replace the same products in Thailand markets. All obtained based on this work lead to transforming or recycling of eggshells to four calcium phosphates with lower or near prices and “green products” compared with the same products in the markets. Therefore, eggshell wastes from anywhere can be used to prepare calcium phosphates by methods reported in this work, which will be alternatives to remove these wastes from environmental problem in the future. The authors suggest that pilot projects are required to evaluate their implementation to a commercial scale by using these cleaner production processes to convert waste into industrially viable resources in the future.
Conclusions
Waste eggshells represented one of the most urging problems of agricultural and food industries as well as communities, due to their enormous amount that must be disposed of in some way nowadays. This research described an alternative way to recycle wastes as added-value products to solve eggshell problem of industries and communities. The as-prepared raw materials (low- and high-purity calcium carbonate (LPC and HPC) and products (triple superphosphate, dicalcium phosphate dihydrate, monocalcium phosphate monohydrate, and tricalcium phosphate) were produced by simple, rapid, cheap, and environmentally benign method. Identification of structural phase and impurity of all as-prepared samples was confirmed by XRF, TGA, XRD and FTIR techniques. The findings indicate that all as-prepared samples can be used in industries of fertilizer, animal feed and food additive, which require massive amounts. Therefore, the study represents alternative ways of the environmental benefits resulting from possible transformation of eggshells to value-added calcium compounds needed for wide uses in many industries. If the eggshells are recycled as described above, then a future of these wastes will be used to produce value-added products, which take care of the environment according to the way of zero waste and corresponding to sustainable development.
References
Oliveira, D. A., Benelli, P. & Amante, E. R. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 46, 42–47. https://doi.org/10.1016/j.jclepro.2012.09.045 (2013).
Christmas, R. B. & Harms, R. H. Utilization of egg shells and phosphoric acid as a source of phosphorus and calcium in the diet of white leghorn cockerels. Poult. Sci. 55, 264–267. https://doi.org/10.3382/ps.0550264 (1976).
Barros, M. C., Bello, P. M., Bao, M. & Torrado, J. J. From waste to commodity: Transforming shells into high purity calcium carbonate. J. Clean. Prod. 17, 400–407. https://doi.org/10.1016/j.jclepro.2008.08.013 (2009).
Cho, M. G., Bae, S. M. & Jeong, J. Y. Egg shell and oyster shell powder as alternatives for synthetic phosphate: Effects on the quality of cooked ground pork products. Korean J. Food Sci. Anim. Resour. 37, 571–578. https://doi.org/10.5851/kosfa.2017.37.4.571 (2017).
Ummartyotin, S. & Manuspiya, H. A critical review of eggshell waste: An effective source of hydroxyapatite as photocatalyst. J. Met. Mater. Miner. 28, 124–135. https://doi.org/10.14456/jmmm.2018.17 (2018).
Arvanitoyannis, I. S. Waste Management for the Food Industries. (Academic Press, 2008). https://doi.org/10.1016/B978-0-12-373654-3.X5001-9.
FAOSTAT statistical database (Food and Agriculture Organization of the United Nations, 2020).
Mookprom, S., Boonkum, W., Kunhareang, S., Siripanya, S. & Duangjinda, M. Genetic evaluation of egg production curve in Thai native chickens by random regression and spline models. Poult. Sci. 96, 274–281. https://doi.org/10.3382/ps/pew326 (2017).
Waheed, M. et al. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 106, 78–90. https://doi.org/10.1016/j.tifs.2020.10.009 (2020).
Waheed, M. et al. Eggshell calcium: A cheap alternative to expensive supplements. Trends Food Sci. Technol. 91, 219–230. https://doi.org/10.1016/j.tifs.2019.07.021 (2019).
Balaz, M. Ball milling of eggshell waste as a green and sustainable approach: A review. Adv. Colloid Interface Sci. 256, 256–275. https://doi.org/10.1016/j.cis.2018.04.001 (2018).
Guo, Y. et al. Enhanced catalytic benzene oxidation over a novel waste-derived Ag/eggshell catalyst. J. Mater. Chem. A 7, 8832–8844. https://doi.org/10.1039/c8ta10822f (2019).
Guru, P. S. & Dash, S. Sorption on eggshell waste-A review on ultrastructure, biomineralization and other applications. Adv. Colloid Interface Sci. 209, 49–67. https://doi.org/10.1016/j.cis.2013.12.013 (2014).
Yoo, S., Hsieh, J. S., Zou, P. & Kokoszka, J. Utilization of calcium carbonate particles from eggshell waste as coating pigments for ink-jet printing paper. Bioresour. Technol. 100, 6416–6421. https://doi.org/10.1016/j.biortech.2009.06.112 (2009).
Laca, A., Laca, A. & Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 197, 351–359. https://doi.org/10.1016/j.jenvman.2017.03.088 (2017).
Tsai, W.-T. et al. Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour. Technol. 99, 1623–1629. https://doi.org/10.1016/j.biortech.2007.04.010 (2008).
Rápó, E. et al. Adsorption of remazol brilliant violet-5R textile dye from aqueous solutions by using eggshell waste biosorbent. Sci. Rep. 10, 8385. https://doi.org/10.1038/s41598-020-65334-0 (2020).
Ahmad, M. et al. Eggshell and coral wastes as low cost sorbents for the removal of Pb2+, Cd2+ and Cu2+ from aqueous solutions. J. Ind. Eng. Chem. 18, 198–204. https://doi.org/10.1016/j.jiec.2011.11.013 (2012).
Park, H. J., Jeong, S. W., Yang, J. K., Kim, B. G. & Lee, S. M. Removal of heavy metals using waste eggshell. J. Environ. Sci. 19, 1436–1441. https://doi.org/10.1016/s1001-0742(07)60234-4 (2007).
Zheng, W. et al. Adsorption of Cd(II) and Cu(II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J. Hazard. Mater. 147, 534–539. https://doi.org/10.1016/j.jhazmat.2007.01.048 (2007).
Mignardi, S., Archilletti, L., Medeghini, L. & De Vito, C. Valorization of eggshell biowaste for sustainable environmental remediation. Sci. Rep. 10, 2436. https://doi.org/10.1038/s41598-020-59324-5 (2020).
Imam, D. M., Moussa, S. I. & Attallah, M. F. Sorption behavior of some radionuclides using prepared adsorbent of hydroxyapatite from biomass waste material. J. Radioanal. Nucl. Chem. 319, 997–1012. https://doi.org/10.1007/s10967-018-06403-7 (2019).
Renu, M. A., Singh, K., Upadhyaya, S. & Dohare, R. K. Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater. Today Proc. 4, 10534–10538. https://doi.org/10.1016/j.matpr.2017.06.415 (2017).
De Angelis, G., Medeghini, L., Conte, A. M. & Mignardi, S. Recycling of eggshell waste into low-cost adsorbent for Ni removal from wastewater. J. Clean. Prod. 164, 1497–1506. https://doi.org/10.1016/j.jclepro.2017.07.085 (2017).
Panagiotou, E. et al. Turning calcined waste egg shells and wastewater to Brushite: Phosphorus adsorption from aqua media and anaerobic sludge leach water. J. Clean. Prod. 178, 419–428. https://doi.org/10.1016/j.jclepro.2018.01.014 (2018).
Liu, X., Shen, F. & Qi, X. Adsorption recovery of phosphate from aqueous solution by CaO–biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 666, 694–702. https://doi.org/10.1016/j.scitotenv.2019.02.227 (2019).
Balaz, M., Bujnakova, Z., Achimovicova, M., Tesinsky, M. & Balaz, P. Simultaneous valorization of polyvinyl chloride and eggshell wastes by a semi-industrial mechanochemical approach. Environ. Res. 170, 332–336. https://doi.org/10.1016/j.envres.2018.12.005 (2019).
Zhang, X., Huang, J., Kang, Z., Yang, D.-P. & Luque, R. Eggshell-templated synthesis of PbS/CaCO3 nanocomposites for ˙CO3− mediated efficient degradation of tetracycline under solar light irradiation. Mol. Catal. 484, 110786. https://doi.org/10.1016/j.mcat.2020.110786 (2020).
Zhang, N. et al. Enhanced bio-methane production from ammonium-rich waste using eggshell-and lignite-modified zeolite (ELMZ) as a bio-adsorbent during anaerobic digestion. Process Biochem. 81, 148–155. https://doi.org/10.1016/j.procbio.2019.03.001 (2019).
Guo, Y. et al. Biogenic Pt/CaCO3 nanocomposite as a robust catalyst toward benzene oxidation. ACS Appl. Mater. Interfaces 12, 2469–2480. https://doi.org/10.1021/acsami.9b18490 (2020).
Zhang, X. et al. Waste eggshell-derived dual-functional CuO/ZnO/eggshell nanocomposites: (photo)catalytic reduction and bacterial inactivation. ACS Sustain. Chem. Eng. 7, 15762–15771. https://doi.org/10.1021/acssuschemeng.9b04083 (2019).
Cao, L., Ding, Q., Liu, M., Lin, H. & Yang, D.-P. Biochar-supported Cu2+/Cu+ composite as an electrochemical ultrasensitive interface for ractopamine detection. ACS Appl. Bio Mater. 4, 1424–1431. https://doi.org/10.1021/acsabm.0c01314 (2021).
He, X. et al. Waste eggshell membrane-templated CuO–ZnO nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem. Eng. J. 369, 621–633. https://doi.org/10.1016/j.cej.2019.03.047 (2019).
Liu, M. et al. Nano-silver-decorated microfibrous eggshell membrane: processing, cytotoxicity assessment and optimization, antibacterial activity and wound healing. Sci. Rep. 7, 436. https://doi.org/10.1038/s41598-017-00594-x (2017).
Rivera, E. M. et al. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 41, 128–134. https://doi.org/10.1016/s0167-577x(99)00118-4 (1999).
Francis, A. A. & Abdel Rahman, M. K. The environmental sustainability of calcined calcium phosphates production from the milling of eggshell wastes and phosphoric acid. J. Clean. Prod. 137, 1432–1438. https://doi.org/10.1016/j.jclepro.2016.08.029 (2016).
Witoon, T. Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceram. Int. 37, 3291–3298. https://doi.org/10.1016/j.ceramint.2011.05.125 (2011).
Mohadi, R., Anggraini, K., Riyanti, F. & Lesbani, A. Preparation calcium oxide (CaO) from chicken eggshells. Sriwijaya J. Environ. 1, 32–35. https://doi.org/10.22135/sje.2016.1.2.32-35 (2016).
Jirimali, H. D. et al. Waste eggshell-derived calcium oxide and nanohydroxyapatite biomaterials for the preparation of LLDPE polymer nanocomposite and their thermomechanical study. Polym. Plast. Technol. Eng. 57, 804–811. https://doi.org/10.1080/03602559.2017.1354221 (2018).
Ok, Y. S. et al. Application of eggshell waste for the immobilization of cadmium and lead in a contaminated soil. Environ. Geochem. Health 33, 31–39. https://doi.org/10.1007/s10653-010-9362-2 (2011).
Balázsi, C., Wéber, F., Kövér, Z., Horváth, E. & Németh, C. Preparation of calcium–phosphate bioceramics from natural resources. J. Eur. Ceram. Soc. 27, 1601–1606. https://doi.org/10.1016/j.jeurceramsoc.2006.04.016 (2007).
Lee, S.-J., Yoon, Y.-S., Lee, M.-H. & Oh, N.-S. Highly sinterable β-tricalcium phosphate synthesized from eggshells. Mater. Lett. 61, 1279–1282. https://doi.org/10.1016/j.matlet.2006.07.008 (2007).
Siva Rama Krishna, D., Siddharthan, A., Seshadri, S. K. & Sampath Kumar, T. S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 18, 1735–1743. https://doi.org/10.1007/s10856-007-3069-7 (2007).
Liao, D. et al. Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J. Hazard. Mater. 177, 126–130. https://doi.org/10.1016/j.jhazmat.2009.12.005 (2010).
Gergely, G. et al. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 36, 803–806. https://doi.org/10.1016/j.ceramint.2009.09.020 (2010).
Chaudhuri, B., Mondal, B., Modak, D. K., Pramanik, K. & Chaudhuri, B. K. Preparation and characterization of nanocrystalline hydroxyapatite from egg shell and K2HPO4 solution. Mater. Lett. 97, 148–150. https://doi.org/10.1016/j.matlet.2013.01.082 (2013).
Cho, J. S., Lee, J.-H. & Kang, Y. C. Large scale production of yolk–shell β-tricalcium phosphate powders, and their bioactivities as novel bone substitutes. Phys. Chem. Chem. Phys. 16, 16962–16967. https://doi.org/10.1039/c4cp01808g (2014).
Ibrahim, A.-R., Wei, W., Zhang, D., Wang, H. & Li, J. Conversion of waste eggshells to mesoporous hydroxyapatite nanoparticles with high surface area. Mater. Lett. 110, 195–197. https://doi.org/10.1016/j.matlet.2013.08.014 (2013).
Wu, S.-C. et al. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 39, 8183–8188. https://doi.org/10.1016/j.ceramint.2013.03.094 (2013).
Wei, Z., Xu, C. & Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 100, 2883–2885. https://doi.org/10.1016/j.biortech.2008.12.039 (2009).
Buasri, A., Chaiyut, N., Loryuenyong, V., Wongweang, C. & Khamsrisuk, S. Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sustain. Energy 1, 7–13. https://doi.org/10.12691/rse-1-2-1 (2013).
Mosaddegh, E. & Hassankhani, A. Application and characterization of eggshell as a new biodegradable and heterogeneous catalyst in green synthesis of 7,8-dihydro-4H-chromen-5(6H)-ones. Catal. Commun. 33, 70–75. https://doi.org/10.1016/j.catcom.2012.12.013 (2013).
Rohim, R., Ahmad, R., Ibrahim, N., Hamidin, N. & Abidin, C. Z. A. Characterization of calcium oxide catalyst from eggshell waste. Adv. Environ. Biol. 8, 35–38 (2014).
Yasar, F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: Its effects on some critical fuel properties and comparison with CaO. Fuel 255, 115828. https://doi.org/10.1016/j.fuel.2019.115828 (2019).
Pandit, P. R. & Fulekar, M. H. Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renew. Energy 136, 837–845. https://doi.org/10.1016/j.renene.2019.01.047 (2019).
Khemthong, P. et al. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 190, 112–116. https://doi.org/10.1016/j.cattod.2011.12.024 (2012).
Kavitha, V., Geetha, V. & Jacqueline, P. J. Production of biodiesel from dairy waste scum using eggshell waste. Process Saf. Environ. Prot. 125, 279–287. https://doi.org/10.1016/j.psep.2019.03.021 (2019).
Borah, M. J., Das, A., Das, V., Bhuyan, N. & Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 242, 345–354. https://doi.org/10.1016/j.fuel.2019.01.060 (2019).
Liu, J. et al. Conversion of Au(III)-polluted waste eggshell into functional CaO/Au nanocatalyst for biodiesel production. Green Energy Environ. https://doi.org/10.1016/j.gee.2020.07.019 (2020).
Boonchom, B. Parallelogram-like microparticles of calcium dihydrogen phosphate monohydrate (Ca(H2PO4)2·H2O) obtained by a rapid precipitation route in aqueous and acetone media. J. Alloys Compd. 482, 199–202. https://doi.org/10.1016/j.jallcom.2009.03.157 (2009).
Rattanai, B., Naratip, V. & Banjong, B. Study on thermal transformation of CuHPO4·H2O obtained by acetone-mediated synthesis at ambient temperature. J. Therm. Anal. Calorim. 110, 625–632. https://doi.org/10.1007/s10973-011-1832-y (2011).
Lim, M., Han, G.-C., Ahn, J.-W. & You, K.-S. Environmental remediation and conversion of carbon dioxide (CO2) into useful green products by accelerated carbonation technology. Int. J. Environ. Res. Public Health 7, 203–228. https://doi.org/10.3390/ijerph7010203 (2010).
Kemperl, J. & Maček, J. Precipitation of calcium carbonate from hydrated lime of variable reactivity, granulation and optical properties. Int. J. Miner. Process. 93, 84–88. https://doi.org/10.1016/j.minpro.2009.05.006 (2009).
Acknowledgements
This work was supported by the Thailand Science Research and Innovation (TSRI) (RE-KRIS/008/64). The authors would like to thanks the Scientific Instruments Center KMITL for supporting TGA, FTIR, XRD, and SEM techniques.
Author information
Authors and Affiliations
Contributions
N.L. carried out the experiments and analysis. B.B. designed the study, contributed in the experiments and analysis and wrote the main manuscript text. S.S. and W.B. contributed in characterization and analysis. K.C. contributed in the experiments and analysis and proved the main manuscript text. All the authors analyzed the results, contributed in discussion and to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laohavisuti, N., Boonchom, B., Boonmee, W. et al. Simple recycling of biowaste eggshells to various calcium phosphates for specific industries. Sci Rep 11, 15143 (2021). https://doi.org/10.1038/s41598-021-94643-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94643-1
- Springer Nature Limited