Abstract
To determine the effect of customized vestibular exercise (VE) and optokinetic stimulation (OS) using a virtual reality system in patients with persistent postural-perceptual dizziness (PPPD). Patients diagnosed with PPPD were randomly assigned to the VE group or VE with OS group. All participants received VE for 20 min using a virtual reality system with a head mount display once a week for 4 weeks. The patients in the VE with OS group additionally received OS for 9 min. We analysed the questionnaires, timed up-to-go (TUG) test, and posturography scores at baseline and after 4 weeks. A total of 28 patients (median age = 74.5, IQR 66–78, men = 12) completed the intervention. From baseline to 4 weeks, the dizziness handicap inventory, activities of daily living (ADL), visual vertigo analogue scale, and TUG improved in the VE group, but only ADL and TUG improved in the VE with OS group. Patients with severe visual vertigo improved more on their symptoms than patients with lesser visual vertigo (Pearson’s p = 0.716, p < 0.001). Our VE program can improve dizziness, quality of life, and gait function in PPPD; however, additional optokinetic stimuli should be applied for individuals with visual vertigo symptoms.
Similar content being viewed by others
Introduction
Persistent postural-perceptual dizziness (PPPD) is a recently defined diagnostic syndrome that unifies key features of chronic subjective dizziness, visual vertigo, phobic postural vertigo, and related disorders1,2. Because PPPD is a chronic functional and portmanteau disorder of the nervous system that presents as dizziness or imbalance due to a neurologic and a medical condition and coupled with psychological distress, a multidisciplinary approach for treatment should be considered3. Medications such as selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, cognitive behavioural psychotherapy, and/or vestibular rehabilitation therapy (VRT) are generally recommended in PPPD4.
The theoretical basis for VRT is sensory reweighing, which is the redistribution of vision, somatosensation, and vestibular sense for balance5, but few studies have been conducted to determine whether specific exercises should be designed to augment an individual’s other sensory strength or to boost vestibular weakness6. In particular, because VRT includes not only compensation training after vestibular loss but also postural exercises in other causes of dizziness or general unsteadiness6, it can be applicable to patients with PPPD, which is entwined by variable pathophysiology3.
Among the sensory components of VRT, visual stimuli using virtual reality environments are not only manoeuvrable but also effective in patients with dizziness and visual vertigo symptoms7,8. Because visual vertigo is a closely related symptom of PPPD2, specific vestibular exercise focused on visual stimuli can be an effective treatment in PPPD; however, few randomized controlled trials on the efficacy of VRT in PPPD have been reported.
We conducted a randomized controlled trial to determine the short-term efficacy of customized vestibular exercise (VE) and optokinetic stimulation (OS) using a mobile virtual reality system in patients with PPPD.
Results
Characteristics at baseline
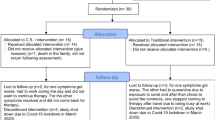
Of the 30 patients enrolled in this study, two patients in the VE group could not attend all training sessions (Fig. 1). Finally, 28 patients (age = median, 74.5, IQR 66–78, men = 12) completed the randomized intervention. There were no significant differences in the demographic characteristics, questionnaire scores, or timed up-to-go-test (TUG) time between the VE and VE + OS groups at baseline (Table 1).
Questionnaire scores and timed up-to-go test time
From baseline to 4 weeks, the dizziness handicap inventory (DHI), activities of daily living (ADL), visual vertigo analogue scale (VVAS), and TUG time significantly improved in the VE group, while in the VE + OS group, only ADL and TUG time improved (Fig. 2). Between the two groups, DHI, ADL, VVAS, and TUG time did not differ after the intervention (Table 1).
Dizziness handicap inventory (DHI, A), vestibular activities of daily living (ADL, B), visual vertigo analogue scale (VVAS, C), and time of timed up-to-go test (TUG, D) before and after treatment. All questionnaires and TUG time scores were significantly improved in the vestibular exercise (VE) group (green); however, only ADL and TUG time were improved in the VE with optokinetic stimulation (OS) group (purple). Every dot is each score or time of patient. The green or purple bars indicate the interquartile range, and the horizontal black bars identify the median value of each group. All p values were assessed with the Wilcoxon signed rank test.
Dynamic posturography
The sensory organization test (SOT) of six conditions on dynamic posturography did not differ at baseline between the VE and VE + OS groups or did not significantly change after the intervention in either group (Table 1). No significant correlations were noted between each posturography score and VVAS at any assessment.
Analysis of the visual vertigo analogue scale
Although complex visual stimuli, including VRT, are helpful for patients with visual vertigo symptoms8, the association between the severity of visual vertigo symptoms and the effectiveness of visual stimuli has not been elucidated. We analysed the correlation between VVAS at baseline and the change in the VVAS score from baseline to 4 weeks in both groups to verify whether the initial severity of visual vertigo symptoms affected the final visual vertigo symptoms. The change in VVAS after completion of 4 weeks of exercise was significantly correlated with the initial VVAS in both the VE (Spearman’s ρ = 0.881, p < 0.001) and VE + OS groups (Spearman’s ρ = 0.544, p = 0.036, Fig. 3). Therefore, the change in the VVAS score of all participants was correlated with the initial visual vertigo symptom (Pearson’s ρ = 0.716, p < 0.001, R2 = 0.513).
The change in VVAS after the 4-week program (ΔVVAS = VVAS at baseline—after 4 weeks, a negative value indicates aggravated visual vertigo) was correlated with VVAS at baseline in the VE group (blue dots, Spearman’s ρ = 0.881, p < 0.001) and the VE + OS group (red dots, Spearman’s ρ = 0.544, p = 0.036). The change in the VVAS score of all participants was correlated with the initial visual vertigo symptom (Pearson’s ρ = 0.716, p < 0.001, R2 = 0.513).
Simulator sickness
Three patients (10%) reported a slight intensity of stimulation sickness on one question of the simulator sickness questionnaire (SSQ), fatigue of the eye during VE.
Discussion
To our knowledge, this study is the first randomized trial utilizing virtual reality-based VRT for the treatment of PPPD. We found that virtual reality-based VRT can improve vestibular symptoms, quality of life, and gait function in patients with PPPD. In both groups, patients with severe visual vertigo showed more significant improvements after completing our program than those with mild visual vertigo.
Sufficient evidence indicates that VRT is a safe and effective treatment for acute and chronic peripheral vestibulopathy9,10. Gaze stability exercises and complex visual stimuli exposure have already been verified and recommended as rehabilitation for patients with chronic peripheral vestibulopathy9; however, VRT should be carefully tailored to the needs of patients with PPPD3. Every day active sports can improve balance function at a young age, even for those with PPPD11, but the individual VRT for promoting habituation has to be attempted by stage because the phase approach is favourable to decrease patients’ high anxiety and excessive vigilance to balance sensation, especially in the elderly3. Although VRT has been proven to improve subjective symptoms and posturographic results in chronic subjective dizziness12,13, which type of exercise is effective specifically for PPPD has not been clarified.
Interestingly, gaze stability exercises can improve the subjective and behavioural performance of patients with unilateral vestibulopathy despite an absent change in VOR gain from semicircular canals15. The remaining otolith function15 and improvement of dynamic visual acuity16 were suggested as possible mechanisms. Likewise, repetitive head and neck movements, including gaze stability exercises, are regarded as effective methods to decrease sensitivity to one’s own movements (adaptation) and substitute other sensory systems for dynamic visual acuity, not restore vestibular loss in PPPD3,5.
Our program was designed to focus on (1) correct head and neck motion during gaze exercise movements based on vestibular adaptation according to the individual’s ability, and (2) adequate visual stimuli that were closest to reality and could arouse interest in promoting habituation and concentration. Because elderly individuals with chronic dizziness have a risk of falling, especially when unaccompanied by a supervisor, we did not include gait training in our exercise program.
Visual stimulation with optokinetic or complex backgrounds would be effective for the improvement of visual vertigo7. In a retrospective survey study, patients with PPPD answered that habituation exercise was effective for the relief of their symptoms14. VRT using complex visual stimuli for visual vertigo has been developed from a simple optokinetic drum and rotating chair to treadmill in a virtual grocery store on a screen7,8,17. However, although optokinetic stimuli are effective in chronic peripheral vestibulopathy regardless of their frequency, velocity, type of texture, and position7, they can be intolerable because optokinetic stimuli that are too intensive and frequent are nauseogenic18.
Optokinetic stimuli using a virtual reality system did not show additional efficacy in our study. Because the realistic background and/or active head-eye movements in our virtual reality-based VRT may fully satisfy the habituation process in the central nervous system of patients with PPPD5, additional optokinetic visual stimuli would be a surplus. The optimal type or feature of visual stimuli has not been settled for VRT, and a visual background such as a crowded square shown on the screen can be sufficient to improve visual vertigo symptoms in patients with unilateral vestibulopathy8. Moreover, marked improvement in our patients with severe visual vertigo reflects that the properties of visual stimuli should be individualized depending on the severity of symptoms in PPPD. Although visual vertigo is closely related to the main symptoms of PPPD, the quantity of dizziness from complex visual stimuli should be considered to develop a VRT strategy for PPPD.
Some studies have shown significant improvement of subjective symptoms or static posturographic assessment by VRT using virtual reality programs in patients with peripheral vestibulopathy or visual vertigo8,19. These studies used variable devices providing virtual reality situations from small and flat monitors to a planetarium with a treadmill. We designed our program to take wireless head mount displays because it does not take up much space, is relatively inexpensive we can operate it more easily and simply than projectors, screens, monitors, or other commercial rehabilitation tools (ex. Balance Rehabilitation Unit or Nintendo Wii), and it can produce very realistic stimuli in comparison to other bulky machines. Because enhanced reality and a high degree of presence can outperform the efficacy of VRT using a virtual reality system, HMD with a well-crafted exercise program would be the best choice for VRT, especially for inducing habituation and motivation in patients with visual vertigo8. Although the SOT of dynamic posturography did not change after our VRT, TUG was significantly improved. The gaze stabilization exercise, the main therapy in our system, can help elderly individuals with dizziness improve their gait function through adaptation of the vestibulo-ocular reflex, even in the absence of any vestibular dysfunction16. Additionally, sufficient habituation depending on the idiosyncratic strategies using these state-of-the-art facilities can be drawn to recover balance function during gait6,19.
The conflict between accommodation and vergence depth cues on stereoscopic displays or complex visual perception without self-motion could lead to significant motion sickness or discomfort when patients are exposed to complex virtual reality environments for a long time18,20. However, therapies using more complicatedly designed virtual reality are effective for anxiety, provoking realistic reactions to feared stimuli and dementia18. In elderly patients (n = 236) without any experience with the virtual reality system, virtual reality cognitive therapy was significantly effective in their depressive mood, but only 1.4% of patients reported severe simulator sickness21. Because our virtual reality program did not include the targets or background objects moving backward and forward, none of our patients complained of severe simulator sickness.
This study has several limitations. First, the duration of VRT and number of sessions might not be enough to improve patients’ symptoms. Because patients enrolled in our study were mostly elderly, they could not visit the hospital easily or frequently. Home-based exercise using our system would be attempted clinically and be more effective for patients than hospital-based exercise. Second, there was no improvement in the SOT of dynamic posturography. The relatively short study duration and lack of balance or gait training in our VRT program may have affected the posturographic results. Additionally, the initial SOT of our patients was not severe because we excluded patients with falling risk.
Our VRT program using a virtual reality system promoting vestibular adaptation and habituation can improve dizziness, quality of life, and gait function in patients with PPPD. Additional visual stimulation using optokinetic stimuli should be carefully provided depending on the quantity of visual symptoms.
Methods
Standard protocol approvals, registrations, and patient consent
The trial was registered at cris.nih.go.kr (KCT0003864, the date of first registration; 24/04/2019). This study was performed in accordance with ethical principles consistent with the Declaration of Helsinki. All of study protocol and informed consent were reviewed and approved by the corresponding health authorities and ethics boards/institutional review boards of Pusan National University Hospital (1903-001-076). Enrolled patients gave written informed consent for participation in the trial.
Subjects
Between April 2019 and April 2020, 30 consecutive patients with a diagnosis of PPPD were recruited from a referred hospital. Subjects were between 51 and 82 years of age (mean ± SD = 71.3 ± 7.8). All participants fulfilled the diagnostic criteria of PPPD2. Patients were excluded if they (1) could not stand alone due to severe imbalance, neurologic deficit, or orthopaedic problem, (2) could not follow instructions of the rehabilitation program due to their cognitive problem, (3) were uncompensated peripheral vestibular loss, in which their dizziness or unsteadiness were provoked as distinct episodes of head motion-induced or physical examination including head thrust, headshake, or stepping tests3, or (4) had previous central vestibulopathy including stroke, infection, or demyelinating diseases, or vestibular migraine.
Study design and randomization
We conducted a prospective randomized controlled study to determine the 4-week therapeutic efficacy of VE and VE + OS using a virtual reality system (Fig. 1). Based on the data from previous studies in chronic subjective dizziness13, we estimated that the proportion of 4-week efficacy of vestibular rehabilitation in chronic subjective dizziness would be 80% in VE and 40% in VE + OS. By adopting 0.9 power to detect a significant difference (p = 0.05, two-sided) and a dropout rate of 20%, 15 patients were required for each treatment arm.
Thirty patients were randomly assigned to the VE or VE + OS groups. The randomization procedure designed in permuted sizes of 4 blocks was based on the web-based program provided by REDCap© (Vanderbilt University, USA).
A physiotherapist conducted the disposed program approximately 20 min weekly for 4 weeks. The patients in the VE + OS group additionally had a crafted OS for 9 min. Before exercise on the visit day and after 4 weeks, we consecutively assessed the questionnaires, TUG test, and Computerized Dynamic Posturography in all participants. The participants also answered questions about simulator sickness after 4 weeks.
Intervention program
Virtual reality equipment
An OCULUS GO virtual reality headset and controller were used for our program. The head mount display (HMD) is mobile and wireless and has a field of view of 110 degrees. The resolution was 2560 × 1440 K, and the refresh rate was 60 Hz. The total hardware weight of hardware was 468 g.
Vestibular exercise program using virtual reality
We developed VE and OS with a virtual reality system and applied a patent collaborating the manufacturer (JCS Co., Ltd.). The exercise protocol was described in Table 2. In a virtual reality environment, the target looks like a robot and moves horizontally and vertically one metre ahead along the centre. The target guided the participants to follow and let them know whether they were exercising exactly 15 degrees around the centre by audio guidance. When the participants successfully completed the exercise 10 times, the speed of the exercise was increased. The optokinetic stimulation program consisted of stars rotating around the yaw, pitch, and roll axes approximately 3 min in each axis in the night sky (Video 1).
Outcome measures
Questionnaires
-
(a)
The DHI contains 25 questions designed to assess a patient’s functional, emotional, and physical symptoms that is validated in Koreans. A score of 0 indicates no discomfort, and a score of 100 indicates a significant self-perceived handicap22.
-
(b)
The ADL contains 28 self-rated questions for measuring the quality of life, which is scaled 10 points and divided into functional, ambulation, and instrumental skills for determining the level of functional limitation or disability in people with vestibular disorders23.
-
(c)
The VVAS consists of nine visual analogue scales, and subjects were asked to rate no dizziness (0) to extreme dizziness (10). Each scale pertains to a specific visual vertigo provoking situation (walking into supermarket, riding in a car, under florescent lights, at an intersection, being in shopping centres, riding on escalators, watching movies at a theatre, on a patterned floor, and watching television)24.
-
(d)
The Beck Anxiety Inventory is a 21-item self-report measure of the level of anxiety a person has experienced during the previous week.
Timed up-to-go test
The participants were instructed to get up from a chair, walk three metres to a wall in an unobstructed room or hall, turn around, walk back, and sit down again. The time (seconds) taken to complete the test was recorded before and after 4 weeks of treatment25.
Computerized dynamic posturography
A computerized dynamic posturography system using the SOT protocol (NeuroCom Smart Equitest system) was performed before and after the intervention. The subject stands on a computerized platform in six conditions (eyes open, eyes closed, surrounding walls moving, platform surface moving, platform surface moving with eyes closed, and walls and platform surface moving together). Three trials are performed in each condition. The computerized platform measures and calculates the subject’s postural stability based on the subject’s input through the platform force plates during the various conditions. If a patient is unable to complete a trial during the test, it is marked as a “fall,” resulting in a score of 0.
Simulator sickness questionnaire
The SSQ was used to assess physical discomfort after exposure to the virtual reality program. The SSQ contains 16 items to measure fatigue, headache, eye strain, difficulty focusing, increased salivation, sweating, feeling of nausea, difficulty concentrating, fullness of the head, blurred vision, dizziness with eyes open/closed, loss of orientation with respect to vertical upright, and general discomfort. The intensity of sickness was measured at four levels: severe, moderate, slight, and none18.
Statistical analysis
The Mann–Whitney U-test and Wilcoxon signed rank test were used to compare the continuous variables, including age, all questionnaires, TUG time, and results from dynamic posturography. Fisher’s exact test was applied for the categorical variables. The relationships between the change in VVAS and VVAS at baseline were assessed with the Spearman or Pearson correlation. All statistical procedures were performed using SPSS statistical software (version 23.0; SPSS, Chicago, IL, USA), and p < 0.05 was considered significant.
Ethical standard
This study followed the tenets of the Declaration of Helsinki and was performed according to the guidelines of the Institutional Review Board of Pusan National University Hospital (1903-001-076).
Data availability
Anonymized data will be shared by request from any qualified investigator.
References
Popkirov, S., Staab, J. P. & Stone, J. Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness. Pract. Neurol. 18, 5–13. https://doi.org/10.1136/practneurol-2017-001809 (2018).
Staab, J. P. et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Barany Society. J. Vestib. Res. 27, 191–208. https://doi.org/10.3233/VES-170622 (2017).
Staab, J. P. Persistent postural-perceptual dizziness. Semin. Neurol. 40, 130–137. https://doi.org/10.1055/s-0039-3402736 (2020).
Seemungal, B. M. & Passamonti, L. Persistent postural-perceptual dizziness: a useful new syndrome. Pract. Neurol. 18, 3–4. https://doi.org/10.1136/practneurol-2017-001817 (2018).
Lacour, M. & Bernard-Demanze, L. Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy: 10 recommendations for optimal functional recovery. Front. Neurol. 5, 285. https://doi.org/10.3389/fneur.2014.00285 (2014).
Tjernstrom, F., Zur, O. & Jahn, K. Current concepts and future approaches to vestibular rehabilitation. J. Neurol. 263(Suppl 1), S65-70. https://doi.org/10.1007/s00415-015-7914-1 (2016).
Pavlou, M. The use of optokinetic stimulation in vestibular rehabilitation. J. Neurol. Phys. Ther. 34, 105–110. https://doi.org/10.1097/NPT.0b013e3181dde6bf (2010).
Pavlou, M. et al. The effect of virtual reality on visual vertigo symptoms in patients with peripheral vestibular dysfunction: a pilot study. J. Vestib. Res. 22, 273–281. https://doi.org/10.3233/VES-120462 (2012).
Hall, C. D. et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American Physical Therapy Association Neurology Section. J. Neurol. Phys. Ther. 40, 124–155. https://doi.org/10.1097/NPT.0000000000000120 (2016).
McDonnell, M. N. & Hillier, S. L. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 1, 397. https://doi.org/10.1002/14651858.CD005397.pub4 (2015).
Nada, E. H., Ibraheem, O. A. & Hassaan, M. R. Vestibular rehabilitation therapy outcomes in patients with persistent postural-perceptual dizziness. Ann. Otol. Rhinol. Laryngol. 128, 323–329. https://doi.org/10.1177/0003489418823017 (2019).
Morisod, B., Mermod, M. & Maire, R. Posturographic pattern of patients with chronic subjective dizziness before and after vestibular rehabilitation. J. Vestib. Res. 27, 305–311. https://doi.org/10.3233/VES-170628 (2018).
Baydan, M., Yigit, O. & Aksoy, S. Does vestibular rehabilitation improve postural control of subjects with chronic subjective dizziness?. PLoS ONE 15, e0238436. https://doi.org/10.1371/journal.pone.0238436 (2020).
Thompson, K. J., Goetting, J. C., Staab, J. P. & Shepard, N. T. Retrospective review and telephone follow-up to evaluate a physical therapy protocol for treating persistent postural-perceptual dizziness: a pilot study. J. Vestib. Res. 25, 97–103. https://doi.org/10.3233/VES-150551 (2015) (quiz 103-104).
Millar, J. L., Gimmon, Y., Roberts, D. & Schubert, M. C. Improvement after vestibular rehabilitation not explained by improved passive VOR gain. Front. Neurol. 11, 79. https://doi.org/10.3389/fneur.2020.00079 (2020).
Hall, C. D., Heusel-Gillig, L., Tusa, R. J. & Herdman, S. J. Efficacy of gaze stability exercises in older adults with dizziness. J. Neurol. Phys. Ther. 34, 64–69. https://doi.org/10.1097/NPT.0b013e3181dde6d8 (2010).
Pavlou, M., Lingeswaran, A., Davies, R. A., Gresty, M. A. & Bronstein, A. M. Simulator based rehabilitation in refractory dizziness. J. Neurol. 251, 983–995. https://doi.org/10.1007/s00415-004-0476-2 (2004).
Park, M. J., Kim, D. J., Lee, U., Na, E. J. & Jeon, H. J. A literature overview of virtual reality (VR) in treatment of psychiatric disorders: recent advances and limitations. Front. Psychiatry 10, 505. https://doi.org/10.3389/fpsyt.2019.00505 (2019).
Micarelli, A., Viziano, A., Augimeri, I., Micarelli, D. & Alessandrini, M. Three-dimensional head-mounted gaming task procedure maximizes effects of vestibular rehabilitation in unilateral vestibular hypofunction: a randomized controlled pilot trial. Int. J. Rehabil. Res. 40, 325–332. https://doi.org/10.1097/MRR.0000000000000244 (2017).
Carnegie, K. & Rhee, T. Reducing visual discomfort with HMDs using dynamic depth of field. IEEE Comput. Graph. Appl. 35, 34–41. https://doi.org/10.1109/MCG.2015.98 (2015).
Chan, J. Y. C. et al. Effects of virtual reality on moods in community older adults. A multicenter randomized controlled trial. Int. J. Geriatr. Psychiatry https://doi.org/10.1002/gps.5314 (2020).
Jacobson, G. P. & Newman, C. W. The development of the Dizziness Handicap Inventory. Arch Otolaryngol. Head Neck Surg. 116, 424–427. https://doi.org/10.1001/archotol.1990.01870040046011 (1990).
Cohen, H. S. Use of the Vestibular Disorders Activities of Daily Living Scale to describe functional limitations in patients with vestibular disorders. J. Vestib. Res. 24, 33–38. https://doi.org/10.3233/VES-130475 (2014).
Dannenbaum, E., Chilingaryan, G. & Fung, J. Visual vertigo analogue scale: an assessment questionnaire for visual vertigo. J. Vestib. Res. 21, 153–159. https://doi.org/10.3233/VES-2011-0412 (2011).
Herdman, S. & Clendaniel, R. A. Vestibular Rehabilitation 4th edn. (F. A Davis Company, 2014).
Acknowledgements
We developed a vestibular exercise and optokinetic stimulation virtual reality system in this study and applied a patent (Number of a patient application: 10-2019-0028899) collaborating JCS Co., Ltd.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07040986).
Author information
Authors and Affiliations
Contributions
S.C. conducted the experiments, analysed and interpreted the data, and wrote the manuscript. J.C., E.H.O., and S.O. conducted the experiments and analysed and interpreted the data. K.C. designed the study, interpreted data, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, SY., Choi, JH., Oh, E.H. et al. Effect of vestibular exercise and optokinetic stimulation using virtual reality in persistent postural-perceptual dizziness. Sci Rep 11, 14437 (2021). https://doi.org/10.1038/s41598-021-93940-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93940-z
- Springer Nature Limited
This article is cited by
-
Compensatory strategies after an acute unilateral vestibulopathy: a prospective observational study
European Archives of Oto-Rhino-Laryngology (2024)
-
Optokinetic stimulation for the treatment of vestibular and balance disorders: a systematic review with meta-analysis
European Archives of Oto-Rhino-Laryngology (2024)
-
Virtual reality-based interventions for the rehabilitation of vestibular and balance impairments post-concussion: a scoping review
Journal of NeuroEngineering and Rehabilitation (2023)
-
Treatment of Persistent Postural-Perceptual Dizziness (PPPD)
Current Treatment Options in Neurology (2023)
-
Effect of vestibular rehabilitation games in patients with persistent postural perceptual dizziness and its relation to anxiety and depression: prospective study
European Archives of Oto-Rhino-Laryngology (2023)