Abstract
The adverse conditions of acidification on sensitive marine organisms have led to the investigation of bioremediation methods as a way to abate local acidification. This phytoremediation, by macrophytes, is expected to reduce the severity of acidification in nearshore habitats on short timescales. Characterizing the efficacy of phytoremediation can be challenging as residence time, tidal mixing, freshwater input, and a limited capacity to fully constrain the carbonate system can lead to erroneous conclusions. Here, we present in situ observations of carbonate chemistry relationships to seagrass habitats by comparing dense (DG), patchy (PG), and no grass (NG) Zostera marina pools in the high intertidal experiencing intermittent flooding. High-frequency measurements of pH, alkalinity (TA), and total-CO2 elucidate extreme diel cyclicity in all parameters. The DG pool displayed frequent decoupling between pH and aragonite saturation state (Ωarg) suggesting pH-based inferences of acidification remediation by seagrass can be misinterpreted as pH and Ωarg can be independent stressors for some bivalves. Estimates show the DG pool had an integrated ΔTA of 550 μmol kg−1 over a 12 h period, which is ~ 60% > the PG and NG pools. We conclude habitats with mixed photosynthesizers (i.e., PG pool) result in less decoupling between pH and Ωarg.
Similar content being viewed by others
Introduction
The myriad biophysical factors that modify estuarine carbonate chemistry often transcend the effects of atmospheric CO2 hydrolysis in seawater (ocean acidification). These include the effects of groundwater flux, fluvial inputs, enhanced biological metabolism, eutrophication, upwelling, and tidal pumping, which interact in complexity resulting in coastal acidification1,2,3,4. The synthesis of these processes is a long-term pH variability that is estimated to be ~ 20 × greater than the open-ocean, where the increasing baseline of dissolved CO2 magnifies the frequency and duration of carbonate chemistry extremes resulting in impeded growth and development of calcifying organisms1,5,6,7,8,9. The deleterious socioeconomic implications of acidification has led to policy initiatives aimed at utilizing phytoremediation (i.e., photosynthetic CO2 uptake) by seagrass and kelp to locally mitigate acidification events10,11,12. This assumes that photosynthesis by macrophytes can reduce the dissolved inorganic carbon (TCO2)—simultaneously raising pH—during daylight hours when photosynthetic rates are high relative to heterotrophic respiration in seagrass beds and kelp forests. Initial research on this topic found that daytime reduction of TCO2 by seagrass is capable of increasing pH on short time scales, however residence time, depth, and enhanced community metabolism in nearshore seagrass habitats were found to dampen mitigation or exacerbate extreme conditions offering only minor, temporary, refuge from acidification9,13,14,15,16,17. These equivocal conclusions of carbonate chemistry variability in seagrass habitats may partially explain the contrasting correlations between bivalve growth and proximity to seagrass patches18,19. Notwithstanding the emerging complexity of phytoremediation, it is clear that further studies are needed to investigate the habitat specificity as it relates to biological communities and the physicochemical oceanographic dynamics of seagrass and kelp ecosystems from an acidification context.
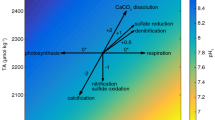
To quantify phytoremediation by seagrass the carbonate system needs to be properly constrained as organismal sensitivities to acidification are specific to individual parameters (e.g., pH and CaCO3 saturation state Ω)—acidification is a multi-stressor20,21. Complicating matters is the nuance of carbonate chemistry variability in coastal margins where the potential for specific parameters to diverge from co-varying positive correlations (i.e., pH and Ω) is high, a phenomenon referred to as a decoupling of the carbonate system1,22. Previous studies have examined seagrass phytoremediation via autonomous pH and O2 sensors complemented by periodic discrete sampling of TCO2 or TA (total alkalinity)9,15,17,18,23, however logistics including site accessibility, timing of tidal cycles, and ability to conduct high-frequency sampling often precludes properly constraining the carbonate system. This can lead to the potential for measurement-estimation discrepancies24, which can equivocate occurrences of carbonate parameter decoupling and quantification of phytoremediation.
To date, most phytoremediation analyses from observations and modelling rely heavily on positive correlations between pH and seagrass density as a means to identify the potential for acidification mitigation13,16,17. Conclusions regarding acidification amelioration are, thus, limited by assumptions of a coupled carbonate system and an opacity to detail the multi-stressor component. In this study, we characterize the carbonate chemistry of adjacent pools in the high intertidal defined as being dense, patchy, or devoid of seagrass (Zostera marina). We measured multiple carbonate chemistry parameters (pH, TCO2, and TA) in each pool over a 17-d period at high frequency to identify tidal and diurnal patterns of carbonate chemistry change as a function of the presence and abundance of seagrass. Our analysis highlights the frequency of carbonate chemistry decoupling of pH and Ωarg as it relates to seagrass density and TA variability. If this decoupling is common in seagrass beds, then previous estimates of phytoremediation are potentially overestimated due to assumptions about positive correlations among parameters (e.g., pH, Ωarg) despite the use of a robust TA-salinity relationship in calculating carbonate chemistry variables.
Results
Timeseries of pool carbonate chemistry
Observations recorded in dense grass (DG: 62% of pool area), patchy grass (PG: 26% of pool area), and no grass (NG: 0% of pool area) pools at the head of Jakolof Bay, AK (Fig. S1), displayed robust hourly changes in TA, TCO2 and pH from 15–27 June (Fig. 1). An increasing dynamic range of ΔTA correlated with residence time, and to a more moderate degree, with ΔTCO2 (Fig. S2). Autocorrelation at lag of 2—corresponding to the troughs of the TA timeseries—was significant for the DG (p = 0.013), PG (p = 0.023), and NG (p = 0.002) pools. Immersion time (i.e., period pools were flooded) decreased from 3.85 to 2.60 h while depth of overlying water at high tide decreased from 1.18 to 0.30 m during the spring to neap tidal transition increasing the magnitude of carbonate chemistry change (Fig. 1). Over the 17-d period the ΔTA (μmol kg−1 h−1) range was greatest in the DG pool with a maximum value 43% greater than the PG pool, and 26% greater than the NG pool (Table S1). The ΔTCO2 maximum rate in the DG pool was double that of its ΔTA and only 23% and 48% greater than the PG and NG pools, respectively (Table S1). Immediately following each flood cycle when pools were emersed and retained an average depth of 6 cm, the TA, TCO2, and pHT signals were approximately equal to ocean measurements and similar to normative estuarine systems. This is despite extremely low salinity (Fig. S3) at the surficial layer of the incoming flood tide which lowered ocean TA only at the surface. Small fluctuations in pool salinity (≤ 1), however, correlated well (slope = − 0.466; R2 = 0.849) with changes in depth (cm) which ranged between 1–2 cm when using the NG pool as reference.
Timeseries of measured TA (a), TCO2 (b), pHT and estimated pHT (c–e) in dense grass (DG), patchy grass (PG), no grass (NG), and ocean (OC). Shaded blue region is predicted tide and shaded grey is night when PAR < 100 μmol photons m−2 s−1. Dashed line indicates tidal height when pools become immersed and tidal height at when samples were taken (blue dots). Three-hour sampling occurred for a period of 24 h starting 24 June 22:30.
Estimated pHT calculated from TCO2 and TA in all pools were robustly different from direct pHT measurements resulting in a measurement-estimation discrepancy (Fig. 1c–e). Measured values were consistently higher where the mean of pHT–pHT_est (± SD) was 0.38 ± 0.35 for DG, 0.22 ± 0.26 for PG, and 0.33 ± 0.23 for NG pools. The NG pool flooded at a height 0.23 m higher than DG and PG pools resulting in an extended emersion period and complete evaporation of the pool on 26 June producing anomalous measurements after the 24th as robust deviations became present (Fig. S3). The PG pool had the greatest overall pHT range from 7.64 to 9.25 while the daily extremes were slightly less from 7.64 to 9.14, and 7.65 to 9.08 for the DG and NG pool, respectively. The daily increase in pHT occurred concomitantly with temperature that ranged from ~ 12.4 to 26.6 (Fig. S3), which would thermodynamically decrease pHT by ~ 0.225 units over the TA and salinity values observed due to the positive correlation between temperature and carbonic acid dissociation constants.
Hourly rates of change during emersion
High-frequency sampling of carbonate chemistry and ancillary parameters (O2, temperature, salinity, and nutrients) characterize a diel modulation for all pools with increases in TA and TCO2 at night (PAR < 100 μmol photons m−2 s−1) and decreases during the day (Fig. 2a–c). Hourly nighttime increases in TA and TCO2 for DG and PG pools were 42.13 and 48.50 (R2 = 0.99 and 0.98), and 44.99 and 173.08 (R2 = 0.99 and 0.92) μmol kg−1 h−1, respectively. The ratio at which TA and TCO2 increased for the DG (1.07) and PG (1.14) pools was ~ 1 whereas the NG pool had a TA:TCO2 ratio of 1.84. In daytime, TA decreased linearly for all pools at a rate ~ 51.0 μmol kg−1 h−1 (R2 = 0.98) for DG and NG pools, and 32.3 (R2 = 0.97) μmol kg−1 h−1 for the PG pool. TCO2 decreased fastest in the PG pool resulting in higher pHT earlier in the day compared to DG and NG pools, and a more rapid shift in carbonate chemistry speciation from HCO3− to CO32−. Supersaturation of CaCO3 (Ωarg) persisted in all pools for the entire emersion period (~ 21 h) regardless of PAR levels (Fig. 2d–f). Estimates of TCO2 modification attributable to photosynthesis and respiration as well as CaCO3 precipitation or dissolution—estimated from changes in TA—show that respiration and photosynthesis was the predominate mechanism of ΔTCO2. The degree of ΔTCO2 appeared to far outpace ΔO2, which peaked in late morning.
Alkalinity and TCO2 (left y-axis) during 21 h emersion sampling period for dense grass (a), patchy grass (b), and no grass (c) pools. The log concentration of CO2, HCO3-, and CO32- are marked as grey on the right y-axis with colored pHT isoclines. Absolute values of ΔTCO2 and ΔO2 during same emersion period where the total ΔTCO2 was estimated based on proportion of change due to biological respiration/photosynthesis or CaCO3 precipitation/dissolution for dense grass (d), patchy grass (e), and no grass (f). Isoclines are Ωarg. Note: The NG pool during this period began to experience increased salinity due to evaporation reducing confidence in the displayed values.
Alkalinity drawdown and associations with seagrass
Logistical curve fits to ΔTA as a function of emersion time was greatest in the DG pool reaching a maximum of ~ 550 μmol kg−1 (RMSE = 82.3) around 10.5 h compared to the NG (RMSE = 46.4) and PG (RMSE = 58.0) pools with ΔTA maxima of ~ 300 μmol kg−1 at 8.5 h (Fig. 3a). At maximum ΔTA, DG was significantly different than both PG and NG pools represented by nonoverlapping model bounds. The ΔTA per proportion of seagrass cover over the same emersion period appeared greater in the PG pool relative to the DG pool, however, the large RMSE (DG = 133.3 and PG = 215.5) bounds suggests these two pools are undistinguishable (Fig. 3b).
Carbonate chemistry decoupling and estimate discrepancies
Aragonite saturation state and [O2] relationships with pHT varied by pool for the entire timeseries. A decoupling from a consistent positive correlation between pHT and Ωarg was observed in the DG and PG pools which displayed Ωarg < 1.5 across a pHT range 7.63–9.10, with a greater proportion of low Ωarg values at high pHT in the DG pool relative to the PG pool (Fig. 4). The [O2] in the DG and PG pools followed a gaussian distribution with an RMSE of 51.4 and 55.7, respectively, where [O2] peaked at a pHT ~ 8.8 and then began to decrease. At this pHT and associated TA:TCO2 ratio, the speciation of TCO2 becomes approximately equal between HCO3− and CO32− concentrations. The trend of O2 decrease at this threshold was not present in the NG pool, and a linear relationship was observed in the ocean signal.
All sample points of Ωarg (a–d) and O2 concentration (e–h) as a function of pHT for dense grass (DG), patchy grass (PG), no grass (NG), and ocean (OC). Color bar is the measured TA:TCO2 ratio for each point in the timeseries. Gaussian fits applied to DG pool with an RMSE of 51.39 and 55.68 for the PG pool. Open circles in panels (c) and (g) indicate measurements taken after 24 June during NG pool evaporation.
Measured TA and TCO2 deviated from estimated values when using two auxiliary carbonate system variables, corroborating decoupling between pHT and Ωarg in all pools. Estimated TA was predominately greater in all pools ranging from 1049 to − 53, 1063 to − 92, and 813 to 18 μmol kg−1 in the DG, PG, and NG pools, respectively (Fig. 5). The converse was true for TCO2 where measured values were majority greater than estimated values. The TA estimates derived from the TA-salinity regression were modest relative to measured values, with ranges below 200 μmol kg−1 for the DG and PG pools, and ~ 450 μmol kg−1 for the NG pool (Fig. 5). We note that the R2 was poor for the DG and PG pools and only moderate in the NG pool (Fig. S4). TCO2 derived values calculated from an estimated TA based on regression with salinity approximately followed TCO2 estimated from measured pHT and TA. Discrepancies as great as ~ 250 μmol kg−1, however, were still observed (Fig. 5). For all estimated values, the timepoints that are most congruent with actual measured values occur at the peaks of the timeseries. These were periods immediately following the flood tide where the more homogenous oceanic signal replaced local pool carbonate chemistry dynamics.
Measured TA (closed squares) along with TA estimated from pHT and TCO2 (open squares) and TA-salinity regression (grey *) for the DG (a), PG (b), and NG (c), pools. TCO2 measured values (closed circles) along with TCO2 estimated from pHT and TA (open circles) and from pHT and TA derived from TA-salinity regression (grey +) for DG (d), PG (e), and NG (f) pools.
Discussion
The extreme diel cyclicity of TA observed in this study is unprecedented for nearshore seagrass habitats in temperate locations and refutes assumptions of its invariability and strong correlation with salinity, a relationship often used to constrain the carbonate system. The modulation of pHT, TCO2, and TA in each of the pools exceeds those that would be derived based on any two of the carbonate chemistry parameters resulting in measurement-estimation discrepancies and an inability to accurately quantify a decoupled system—a situation likely overlooked by previous studies15,16,17,23,25. Extreme decoupling of the carbonate system was present in the DG pool only, where pHT ranged from 7.65 to 9.19 while maintaining a salinity > 26 and Ωarg < 1.5—a threshold at which acute stress occurs in certain bivalve larva26. The two seagrass pools (DG, PG) displayed fundamental differences as it relates to extremes in carbonate chemistry variability, characteristic of previous findings detailing an exacerbation of extremes in seagrass habitats9. Despite abundant filamentous macroalgae and observed microphytobenthos (Fig. S1), the NG pool exhibited a reduced magnitude of variability and maintained a mostly positive correlation between pHT and Ωarg. The OC signal experienced more modest decoupling, however this was due to the freshwater lens at the surface upon the incoming flood tide. While phytoremediation may appear present during occasions with extremely high pHT and Ωarg, the reduction of TCO2 and bioavailable carbon for calcification remained extremely low, potentially impeding organismal calcification27,28,29, inducing a result opposite of phytoremediation. While these conclusions are based on in situ timeseries sampling without replication, the consistent behavior and difference between each pool and autocorrelation over the timeseries gives confidence in our conclusions.
Model estimates of daytime ΔTA as a function of residence time suggest that the mixed autotroph PG pool resulted in a lower integrated TA decrease but a faster rate of TCO2 drawdown shifting the distribution of carbonate chemistry speciation to limited CO2 availability earlier in time (Fig. 6). Based on our results, we hypothesize that higher photosynthetic rates by non-seagrass photosynthesizers (e.g., microphytobenthos) in mixed seagrass communities can raise pH and drawdown TCO2 faster leading to a more rapid increase in TA:TCO2 due to the TCO2 uptake physiology by those autotrophs14,30,31. This is counter to other assertions suggesting greater seagrass density (leaf area index) leads to a greater potential of acidification remediation13; it is clear though, that a more rigorous characterization of the carbonate system is needed to address the efficacy of mitigation and potential decoupling of the system as demonstrated by this study.
Conceptual model plots of ΔTA as a function of residence time and the concomitant change in carbonate chemistry speciation (shaded in grey) for dense grass (green) and patchy grass (yellow) based on measurements presented in Figs. 3 and 4. Dashed lines on plots indicate specific time points at which TA decreases: (a) TA begins to decrease at 5 h when [CO2] is still moderate, (b) TA begins to decrease at 6.5 h when [CO2] is nearly exhausted, (c) 3–6 h residence time TCO2 decreases at rate of ~ − 85 µmol kg−1 h−1, and (d) 3–6 h residence time TCO2 decreases at rate of − 205 µmol kg−1 h−1. Stoichiometric equations and the effects on TA are shown in purple box with hypothesized processes modulating TA based on this study in dark blue text.
The effects of extreme TA diel cycling modify the acid–base chemistry and, thus, the sensitivity of Ωarg, pH, and PCO2 to subsequent fluctuations in TA and TCO232. The TA:TCO2 ratio ranged from 1.05 to 1.80 with highs correlated to longer residence times during neap tidal periods that affected the duration of immersion and emersion. Studies that have previously recorded such changes in TA identify benthic flux or CaCO3 dissolution as the driver of diel variability, however the attributes of the sediment in those habitats were permeable, medium sand or coarse, and rich in CaCO3—conducive conditions for enhanced diffusive and advective efflux from porewater33,34,35. While decreases in TA can be a result of calcification by epibionts or seagrass leaves themselves, those instances occurred in tropical environments with waters extremely high in CaCO3, or in temperate nearshore waters where riverine inputs carried high [TCO2] and [TA], and Ωarg > 10 in the submerged aquatic habitats; however, neither reported diel cycling of TA or found the unique TA:TCO2 ratios observed here36,37. In this study site, the sediment in the study pools was comprised of slate and greywacke, comingled with densely packed fine-grained clay and silt, attenuating in-sediment permeability. Porewater profiles at 1, 2, and 3 cm depths depicted [TCO2] > [TACarb] and were consistently higher than the concentrations in the overlying water (Fig. S5). Given this orientation, efflux of TA and TCO2 from porewater should be persistent, particularly as the concentration gradient would increase during the day as TA and TCO2 decreased in the pools.
If we assume the solute exchange between porewater and overlying water was the mechanism for observed diel TA variability, we can estimate an integrated benthic flux at night in the DG pool of 4.4 mmol m−2 (~ 6 h) and ~ 10 mmol m−2 (~ 12 h) during the day, with daytime rates for the PG and NG pools slightly lower at ~ 5.5 mmol m−2. These rate estimates are similar to those reported in tropical environments where TA flux occurs concomitantly with dissolution in permeable sediments33. There was no evidence of high CaCO3 in the muddy sediments at this site, however, nor of calcifying epiphytes as this region is dominated by cyanobacteria and diatoms at the sediment surface38—epiphytic growth, overall, was surprisingly minimal. The linear changes in TA over the 21 h period would assume a diffusive flux with a likely stagnant boundary layer because advective processes that can enhance flux rates were limited to bio-irrigation and- turbation—which visually appeared minimal—due to lack of other forces (e.g., wave, current, tide). The characteristics of TA variability were expressed as linear rates of change during the day and at night, whereas TCO2 changes were nonlinear during the day and linear at night. At night TA and TCO2 increased ~ 1:1 in the DG and PG pools, which exemplified a possible scenario of CaCO3 dissolution (2:1 change) coupled with respiration that would change TA:TCO2 0:1. If plausible, this would have to occur in superstrated waters (Ωarg was > 1) where the ΔO2 roughly matches the estimated—respiration only—ΔTCO2. This was not the case, however, as the ΔTCO2 was < ΔO2 after 3 h (ratio of 0.55 and 0.44, respectively) and mixed after 6 h with a ratio of 0.75 and 2.0 in the DG and PG pools, respectively. It is possible denitrification-nitrification processes could account for this excess O2 by lowering the community respiration quotient as has been seen in seagrass communities39. While we note that biogenic CaCO3 dissolution occurring at Ωarg > 1 is possible, the rate of dissolution is minimal in supersaturated waters and dependent on specific polymorphs40. Additionally, the rate of ΔTA is consistent despite the temperature fluctuation in the pools, which would enhance the rate of dissolution increasing the rate of TA change: this was not observed. These speculations for TA variability of course assume ΔTCO2 could be partitioned by precipitation/dissolution, of which there is no strong evidence.
Nutrient assimilation and remineralization as well as organic alkalinity can also contribute to changes in TA41,42,43,44,45, but not at the scale of change observed here. Over the high frequency sampling period when nutrients were measured, the stochasticity and low magnitude of change in PO43−, SiO2, NOx, and NH4+ were not found to correlate with ΔTA (Table S2). The changes in TA observed were 1–2 orders of magnitude greater than changes in nutrient concentrations resulting in a trivial addition to ΔTA. In addition, presence of organic alkalinity derived from phytoplankton or humic organic compounds would result in measured TA values > estimated values, but this was not the case. This further complicates the identification of processes responsible for TA variability and, thus, provokes the speculation of non-dissolution and nutrient cycling mechanisms.
The modulation of TA and TCO2 at a 1:1 ratio during night and a decreasing ratio during the day as carbon becomes potentially limiting for seagrass gives credence to HCO3- as a potential source of TA cycling. Specific seagrass species including Z. marina and other macrophytes are known to utilize HCO3- as a carbon source46,47,48. Evidence suggests the active uptake of HCO3− occurs via H+ symport in P. oceanica, where electroneutrality is likely preserved by a Cl− or NO3− efflux and Na+/H+ antiport49,50. The accumulation of HCO3- in P. oceanica aids in the establishment of a robust electronegative potential in the leaves, which is also a phenomenon present in Z. marina50. The accumulation of HCO3- within the cell wall may occur at a faster rate than the dehydration to CO2 in the cytoplast catalyzed by carbonic anhydrase, which could lead to efflux of undehydrated HCO3− at night when light energy is limiting. This could also explain the disparity between O2 generation and TCO2 drawdown during the day as hysteresis can occur on the timescale of hours51, which could be further modified by photorespiration52. The evidence here, along with the suggested mechanisms by which electroneutrality is preserved when HCO3− uptake occurs could explain the decrease in TA observed in these pools when TCO2 is limiting. Photosynthesis, however, is not presumed to affect TA even if HCO3− is utilized because it is expected that uptake would be compensated with H+ or OH− exchange43. More evidence is needed though to determine if this is the case in higher order photosynthesizers because research shows electroneutrality preservation by OH− and H+ may be replaced by NA+, Cl− and NO3− in some seagrasses48,49,53. Anomalous to this conclusion would be the observed TA variability in the NG pool. The Ulva spp., which was observed in the NG pool, however, can also take up HCO3− via ion exchange, potentially with OH- or Cl−47,54. While further investigation is needed to identify the mechanism of TA variability in these pools, similarly low TA values and cycling have been recorded in enclosed bays with long residence times and abundant seagrass, and even speculation of HCO3- was noted as the potential driver of the observed TA variability35,52,55. These incidences of low TA and its diel cycling, however, were not assessed from a phytoremediation standpoint, thus the implications hitherto remained unappreciated.
The pools in this study are representative microcosms of larger systems, replicating carbonate chemistry variability in seagrass habitats on a magnified scale. Perched estuaries, lagoons, and enclosed bays with long residence times have recorded extremely high pH values similar to those found in this study, including instances of reduced TA: correlated to the proximity of oceanic influence52,55,56. In these systems, biological metabolism and evaporation become the dominate mechanisms that modify pH5,56,57. It would be remiss to note, however, that the thermodynamic controls on carbonate chemistry also become enhanced at long residence times as was seen in this study. Observed temperature fluctuations ranged from ~ 12.5–26.5 °C increasing the carbonic acid dissociation constants resulting in an estimated pH drop of ~ 0.21 units across the recorded pH and TA range (Fig. S2); however, this was superseded by photosynthetic modification of pH. Evaporation appeared to have little effect on the DG and PG pools with respect to salinity as values remained fairly constant even at extreme temperatures (Fig. S2). Noticeable was the complete evaporation of the NG pool and the rapid salinity increase, suggesting that small variations in the volume of tidal immersion (i.e., depth of overlying water) can modulate the degree to which estuary carbonate chemistry is affected by the slope of the tidal region.
Findings here show unequivocal, frequent, decoupling of carbonate chemistry that would otherwise be overlooked without properly constraining the system and absolving measurement-estimation discrepancies. This can lead to an overestimation of phytoremediation when conclusions are based on pH variability rather than the total carbonate acid–base chemistry system17,23. This can occur despite a robust TA-salinity relationship or because of a mischaracterization of TA variability if results are derived from discrete measurements based on specific sampling times corresponding to tidal period, proximity to macrophyte habitat (above or in canopy) and coupled with pH-only monitoring. In accordance with previous studies, findings here show that long residence times and spatial variability along with tidal and current mixing are strong drivers for determining carbonate chemistry variability in seagrass13,15, however, we show that decoupling of the carbonate system in dense seagrass occurs rapidly, is independent of low salinity, and is likely related to extreme TA variability potentially caused by a hysteresis of how HCO3- cycles within the medium in a carbon-limited system.
Materials and methods
Site description and assessment
Jakolof Bay is located in the outer portion of Kachemak Bay where local oceanographic conditions and carbonate chemistry are driven by exogenous characteristics from the Gulf of Alaska and autochthonous biological metabolism58. Jakolof Bay is a small fjord (3.5 km in length) that opens up into Kasitsna Bay, fed terrestrially by Jakolof Creek which runs along the north and south boarding the elevated salt marsh and the higher tidal flat—interspersed with depressions—where study site pools were located. The topography of the site suggests that minimal terrestrial subterranean groundwater reaches the pools which are at elevation from the land side creek. Thus, porewater intrusion likely originates from the oceanic front during the floodtide pressure gradient. The geology of Jakolof Bay consists primarily of highly metamorphosed slate and graywacke, likely from the Triassic Period59. The sediment in each pool was muddy with interspersed slate gravel. The outer region of Jakolof Bay sediment is ~ 60% fine grained silt and clay60, a similar sediment characteristic to that found in the pools. Species characterization of the intertidal in Jakolof is scarce, but shallow subtidal assessments identified dominant taxa as Polychaetes, Malacostraca, Gastropods, and Bivalves, however, the diversity of these groups was fairly low relative to other fjords in the region and the deeper areas of Jakolof Bay60. Pacific blue mussels (Mytilus trossulus) were observed in the creek channels, while scattered amphipods and sparse Littorina spp. feeding on the fleshy macroalgae appeared to be the only potential calcifiers in the pools, which were transported in-and-out during flood and ebb. This was the extent of the fauna characterization, although presence appeared to be minimal as well as epiphytic growth on seagrass (Fig. S1).
Sample collection and processing
Three shallow, adjacent pools with varying depth on the edges, within 50 m of one another were selected as sample sites and characterized as dense grass (DG), patchy grass (PG), and no grass (NG) with ellipse areas ~ 330.9, 180.0, 339.9 m2 with average center depths 5.1, 6.2, and 7.1 cm, respectively, in the high intertidal of Jakolof Bay, AK: 59°26′54.09″ N, 151°29′49.96″ W (Fig. S1). TA, TCO2, pHT, O2, and salinity samples were collected toward the center of pools at maximum depth at intervals ~ 16 and 8 h for 17 d, with high-frequency sampling occurring every 3 h on 24 June 22:30 AKT during a 21 h emersion period. TA, pHT and O2 samples were collected in two separate 150 mL borosilicate bottles (one with optical dot), while measurements for TCO2 were collected in a 5 mL centrifugal tube and poisoned for preservation with 10 μL of saturated HgCl2. Temperature measurements were made in situ with an Omega HH81A digital thermometer, while TA, pHT, salinity, and O2, were measured at Kasitsna Bay Laboratory within 30 min of collection. TA was measured using an Apollo SciTech AS-ALK2 with duplicate titrations performed haphazardly throughout the entire sample collection (average duplicate uncertainty 6.71, SD ± 7.48), and CRMs (certified reference material: batch #181) measured before and after each machine calibrated run. pHT was measured potentiometrically with a Thermo Scientific ROSS Ultra electrode calibrated at total scale with Tris buffer and corrected with an offset that was derived from a regression of 25 samples between potentiometric and spectrophotometric measurements using a Shimadzu UV-1900: this offset was 0.019 units (Fig. S5). O2 measurements were performed with a PreSens Fibox 4 (using factory calibration) after each sample was collected and immediately stored in a dark box in the field until measurement ~ 30 min later. Salinity was measured with a YSI 3100 conductivity meter. All TCO2 samples were run at Shannon Point Marine Center, WA, on an Apollo SciTech AS-C3 along with several CRMs (batch #179) interspersed after calibration. Each TCO2 measurement reported was the average of the three closest analytical measurements that were < 10 μmol kg−1 between each measurement. The uncertainty for measured TA (7.58 ± 8.78 SD) and TCO2 (6.77 ± 6.32 SD) was the average difference between known CRM and measured CRM across all samples.
Estimated pHT and Ωarg were calculated using CO2SYS (Matlab V1.1) with inputs TA and TCO2 using the carbonic acid dissociation constants from Lueker et al.61, the bisulfate dissociation constant of Dickson et al.62, and the boron constant from Uppström63. Assuming the ΔTCO2 over the 21 h sampling period was a response to photosynthesis/respiration and CaCO3 precipitation/dissolution, estimates of partitioned ΔTCO2 were calculated using the absolute hourly rate of change for TCO2 and TA during each time point. Where ΔTA*0.5 (based on the alkalinity anomaly) was equal to the ΔTCO2 as a result of precipitation or dissolution, and the remainder of ΔTCO2–ΔTA*0.5 was a due to biological metabolism. Proportion of cover was calculated by measuring the length and width of each pool and then using photographed images to define the proportion of pool area covered by Z. marina present as a ratio of pool size using ImageJ (v. 1.53a). Significance of autocorrelation lag points on TA timeseries for each pool was determined using a Ljung-Box Q-test.
Model construction
A three-parameter logistic curve fitting routine was applied to each pool correlating ΔTA with emersion time when samples were collected immediately after and before flood tide. This ended up being 12 time points for DG and PG pools and 10 for the NG pool (pool evaporated at day 15) during the change in tidal cycle from spring to neap. A conceptual model of carbonate chemistry dynamics for the DG and PG pools was determined by using the estimated values of ΔTA across the entire time series (described above) and the changes in carbonate chemistry speciation derived from the high frequency 21 h sampling period. Since the ΔTA during the 21 h sampling period was integrated into the entire timeseries estimates, the carbonate speciation changes are representative of the expected dynamics that would be visible at other time points.
Porewater sampling
Porewater collectors were assembled using Super Speedfit polypropylene 6.35 mm press-connect fittings. T-shaped connectors were fit with two, ~ 3.8 cm length pieces of food grade plastic tubing, with open ends sealed with thermoplastic hot melt adhesive. Two sides of each piece of tubing were punctured five times with a 16-gauge needle, wrapped with a 0.45 micron PES membrane filter and adhered with thermoplastic adhesive to keep out sediment. Three collectors for each pool were buried at 1, 2, and 3 cm depths (Fig. S1), ~ 36 h and 3 tidal cycles before the first samples were collected. The top of each T-connector—which protruded above the sediment—was sealed with a Super Speedfit polypropylene plug.
Prior to collection, 5 mL centrifugal tubes were placed in a glove bag purged of O2 and filled with N2. Vials sat in a glove bag with caps off for 1–2 h while being filled with N2 and shaken haphazardly. The caps of each vial had a 6.35 mm hole drilled in the top and then covered with electrical tape. Caps were secured from inside glove bag and vials then stored and transported in a plastic bag. A 10 mL serological pipette fitted with a 6.35 mm piece of rigid tubing was fit into the top of each press-connect securing a tight connection and porewater slowly extracted. The first 2 mL of water was discarded and 4–5 mL transferred to the vial by removing tape temporarily and injecting the collected porewater into the vial. Samples were measured immediate for pHT in glove bag using a Thermo Scientific ROSS Ultra electrode calibrated with Tris buffer and reported on the total scale. A 0.019 correction factor derived from a 25-sample regression between potentiometric and spectrophotometric measurements using a Shimadzu UV-1900 was applied. After pH measurement, samples were poisoned with 10 μL of saturated HgCL2 and capped with a new screw top until TCO2 was measured at Shannon Point Marine Center, Anacortes, WA, on an Apollo SciTech AS-C3. Samples were collected every 3 d.
Nutrient sampling
Nutrient NOx, NH4+, PO43−, and SiO4 samples were collected in each pool every 3 h during the 21 h sampling period starting 24 June 2019. Water samples were collected with a 60 mL syringe and filtered through a GFF with a particle retention rate of 1.2 µm into a 20 mL scintillation vial. Samples were frozen at – 4 °C within 30 min of collection until analysis. Samples were processed on a SmartChem multi-element analyzer at Oregon State University.
References
Waldbusser, G. G. & Salisbury, J. E. Ocean acidification in the coastal zone from an Organism’s perspective: Multiple system parameters, frequency domains, and habitats. Annu. Rev. Mar. Sci. 6, 221–247 (2014).
Duarte, C. M. et al. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36, 221–236 (2013).
Johnson, Z. I. et al. Dramatic variability of the carbonate system at a temperate coastal ocean site (Beaufort, North Carolina, USA) is regulated by physical and biogeochemical processes on multiple timescales. PLoS ONE 8, e85117 (2013).
Baumann, H. & Smith, E. M. Quantifying metabolically driven ph and oxygen fluctuations in US nearshore habitats at diel to interannual time scales. Estuaries Coasts 41, 1102–1117 (2018).
Carstensen, J. & Duarte, C. M. Drivers of pH variability in coastal ecosystems. Environ. Sci. Technol. 53, 4020–4029 (2019).
Clark, H. R. & Gobler, C. J. Diurnal fluctuations in CO2 and dissolved oxygen concentrations do not provide a refuge from hypoxia and acidification for early-life-stage bivalves. Mar. Ecol. Prog. Ser. 558, 1–14 (2016).
Mangan, S., Urbina, M. A., Findlay, H. S., Wilson, R. W. & Lewis, C. Fluctuating seawater pH/pCO2 regimes are more energetically expensive than static pH/pCO2 levels in the mussel Mytilus edulis. Proc. R. Soc. B Biol. Sci. 284, 20171642 (2017).
Hauri, C., Gruber, N., McDonnell, A. M. P. & Vogt, M. The intensity, duration, and severity of low aragonite saturation state events on the California continental shelf. Geophys. Res. Lett. 40, 3424–3428 (2013).
Pacella, S. R., Brown, C. A., Waldbusser, G. G., Labiosa, R. G. & Hales, B. Seagrass habitat metabolism increases short-term extremes and long-term offset of CO2 under future ocean acidification. Proc. Natl. Acad. Sci. U. S. A. 115, 3870–3875 (2018).
Adelsman, H. & Whitely Binder, L. Ocean Acidification: From Knowledge to Action, Washington State’s Strategic Response. Washington State Blue Ribbon Panel on Ocean Acidification. (Washington Department of Ecology, Olympia, Washington, 2012).
Nielsen, K. et al. Emerging Understanding of the Potential Role of Seagrass and Kelp as an Ocean Acidification Management Tool in California (California Ocean Science Trust, Oakland, CA, 2018).
Ekstrom, J. A. et al. Vulnerability and adaptation of US shellfisheries to ocean acidification. Nat. Clim. Change 5, 207–214 (2015).
Koweek, D. A. et al. Expected limits on the ocean acidification buffering potential of a temperate seagrass meadow. Ecol. Appl. 28, 1694–1714 (2018).
Miller, C. A., Yang, S. & Love, B. A. Moderate increase in TCO2 enhances photosynthesis of seagrass Zostera japonica, but not Zostera marina: Implications for acidification mitigation. Front. Mar. Sci. 4, 2 (2017).
Cyronak, T. et al. Short-term spatial and temporal carbonate chemistry variability in two contrasting seagrass meadows: Implications for pH buffering capacities. Estuaries Coasts 41, 1282–1296 (2018).
Unsworth, R. K. F., Collier, C. J., Henderson, G. M. & McKenzie, L. J. Tropical seagrass meadows modify seawater carbon chemistry: Implications for coral reefs impacted by ocean acidification. Environ. Res. Lett. 7, 024026 (2012).
Hendriks, I. E. et al. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11, 333–346 (2014).
Greiner, C. M., Klinger, T., Ruesink, J. L., Barber, J. S. & Horwith, M. Habitat effects of macrophytes and shell on carbonate chemistry and juvenile clam recruitment, survival, and growth. J. Exp. Mar. Biol. Ecol. 509, 8–15 (2018).
Groner, M. L. et al. Oysters and eelgrass: Potential partners in a high pCO2 ocean. Ecology 99, 1802–1814 (2018).
Waldbusser, G. G. et al. Ocean acidification has multiple modes of action on bivalve larvae. PLoS ONE 10, e0128376 (2015).
Hurd, C. L. et al. Ocean acidification as a multiple driver: How interactions between changing seawater carbonate parameters affect marine life. Mar. Freshw. Res. 71, 263–274 (2020).
Hales, B., Suhrbier, A., Waldbusser, G. G., Feely, R. A. & Newton, J. A. The carbonate chemistry of the “Fattening Line,” Willapa Bay, 2011–2014. Estuaries Coasts 1–14 (2016).
Ricart, A. M. et al. Coast-wide evidence of low pH amelioration by seagrass ecosystems. Glob. Change Biol. 27, 2580–2591 (2021).
Hoppe, C. J. M., Langer, G., Rokitta, S. D., Wolf-Gladrow, D. A. & Rost, B. Implications of observed inconsistencies in carbonate chemistry measurements for ocean acidification studies. Biogeosciences 9, 2401–2405 (2012).
Buapet, P., Gullström, M. & Björk, M. Photosynthetic activity of seagrasses and macroalgae in temperate shallow waters can alter seawater pH and total inorganic carbon content at the scale of a coastal embayment. Mar. Freshw. Res. 2, 2 (2013).
Waldbusser, G. G. et al. Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat. Clim. Change 5, 273–280 (2015).
Comeau, S., Carpenter, R. C. & Edmunds, P. J. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. R. Soc. B-Biol. Sci. 280, 20122374 (2013).
Kawahata, H. et al. Perspective on the response of marine calcifiers to global warming and ocean acidification—Behavior of corals and foraminifera in a high CO2 world “hot house”. Prog. Earth Planet. Sci. 6, 5 (2019).
Ries, J. B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064 (2011).
Raven, J. A., Giordano, M., Beardall, J. & Maberly, S. C. Algal evolution in relation to atmospheric CO2: Carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos. Trans. R. Soc. B Biol. Sci. 367, 493–507 (2012).
Vieira, S., Cartaxana, P., Máguas, C. & Marques da Silva, J. Photosynthesis in estuarine intertidal microphytobenthos is limited by inorganic carbon availability. Photosynth. Res. 128, 85–92 (2016).
Egleston, E. S., Sabine, C. L. & Morel, F. M. M. Revelle revisited: Buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Glob. Biogeochem. Cycles 24, GB1002 (2010).
Cyronak, T., Santos, I. R., McMahon, A. & Eyre, B. D. Carbon cycling hysteresis in permeable carbonate sands over a diel cycle: Implications for ocean acidification. Limnol. Oceanogr. 58, 131–143 (2013).
Burdige, D. J. & Zimmerman, R. C. Impact of sea grass density on carbonate dissolution in Bahamian sediments. Limnol. Oceanogr. 47, 1751–1763 (2002).
Chou, W.-C. et al. A Unique Diel Pattern in Carbonate Chemistry in the Seagrass Meadows of Dongsha 1 Island: implications for ocean acidification buffering. ESSOAr. https://doi.org/10.1002/essoar.10504715.1 (2020).
Su, J. et al. Chesapeake Bay acidification buffered by spatially decoupled carbonate mineral cycling. Nat. Geosci. 13, 441–447 (2020).
Enríquez, S. & Schubert, N. Direct contribution of the seagrass Thalassia testudinum to lime mud production. Nat. Commun. 5, 3835 (2014).
Currin, C., Brewer, J. & Delano, P. Tide Flat Microphytobenthos: Biomass Distribution, Community Composition and Trophic Role in a Macrotidal Alaskan Estuary (National Centers for Coastal Ocean Science, Beaufort, NC, 2002).
Martin, S. et al. Comparison of Zostera marina and maerl community metabolism. Aquat. Bot. 83, 161–174 (2005).
Ries, J. B., Ghazaleh, M. N., Connolly, B., Westfield, I. & Castillo, K. D. Impacts of seawater saturation state (ΩA = 0.4–4.6) and temperature (10, 25 °C) on the dissolution kinetics of whole-shell biogenic carbonates. Geochim. Cosmochim. Acta 192, 318–337 (2016).
Brewer, P. G. & Goldman, J. C. Alkalinity changes generated by phytoplankton growth1. Limnol. Oceanogr. 21, 108–117 (1976).
Gazeau, F., Urbini, L., Cox, T. E., Alliouane, S. & Gattuso, J.-P. Comparison of the alkalinity and calcium anomaly techniques to estimate rates of net calcification. Mar. Ecol. Prog. Ser. 527, 1–12 (2015).
Wolf-Gladrow, D. A., Zeebe, R. E., Klaas, C., Körtzinger, A. & Dickson, A. G. Total alkalinity: The explicit conservative expression and its application to biogeochemical processes. Mar. Chem. 106, 287–300 (2007).
Cai, W.-J., Wang, Y. & Hodson, R. E. Acid-base properties of dissolved organic matter in the estuarine waters of Georgia, USA. Geochim. Cosmochim. Acta 62, 473–483 (1998).
Ko, Y. H., Lee, K., Eom, K. H. & Han, I.-S. Organic alkalinity produced by phytoplankton and its effect on the computation of ocean carbon parameters. Limnol. Oceanogr. 61, 1462–1471 (2016).
Invers, O., Zimmerman, R. C., Alberte, R. S., Pérez, M. & Romero, J. Inorganic carbon sources for seagrass photosynthesis: an experimental evaluation of bicarbonate use in species inhabiting temperate waters. J. Exp. Mar. Biol. Ecol. 265, 203–217 (2001).
Sand-Jensen, K. & Gordon, D. M. Differential ability of marine and freshwater macrophytes to utilize HCO3- and CO2. Mar. Biol. 80, 247–253 (1984).
Larkum, A. W. D., Davey, P. A., Kuo, J., Ralph, P. J. & Raven, J. A. Carbon-concentrating mechanisms in seagrasses. J. Exp. Bot. 68, 3773–3784 (2017).
Rubio, L. et al. Direct uptake of HCO3- in the marine angiosperm Posidonia oceanica (L.) Delile driven by a plasma membrane H+ economy. Plant Cell Environ. 40, 2820–2830 (2017).
Fernández, J. A., García-Sánchez, M. J. & Felle, H. H. Physiological evidence for a proton pump and sodium exclusion mechanisms at the plasma membrane of the marine angiosperm Zostera marina L. J. Exp. Bot. 50, 1763–1768 (1999).
Berg, P. et al. Dynamics of benthic metabolism, O2, and pCO2 in a temperate seagrass meadow. Limnol. Oceanogr. 64, 2586–2604 (2019).
Buapet, P., Rasmusson, L. M., Gullstrom, M. & Bjork, M. Photorespiration and carbon limitation determine productivity in temperate seagrasses. PLoS ONE 8, e83804 (2013).
Garcı́a-Sánchez, M. J., Jaime, M. P., Ramos, A., Sanders, D. & Fernández, J. ,. Sodium-dependent nitrate transport at the plasma membrane of leaf cells of the marine higher plant Zostera marina L. Plant Physiol. 122, 879–886 (2000).
Drechsler, Z. & Beer, S. Utilization of inorganic carbon by Ulva lactuca. Plant Physiol. 97, 1439–1444 (1991).
Ribas-Ribas, M. et al. Effects of upwelling, tides and biological processes on the inorganic carbon system of a coastal lagoon in Baja California. Estuar. Coast. Shelf Sci. 95, 367–376 (2011).
Omarjee, A., Taljaard, S., Weerts, S. P. & Adams, J. B. The influence of mouth status on pH variability in small temporarily closed estuaries. Estuar. Coast. Shelf Sci. 246, 107043 (2020).
McCutcheon, M. R., Staryk, C. J. & Hu, X. Characteristics of the carbonate system in a semiarid estuary that experiences summertime hypoxia. Estuaries Coasts. 42, 1509–1523 (2019).
Miller, C. A. & Kelley, A. L. Seasonality and biological forcing modify the diel frequency of nearshore pH extremes in a subarctic Alaskan estuary. Limnol. Oceanogr. 66, 1475–1491 (2021).
Moxham, R. M. & Nelson, A. E. Trace Elements Reconnaissance in the Jakolof Bay Area, Southern Alaska (United States Department of Interior, Geological Survey, 1950).
Hartwell, S. I., Dasher, D. & Lomax, T. Characterization of Benthic Habitats and Contaminant Assessment in Kenai Peninsula Fjords and Bays (NOAA Technical Memorandum NOS NCCOS, 2016).
Lueker, T. J., Dickson, A. G. & Keeling, C. D. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 70, 105–119 (2000).
Dickson, A. Thermodynamics of the Dissociation of Boric-Acid in Synthetic Seawater from 273.15-K to 318.15-K. Deep-Sea Res. Part Oceanogr. Res. Pap. 37, 755–766 (1990).
Uppström, L. R. The boron/chlorinity ratio of deep-sea water from the Pacific Ocean. Deep Sea Res. Oceanogr. Abstr. 21, 161–162 (1974).
Acknowledgements
We would like to thank Edwin (Ted) Grosholz and J. Jay Stachowicz for providing feedback and comments to previous iterations, as well as two anonymous reviewers whose comments have improved this manuscript. We would also like to thank Brooke Love and Gene McKeen at Shannon Point Marine Center and George Waldbusser at Oregon State University for use of analytical instrumentations to process samples. This work was partially funded by NSF award number OIA-1757348.
Author information
Authors and Affiliations
Contributions
C.A.M. and A.L.K. designed the study. C.A.M. performed all sample collection, processing, and data analysis. C.A.M. led the writing with editing and contributions from A.L.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, C.A., Kelley, A.L. Alkalinity cycling and carbonate chemistry decoupling in seagrass mystify processes of acidification mitigation. Sci Rep 11, 13500 (2021). https://doi.org/10.1038/s41598-021-92771-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92771-2
- Springer Nature Limited
This article is cited by
-
The Role of Submarine Groundwater Discharge to the Input of Macronutrients Within a Macrotidal Subpolar Estuary
Estuaries and Coasts (2023)