Abstract
The factors associated with the timely creation of distal vascular access for hemodialysis initiation are unclear. We aimed to explore the association between the slope of estimated glomerular filtration rate (eGFR) and the successful usage of vascular access upon hemodialysis initiation. This single center retrospective cohort study enrolled chronic kidney disease patients who undertook a multidisciplinary care program from 2003 to 2016. Using eGFR slope as predictor, we evaluated the vascular access created timely upon hemodialysis initiation. Among the 987 patients, vascular access was created at a median eGFR of 5.8 min/ml/1.73 m2, with a median duration of 3.1 months before hemodialysis. The proportions of vascular access created timely, created not timely (vascular access immature), and not created were 68.5%, 8.8%, and 22.7%, respectively. There was a significant negative association of eGFR upon vascular access creation with eGFR slope (r = − 0.182, P < 0.001). The fastest eGFR slope patients (the first quartile or < − 10 min/ml/1.73 m2/year) had the lowest percentage of vascular access created timely. In the multivariable logistic regression analysis, only higher eGFR upon vascular access creation (P = 0.001) and eGFR slope (P = 0.009) were significantly associated with vascular access created timely. The adjusted odds ratios of each quartile of eGFR slopes for vascular access created timely were 0.46 (95% confidence interval 0.27–0.86), 1.30 (0.62, 2.72), 1.00 (reference), and 0.95 (0.48–1.87), respectively. eGFR slope is associated with the timely creation of vascular access for the initiation of hemodialysis in a reverse-J-shaped pattern and may help determine the time of vascular access creation.
Similar content being viewed by others
Introduction
The timely creation of distal vascular access (VA) upon initiation of dialysis has been a major recommendation in existing guidelines for the planned initiation of hemodialysis (HD)1,2, based on the evidence that VA has lower complications and cost, and improved survival3. Unplanned initiation of HD leads to additional burdens on healthcare systems as well as adverse outcomes4. However, an updated report from the Dialysis Outcomes and Practice Patterns study and many others still showed that numerous countries had more than 50% of patients using a temporary catheter upon initiation of HD5,6,7. Many factors have been proposed to explain the gap mentioned above, including mainly late referral, no disease insight, not being under regular pre-dialysis care, and an unpredicted clinical course8,9,10. To overwhelm this gap, the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guideline suggests starting patient education on all modalities of kidney replacement options at an estimated glomerular filtration rate (eGFR) of 30 mL/min/1.73 m2. It further recommends referral for VA assessment and subsequent creation when the eGFR is 15–20 mL/min/1.73 m2, given that it is unlikely to predict the time of dialysis initiation11. In past decades, multidisciplinary chronic kidney disease (CKD) care programs have emerged and resulted in more effective prescriptions, a lower renal progression rate, a decrease in temporary dialysis catheter usage, and lower medical expenses12,13,14,15,16. Under the care of such a program, later referral and regular monitoring of renal progression were more under control; this made it easier to evaluate the time of determining when to create VA and how it affected the initiation of HD without using a central venous catheter. Thus, we conducted this retrospective study to explore the factors associated with the timely preparation of VA creation in a CKD population undertaking a multidisciplinary CKD care program, focusing on the renal progression rate by testing the hypothesis that slower eGFR slope is associated with better VA creation timely.
Methods
Study design
This retrospective cohort study included CKD patients who had followed up at a tertiary medical center in Taiwan from 2003 to 2016. The eligible subjects were those who had received multidisciplinary CKD care for more than 6 months and transitioned to HD. We excluded patients who had previously undergone kidney transplantation or peritoneal dialysis. The study protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-G(II)-20160024). Informed consent was obtained in written form and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Multidisciplinary care program
We started a multidisciplinary CKD care program in 2003. Management and education were dependent on the stages of CKD, according to the 2002 NKF-KDOQI guidelines17, and the reimbursement policies of National Health Insurance (NHI). Detailed contents of this program have been described somewhere in our previous studies12,18. In 2006, Taiwan NHI launched a pay-for-performance program with indicators including CKD management, patient education, continuous care, remission of proteinuria, and maintenance of renal function for pre-End Stage Kidney Disease (ESKD) care19,20.
Outcomes of VA preparation for HD
The introduction of renal replacement therapy (RRT) modality will be implemented for all patients in the multidisciplinary CKD care program when their eGFRs drop below 10 ml/min/1.73 m2. Usually, VA creation will be conducted for no longer than 2 weeks after surgeon consultation in this hospital. A cardiovascular surgeon consultation visit will be arranged before the creation with ultrasound mapping done at the same time if indicated. Two experienced cardiovascular surgeons performed all VA creation for most of our patients, with a surgical success rate of more than 95%. For fistula creation, local anesthesia was selected while general anesthesia was chosen for graft creation. In general, the surgeons performed end to side arterial anastomosis in both fistula and graft creation, and end to end venous anastomosis in graft creation. Occasionally, superficialization and transposition of deep fistula were needed21. In our hospital, it usually took 2 weeks from the cardiovascular surgeon referral to the VA creation.
Based on the status of VA used at the beginning of HD, we divided the study patients into three groups: VA created timely, VA created not timely, and VA not created. The group of VA created timely comprised patients using distal VA, including both fistula and graft, in the first three sessions of HD. The group of VA created not timely comprised patients using a temporary catheter for any one of the first three sessions of HD with distal VA created but immature. Meanwhile, the group of VA not created comprised patients using a temporary catheter for the first HD without distal VA created.
eGFR calculation and slope
The eGFR in the study was calculated using the Modification of Diet in Renal Disease (MDRD) study equation22 and the Cockcroft and Gault (CG) formula23. In this hospital, physicians have access to all eGFR data when patients are attending clinical visits, and the eGFR slope is available at the same time. We defined the eGFR slope used in this study as using all eGFRs between the timing of CKD program enrollment and VA creation or starting HD—whichever came first. If there were fewer than three eGFR measurements within the aforementioned duration, we would include all eGFR measurements within 1 year prior to VA creation or starting HD—whichever came first. Through either quartile or clinical recommendations using the three cut values of − 10, − 5 and − 3 ml/min/1.73 m2 per year, we divided eGFR slopes into four groups for further analysis. In order to evaluate the effect of eGFR slope on VA maturation, we only included those stayed at program longer than 6 months.
Covariates
Our register record or the medical informatics system within the hospital was used to obtain demographic information, namely age, sex, marital status (unmarried, married, or other), education level (years of education: 0–6, 7–12, equal to or more than 13), employment status (no, yes or retirement), smoking status (yes or no), underlying disease (chronic glomerulonephritis, diabetes mellitus, hypertension, or other), renal function upon timing of VA creation, types of VA for long-term dialysis (arteriovenous fistula (AVF), arteriovenous graft (AVG), or permanent catheter), renal function upon multidisciplinary CKD care program enrollment, renal function upon VA creation, renal function upon first dialysis, duration in multidisciplinary CKD care program, and time between first dialysis and shunt creation.
Statistical analysis
The baseline characteristics were summarized as the mean and standard deviation or the median (interquartile range) for continuous variables and count (percentages) for categorical variables, respectively. Differences among groups were compared using the chi-squared test for categorical variables and one-way ANOVA, the Mann–Whitney U test or the Kruskal–Wallis test for continuous variables. To determine the significant differences in continuous variables between the groups, Fisher’s least significant difference test was applied after significant results were obtained from one-way ANOVA. The trend of VA preparation was described by the proportion of patients with their VA status upon the onset of HD, and shifts in trends were assessed using the Cochran–Armitage trend test. We used scatter plots with the Pearson correlation coefficient and test to investigate the linear associations between creatinine levels upon VA creation and eGFR slope, and eGFR by MDRD and CG equations upon VA creation and eGFR slope. Multivariable logistic regression analysis with backward selection was performed to determine factors associated with VA created timely. Statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). A two-sided P < 0.05 indicates statistical significance.
Results
Patient selection
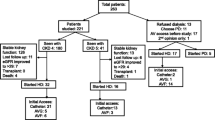
Our study population was derived from a cohort of 3913 patients enrolled in the multidisciplinary CKD care program between 1 January 2003 and 31 December 2016. Of this cohort, 1435 patients entered maintenance HD. We excluded those who enrolled less than 180 days before the first HD (n = 411), whose VA status was unknown upon the first HD (n = 7), who had VA creation before CKD care program enrollment (n = 18), and those with fewer than three eGFR measurements available (n = 12). The final cohort consisted of 987 patients. Besides, 382 subjects died at the program, with 29 had VA created. Figure 1 presents a flow chart of the study cohort.
Patient characteristics by the outcome of VA preparation
The baseline characteristics of the study cohort by consequences of VA preparation are presented in Table 1. Among these patients, 676 (68.5%) belonged to VA created timely, 87 (8.8%) belonged to VA created not timely, and 224 (22.7%) of them were in VA not created. There was no difference in demographic characteristics among the aforementioned three groups. Compared with the other groups, the VA created timely had slower CKD progression, with an eGFR slope median value of − 4.30 ml/min/year. The VA created timely had more AVF creation and, as expected, a higher eGFR level when receiving VA creation surgery. Similarly, the time between VA creation and the first HD was greater in this group, with a median of 105 days and a mean of 180 days. All groups had similar residual renal function when starting the first HD, with a median eGFR value of around 4.5 ml/min/1.73 m2, and the VA created not timely even had values below 4.0. Meanwhile, the VA created timely had the longest stay in the multidisciplinary CKD care program, with a median duration of more than 2 years. Figure 2 shows the proportion of patients with their VA status according to the calendar years. The comparisons among these three different time cohorts are displayed in Table 2.
eGFR slope and timely VA creation
VA was created at a median eGFR of 5.8 min/ml/1.73 m2, with a median duration of 3.1 months before HD. Figure 3 reveals the association between eGFR slope and renal function at the time of VA creation. In both the groups comprising VA created timely and not timely, there was a negative association between eGFR slope and eGFR, which was determined either by MDRD or CG formulae at the time of VA creation (Fig. 3b,c, respectively; both P < 0.001). However, this negative association disappeared when creatinine (Cr) was used to replace the eGFR (Fig. 3a, P = 0.425). Table 3 reveals the distribution of VA maturation among various eGFR slope ranges. As expected, the fastest-progressing group, whether cut by the first quartile or − 10 ml/min/1.73 m2/year, had the lowest percentage of VA created timely, while the remaining groups shared similar success rates. In contrast with the longer duration between VA creation and the first HD as the eGFR slope becomes more flattened in the group comprising VA created timely, eGFRs upon VA creation were not significantly different among various eGFR slope ranges in both the groups consisting of VA created timely and not timely (Fig. 4). The eGFR differences upon VA creation between VA created timely and not timely, at approximately 0.9 ml/min/1.73 m2, were not affected by the various eGFR slope groups (Fig. 4a,b), while the time difference between VA creation and the first HD increased as eGFR slope became slower (Fig. 4c,d). Supplementary Figure S1 further displays the distribution of percentages of VA created timely by eGFR slope, with an interval of 2 ml/min/1.73 m2/year. We identified that there was a trend whereby slower eGFR slopes were associated with higher VA created timely, even though the middle progression with eGFR slope between − 4 and − 6 ml/min/1.73 m2/year had a slightly higher percentage of VA created timely. Table 4 shows the results of the multivariate analysis regarding factors and their interactions associated with VA created timely. After adjusting with covariates, only renal function upon VA creation and eGFR slope were associated with the timely creation of VA. When renal function was expressed by Cr, sex and age upon VA creation were significant predictors for VA created timely. But when the eGFR was used to express renal function upon VA creation, sex and age were no longer significant predictors. Using the third quartile of eGFR slope (− 4.30 ~ < − 2.79 ml/min/1.73 m2/year) as a reference, patients with slightly steeper eGFR slope (− 7.79 < − 4.30 ml/min/1.73 m2/year) were 30% more likely, albeit not statistically significantly, to have VA created timely. For those with the steepest eGFR slope (< − 10 ml/min/1.73 m2/year), the likelihood of having VA created timely was around only 50%. The patients with the flattest eGFR slope (≥ − 2.79 ml/min/1.73 m2/year) had similar results to those of the reference group. If we used the cutting ranges recommended by guidelines, consistent findings were produced, as shown in Supplementary Fig. S2.
Correlation between renal function at vascular access (VA) creation and estimated glomerular filtration rate (eGFR) slope by VA created timely or not timely (n = 763). Renal function expressed by creatinine (Cr) (a), estimated glomerular filtration rate (eGFR) by modification of diet in renal disease (MDRD) (b) calculated by the equation: 186 \(\times\) serum creatinine−1.154 \(\times\) age−0.203 \(\times\) 1.212 (if patient is black) \(\times\) 0.742 (if female)22, and eGFR by Cockcroft–Gault (CG) (c) calculated by the equation: [(140 − age)] \(\times\) weight \(\times\) 0.85 (if female)/(72 \(\times\) serum creatinine), adjusted for BSA/1.73 m2. \(BSA = weight~_{{{\text{kg}}}}^{{0.425}} \times height~_{{{\text{cm}}}}^{{0.725}} \times 0.007184\)23.
Distribution of estimated glomerular filtration rate (eGFR) and time between vascular access (VA) creation and first hemodialysis in VA created timely and not timely by eGFR slope group. eGFR slope by 75th, 50th and 25th percentiles (a,c) and eGFR slope by clinical recommendation (b,d). Asterisk in (a) represents the significant difference between slope groups in the group comprising VA created timely (P = 0.02), and there was a non-significant difference between slope groups in the group comprising VA created not timely by the Kruskal–Wallis test. Asterisk in (c) and (d) display the significant difference between slope groups in the group comprising VA created timely (P < 0.001), while there was a non-significant difference between slope groups in the group comprising VA created not timely by the Kruskal–Wallis test.
Discussion
This is the first report showing, through the multidisciplinary CKD program, that a planned initiation of maintenance HD without a temporary catheter can be constantly achieved in more than 70% of all enrolled patients. The median days between VA creation and the first dialysis constituted almost 3 months for those with timely creation, while the mean days constituted around 6 months. In addition to a higher eGFR at the time of VA creation, eGFR slope played an important role in our findings with regard to VA created timely. Fast progression of CKD led to much less VA created timely, but slow-progression groups did not achieve as high a percentage of VA created timely as that achieved by the middle-progression group. Our findings in this observational study could provide a practical recommendation when determining the time of VA creation in the care of late CKD patients.
Registry data indicating timely VA creation varies among countries from less than 20% to almost 80%7,24,25,26. Given the many advantages of not using temporary central catheters to initiate dialysis but the unsatisfactory results worldwide27,28, a strategy with which to increase VA created timely should be well examined in every CKD care unit. To create VA timely for the initiation of dialysis, referral in time, regular follow-up of CKD progression, conducting shared decision making on the choice of RRT modality, and determining the time of VA creation are all needed, in that order14,18,29. Thus, it seems very reasonable to consider timely VA creation to be a potential candidate for the quality index of pre-dialysis CKD care. Through the multidisciplinary CKD program, our cohort showed a continuous improvement in the proportion of patients starting HD with distal VA of more than 70% and whose results had been consistent for the 6 years running up to 2016. A few HD centers have a similar performance at such a percentage or even higher7,30, which suggests that the figure of 70% might be an achievable goal for many other centers to reach.
The European Best Practice Guidelines recommend arteriovenous fistula creation at least 2–3 months prior to HD, whereas the NKF-KDOQI used to suggest at least 6 months prior to HD, but recently have recommended that physicians begin VA creation preparation with patients when entering late CKD stage 4, as it is impractical to arrange VA creation based on predicting the time when HD is needed11,31,32. Taiwan has relatively late dialysis initiation, with an eGFR of 5.9 ml/min/1.73 m2 in 201733. Amongst this 15-year cohort, patients received VA creation at an eGFR of 4.1–7.1 (Q1–Q3) ml/min/1.73 m2 and started HD at an eGFR of 3.4–5.5 (Q1–Q3) ml/min/1.73 m2, with the median duration between VA creation and dialysis starting at approximately 3 months. The negative association between eGFR slope and the eGFR upon VA creation demonstrated that nephrologists in our program had used eGFR slope as a guide in determining when VA creation was needed. The high successful rate of VA creation timely implied that it is practical to prepare VA creation based on predicting the onset of dialysis. The present NKF-KDOQI guideline recommendation of starting VA creation when entering CKD stage 4 with an eGFR of 15–20 ml/min/1.73 m2 raises concern surrounding the duration between VA creation and initiation of dialysis would become much longer. The longer duration referred to above may increase risks such as having VA fail and death before use29, not to mention having a higher cardiac burden from VA shunting. In addition, unit would consume much more human resource in convincing patients for earlier schedules. In brief, we used our clinical results to support the contention that using eGFR slope for preparing VA creation by predicting the occurrence of HD is feasible with a high probability of success. The guidelines could give more precise individual suggestions regarding the timing of VA creation with putting eGFR slope into consideration.
Fistula first is our policy for a very long time, with the distribution of AVF (85%) and AVG (15%) in our study cohort of 2003–2016 (Fig. 2). In our hospital, the surgeon is the one who suggests whether fistula or graft should be created based on the vessel condition, and the nephrologist only suggests permanent catheter insertion if a short life span or temporary dialysis duration is expected. Again, our data support this practice pattern as the AVG had the same distribution in both created timely and not timely groups. In our practice, we did not arrange AVG creation for the reason of early cannulation. A temporary or permanent catheter will be used once early cannulation is required. With the introduction of ESKD Life-Plan by KDOQI in 201911, it recommends taking VA creation and care as a continuous process from predialysis until the whole ESKD. For the first access creation, a patient-centered approach for dialysis modality choice, vessel condition evaluation, VA creation, and maturation for cannulation have all been well documented. Fistula-First might be no longer suited to everyone who chose HD. When preparing the distal VA in the elderly, the more inferior vessel quality, higher comorbidity burden, and shorter life expectancy even further addressed the necessity of ESKD Life-Plan in this population. To complete all the processes as mentioned above, starting the preparations at CKD stage 4 is encouraged. We believe the eGFR slope can help the care team take the comprehensive steps in VA creation suggested by ESKD Life-Plan at proper timings regarding local practice patterns.
The eGFR rather than creatinine, like detecting CKD34, should be used to make the decision regarding VA creation simpler according to our model suggestion. In addition to being a surrogate of renal outcomes35, the role of eGFR slope in timely VA preparation should be addressed how it could predict the initiation of dialysis. When exhibiting an eGFR slope steeper than − 10 ml/min/1.73 m2/year, patients would have VA creation scheduled much earlier based on prior recommendations11; now we offer the evidence which can be used to support this expert consensus. In contrast to our retrospective observation, Al-Balas et al. have published a study on the optimal timing of predialysis AVF creation with a prospective approach29. With night-tenth of AVF creation at eGFR higher than 10 ml/min/1.73 m2, only 47% of the participants used this permanent access on the first HD session, and 15% died before dialysis initiation. They can't find any factors predicting the use of mature AVF on dialysis initiation, but the eGFR at AVF creation, diabetes mellitus, eGFR slope, and degree of proteinuria are associated with HD initiation within two years after the AVF creation. In addition, the non-significant difference of the eGFR upon VA creation among the remaining various eGFR slope groups implied, even with satisfactory success probability, that other factors may contribute to the prognosis. We speculate that patients’ decision making might play a role in delayed VA creation for the following findings: firstly, the very short duration before the first HD in VA created not timely among every eGFR slope group, secondly, that the eGFR differences upon VA creation between VA created timely and not timely were fixed at various eGFR slopes, and, thirdly, that VA created not timely had a lower eGFR than did VA created timely when starting the first HD. Then, starting VA preparation earlier may have few effects on improving the probability of success.
There were several limitations in our study, especially due to its retrospective and observational study design. Firstly, the generalization of our findings may be limited regarding our VA created at a relatively low eGFR level and fast surgical assistance, and we only included those late CKD patients under multidisciplinary CKD program for more than 6 months. However, the consistent high timely creation of VA in our study, and in certain other studies, might support the notion that the majority of CKD teams can initiate planned HD through distal VA with a certain probability of success. Secondly, how the patients made their decision was not measured, which could be a main factor contributing to the timely creation of VA. There was no difference in both eGFR slope before HD and eGFR values upon starting HD between patients receiving VA creation and those not; this implied that patients’ refusal of VA creation might play a determining role for those with VA not created. Thus, including only those having VA creation within analysis means that the results are less influenced by patients’ compliance. Thirdly, the disparity of clinical experience among nephrologists is another unmeasurable factor when running the prediction model. Finally, factors other than eGFR slope, such as the degree of proteinuria, are not included in the analysis to explain the timely VA creation. Although the eGFR slope itself is a very dominant predictor of CKD progression, several studies consider it as CKD progression; other potential indexes are still having the chance for better prediction power. Nevertheless, the standard procedures within the multidisciplinary care did mean that the majority of in-program patients received similar management. Although the eGFR slope itself is a very dominant predictor of CKD progression, several studies consider it a CKD progression35,36, more potential indexes are needed for better prediction power.
Conclusion
Our study revealed that, through the multidisciplinary CKD program, participants can constantly have timely VA creation of more than 70%. Factors associated with timely VA creation include receiving VA creation at a higher eGFR and flattening the eGFR slope. The eGFR slope should be highlighted when advanced CKD patients are scheduled for VA creation.
Abbreviations
- AVF:
-
Arteriovenous fistula
- AVG:
-
Arteriovenous graft
- CG:
-
Cockcroft and gault
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- HD:
-
Hemodialysis
- MDRD:
-
Modification of diet in renal disease
- NHI:
-
National Health Insurance
- NKF-KDOQI:
-
National Kidney Foundation-Kidney Disease Outcomes Quality Initiative
- RRT:
-
Renal replacement therapy
- VA:
-
Vascular access
References
Fluck, R. & Kumwenda, M. Renal Association Clinical Practice Guideline on vascular access for haemodialysis. Nephron. Clin. Pract. 118(Suppl 1), c225-240. https://doi.org/10.1159/000328071 (2011).
Vascular Access Work Group. Clinical practice guidelines for vascular access. Am. J. Kidney Dis. 48(Suppl 1), S176-247. https://doi.org/10.1053/j.ajkd.2006.04.029 (2006).
Ravani, P. et al. Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J. Am. Soc. Nephrol. 24, 1668–1677. https://doi.org/10.1681/ASN.2012121234 (2013).
Ravani, P. et al. Associations between hemodialysis access type and clinical outcomes: A systematic review. J. Am. Soc. Nephrol. 24, 465–473. https://doi.org/10.1681/ASN.2012070643 (2013).
Hughes, S. A., Mendelssohn, J. G., Tobe, S. W., McFarlane, P. A. & Mendelssohn, D. C. Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol. Dial. Transplant. 28, 392–397. https://doi.org/10.1093/ndt/gfs431 (2013).
Mendelssohn, D. C. et al. Suboptimal initiation of dialysis with and without early referral to a nephrologist. Nephrol. Dial. Transplant. 26, 2959–2965. https://doi.org/10.1093/ndt/gfq843 (2011).
Pisoni, R. L., Zepel, L., Port, F. K. & Robinson, B. M. Trends in US vascular access use, patient preferences, and related practices: An update from the US DOPPS practice monitor with international comparisons. Am. J. Kidney Dis. 65, 905–915. https://doi.org/10.1053/j.ajkd.2014.12.014 (2015).
Astor, B. C. et al. Timing of nephrologist referral and arteriovenous access use: The CHOICE Study. Am. J. Kidney Dis. 38, 494–501 (2001).
Avorn, J. et al. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J. Clin. Epidemiol. 55, 711–716 (2002).
Lin, M. Y. et al. Effects of physician’s specialty on regular chronic kidney disease care in predialysis: A population-based cross-sectional study. Medicine 97, e11317 (2018).
Lok, C. E. et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am. J. Kidney Dis. 75, S1–S164 (2020).
Chen, J. H. et al. Effect of nephrology referrals and multidisciplinary care programs on renal replacement and medical costs on patients with advanced chronic kidney disease: A retrospective cohort study. Medicine 98, e16808 (2019).
Chen, P. M. et al. Multidisciplinary care program for advanced chronic kidney disease: reduces renal replacement and medical costs. Am. J. Med. 128, 68–76. https://doi.org/10.1016/j.amjmed.2014.07.042 (2015).
Chen, Y. R. et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: A 3-year prospective cohort study. Nephrol. Dial. Transplant. 28, 671–682. https://doi.org/10.1093/ndt/gfs469 (2013).
Chen, Y. R. et al. Multidisciplinary care improves clinical outcome and reduces medical costs for pre-end-stage renal disease in Taiwan. Nephrology (Carlton) 19, 699–707. https://doi.org/10.1111/nep.12316 (2014).
Wang, S.-M. et al. Multidisciplinary care in patients with chronic kidney disease: A systematic review and meta-analysis. Eur. J. Intern. Med. 26, 640–645 (2015).
Levey, A. S. et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 139, 137–147 (2003).
Wei, S. Y. et al. Chronic kidney disease care program improves quality of pre-end-stage renal disease care and reduces medical costs. Nephrology 15, 108–115 (2010).
Lin, M. Y. et al. Effect of national pre-ESRD care program on expenditures and mortality in incident dialysis patients: A population-based study. PLoS ONE 13, e0198387 (2018).
Hsieh, H. M. et al. Economic evaluation of a pre-ESRD pay-for-performance programme in advanced chronic kidney disease patients. Nephrol. Dial. Transplant. 32, 1184–1194. https://doi.org/10.1093/ndt/gfw372 (2017).
Lin, Y.-C. et al. Long-term outcomes of lateral tunneled transposed brachiobasilic arteriovenous fistulas in elderly hemodialysis patient. J. Vasc. Access 20, 557–562 (2019).
Levey, A. S. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 145, 247–254. https://doi.org/10.7326/0003-4819-145-4-200608150-00004 (2006).
Cockcroft, D. W. & Gault, M. H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41. https://doi.org/10.1159/000180580 (1976).
Australia and New Zealand Dialysis and Transplant Registry. The 42nd Annual ANZDATA Report 2019 (Data to 2018). (Adelaide, Australia, 2019).
Noordzij, M. et al. Use of vascular access for haemodialysis in Europe: A report from the ERA-EDTA Registry. Nephrol. Dial. Transplant. 29, 1956–1964 (2014).
United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018).
Hasegawa, T. et al. Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin. J. Am. Soc. Nephrol. 4, 595–602. https://doi.org/10.2215/CJN.03540708 (2009).
Tennankore, K. K., Soroka, S. D. & Kiberd, B. A. The impact of an “acute dialysis start” on the mortality attributed to the use of central venous catheters: A retrospective cohort study. BMC Nephrol. 13, 72. https://doi.org/10.1186/1471-2369-13-72 (2012).
Al-Balas, A., Lee, T., Young, C. J., Barker-Finkel, J. & Allon, M. Predictors of initiation for predialysis arteriovenous fistula. Clin. J. Am. Soc. Nephrol. 11, 1802–1808 (2016).
Ozeki, T. et al. The type of vascular access and the incidence of mortality in Japanese dialysis patients. Intern. Med. 56, 481–485 (2017).
Tordoir, J. et al. EBPG on vascular access. Nephrol. Dial. Transplant. 22, 88–117 (2007).
Schwab, S. et al. NKF-DOQI clinical practice guidelines for vascular access. Am. J. Kidney Dis. 30, S150–S191 (1997).
Taiwan Renal Data System. 2019 Annual Report on Kidney Disease in Taiwan. (National Health Research Institutes and Taiwan Society of Nephrology, 2019).
Swedko, P. J., Clark, H. D., Paramsothy, K. & Akbari, A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch. Intern. Med. 163, 356–360. https://doi.org/10.1001/archinte.163.3.356 (2003).
Lambers Heerspink, H. J. et al. GFR decline and subsequent risk of established kidney outcomes: A meta-analysis of 37 randomized controlled trials. Am. J. Kidney Dis. 64, 860–866. https://doi.org/10.1053/j.ajkd.2014.08.018 (2014).
Inker, L. A. et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J. Am. Soc. Nephrol. 30, 1735–1745. https://doi.org/10.1681/asn.2019010007 (2019).
Funding
The authors would like to sincerely thank the Taiwan Ministry of Science and Technology (Grant numbers: MOST: 103-2314-B-037-033-, MOST: 104-2314-B-037-054, MOST: 105-2314-B-037-065-, MOST: 106-2314-B-002-253-, MOST:107-2314-B-037-122-, MOST: 108-2314-B-037-110-, and MOST109-2314-B-037-099), the Taiwan Ministry of Health and Welfare (Grant number: MOHW107-TDU-B-212-123006), the Taiwan National Health Research Institutes (Grant numbers: NHRI-EX107-10717PI and NHRI-EX108-10505PI), Kaohsiung Medical University Hospital (Grant numbers: KMUH103-3R10 and KMUH104-4R11), and Center for Big Data Research for supporting this study. The funders had no role in the design and implementation of the study, nor in the collection, management, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.Y.L., and Y.W.C.; methodology: M.Y.L., and Y.W.C.; writing—original draft preparation: L.M.L., M.Y.L., and Y.W.C.; writing—review and editing: L.M.L., M.Y.L., and Y.W.C.; supervision: S.J.H., H.C.C., and Y.W.C. All authors have reviewed and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, LM., Lin, MY., Hwang, SJ. et al. Association of glomerular filtration rate slope with timely creation of vascular access in incident hemodialysis. Sci Rep 11, 13137 (2021). https://doi.org/10.1038/s41598-021-92359-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92359-w
- Springer Nature Limited
This article is cited by
-
Predictors of post-kidney transplantation complications among Egyptian recipients—1-year follow-up
The Egyptian Journal of Internal Medicine (2024)