Abstract
In retail meat products, Campylobacter jejuni, C. coli, and Staphylococcus aureus have been reported in high prevalence. The polymicrobial interaction between Campylobacter and other bacteria could enhance Campylobacter survival during the adverse conditions encountered during retail meat processing and storage. This study was designed to investigate the potential role of S. aureus from retail meats in enhancing the survival of Campylobacter exposed to low temperature, aerobic conditions, and biofilm formation. Results indicated that viable S. aureus cells and filter-sterilized cell-free media obtained from S. aureus prolonged the survival of Campylobacter at low temperature and during aerobic conditions. Biofilm formation of Campylobacter strains was significantly enhanced in the presence of viable S. aureus cells, but the results were inconclusive when extracts from cell-free media were used. In conclusion, the presence of S. aureus cells enhances survivability of Campylobacter strains in adverse conditions such as low temperature and aerobic conditions. Further investigations are warranted to understand the interaction between Campylobacter and S. aureus, and effective intervention strategies are needed to reduce the incidence of both foodborne pathogens in retail meat products.
Similar content being viewed by others
Introduction
The high prevalence of Campylobacter spp. and Staphylococcus aureus in retail meat products1,2,3,4 remains a challenge for food safety despite the improvement in retail meat production systems. Sporadic outbreaks of campylobacteriosis and staphylococcal food poisoning after consumption of contaminated food products has been reported worldwide5,6,7,8. A recent report from the CDC has documented an incremental increase in campylobacteriosis cases in the USA in recent years9. Campylobacter is a microaerobic, fastidious organism that lacks genetic mechanisms to cope with various stressors10,11,12; however, it persists in food products even with exposure to harsh environmental conditions during processing and storage1,2,13. Temperature fluctuations beyond its optimal growth temperature (37–42 °C) and exposure to aerobic conditions (oxidative stress) are major stressors that Campylobacter encounters during food processing and storage14,15. Survival mechanisms of Campylobacter include the viable but non-culturable condition (VBNC), biofilm formation, aerotolerance, and acquisition of cryoprotectant molecules14,15. Biofilm production by foodborne pathogens in retail meat processing surfaces and environments facilitates their survival16,17 and ensures the contamination of retail meat products. In situations where enhanced survival and biofilm formation have been reported in retail meat and liver products, the food matrix environment influenced the survival of Campylobacter18,19. Meanwhile, the higher prevalence of aerotolerant Campylobacter strains from food products also ensures their survival during oxidative stress20,21.
The high prevalence of S. aureus as a co-contaminant with Campylobacter in the same retail meat source has been previously reported by our laboratory1,2,3,4,13,22. Meanwhile, many other co-contaminants, including Pseudomonas spp., Salmonella spp., Staphylococcus spp., hepatitis E, Escherichia spp. and Yersinia spp. are also prevalent in retail meat and liver products23,24,25. Likewise, many spoilage bacteria have been identified in biofilms in meat processing environments that are known to contaminate retail meat products16,17. The presence of other co-contaminants could enhance survival of Campylobacter spp. during adverse environmental conditions26,27, which would ensure its persistence in the retail meat environment. Campylobacter has been characterized as a secondary colonizer in pre-existing biofilms26. However, there are very few studies documenting higher biofilm formation and enhanced survival of Campylobacter in adverse environments that include polymicrobial interactions26,27,28,29,30.
Staphylococcus aureus can grow aerobically within a wide range of temperatures31; consequently, it might provide a better environment for survival of co-contaminant Campylobacter during food processing and storage. A few reports exist documenting the coexistence of C. jejuni and S. aureus in biofilms28,29; however, the influence of S. aureus strains from retail meats on the survival of C. jejuni and C. coli at low temperatures and aerobic conditions is unclear. In this study, we studied the influence of S. aureus strains from retail meat products on Campylobacter survival at low temperature, in aerobic conditions and within biofilms. Cell-free S. aureus were included to investigate the effect of extracellular metabolites on Campylobacter survival during adverse environmental conditions.
Materials and methods
Bacterial strains and culture conditions
Five Campylobacter strains, including two and three strains of C. jejuni and C. coli, respectively, and two S. aureus strains were sourced from retail meat products and used in this study (Table 1). These strains were previously isolated, characterized and sequenced in our laboratory1,2,3,4,12,13,22,32,33,34,35,36,37. These strains were selected because the genomic sequences were available, and they represent different sources of retail meats. The clinical strain C. jejuni NCTC11168 was used as a reference strain. Campylobacter strains were subcultured from stock culture (stored at − 70 °C) in Mueller Hinton Agar (MHA) supplemented with 5% laked horse blood (at 42 °C) in microaerobic conditions (Oxoid CampyGen 3.5 L sachet, Thermo Scientific or Thermo Forma tri-gas incubator) for 48 h. Subcultures of Campylobacter cultures were maintained on MHA plates or Mueller Hinton Broth (MHB) as needed. S. aureus strains were grown and subcultured in Mannitol Salt Agar (MSA) at 37 °C in aerobic conditions.
Preparation of cell-free extracts from S. aureus
Staphylococcus aureus strains B4-59C and B6-55A were grown in MSA for 24 h; cells were then subcultured in freshly-prepared MHB (50 ml) and incubated at 37 °C at 100 rpm for 24 h with aerobic conditions. Cell pellets were obtained after centrifugation (5000 rpm, 5 min), washed in PBS (pH 7.4), suspended in fresh MHB, and adjusted to OD600 = 1.0. Twenty milliliters of cell suspension was added to 200 ml MHB and incubated at 4 °C for 48 h. Similarly, 10 ml of cell suspension was added to 200 ml MHB in conical flasks (250 ml) and incubated separately at 25 °C and 37 °C with 100 rpm agitation for 24 h. After incubation, inoculated MHB media were filter-sterilized (0.45 µm, Nalgene Rapid-Flow), and sterility was verified on MHA, MHA supplemented with 5% laked horse blood and MSA incubated in aerobic and microaerobic conditions at 37 °C and ambient temperature for 48 h. For each temperature setting (4 °C, 25 °C and 37 °C), experiments were performed in triplicate; filter-sterilized media were mixed and stored at − 20 °C. These sterilized media included: S. aureus grown in MHB at 4 °C (strains B4-49C-4 and B6-55A-4), S. aureus grown in MHB at 25 °C (strains B4-59C-25 and B6-55A-25), S. aureus grown in MHB at 37 °C (strains B4-59C-37 and B6-55A-37), and non-inoculated MHB (control); these media were used in survival, biofilm and aerotolerance assays of Campylobacter strains. The protein concentration of inoculated and non-inoculated MHB was measured by the BCA protein Assay kit (Pierce™).
Fractionation of media used to cultivate S. aureus
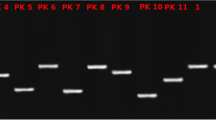
Filter-sterilized media from S. aureus cultures were stored at − 20 °C and thawed overnight at 4 °C prior to use. Filter-sterilized media were fractionated using a Ultracel-30 kDa filter (Millipore, Burlington, Massachusetts, USA), and the eluate (≤ 30 kDa) was centrifuged for 30 min at 5000 rpm. For all samples, 20 µl of non-inoculated MHB and both fractions (≤ 30 kDa and ≥ 30 kDa) were separated in 12% polyacrylamide gels by electrophoresis.
Campylobacter survival assays at 4 °C
Survival assays using cell-free extracts from S. aureus
Campylobacter jejuni NCTC11168, T1-21 and OD2-67 and C. coli HC2-48, ZV1-224 and WA3-33 were cultured overnight on MHA supplemented with 5% laked horse blood. Cells were removed, suspended to OD600 = 0.1 in MHB, and then diluted tenfold in either MHB or cell-free extracts of S. aureus that were chilled to (4 °C). The cell-free extracts included S. aureus B4-59C grown at 4, 25, and 37 °C (B4-59C-4, B4-59C-25, B4-59C-37) and S. aureus B655A grown at 4, 25, and 37 °C (B655A-4, B6-55A-25 and B6-55A-37). Triplicate sets of Campylobacter-inoculated media (8 ml) were incubated at 4 °C in polystyrene culture tubes. At 0, 24, 48, 72 and 120 h, 40 µl samples were removed and serial dilutions were made in PBS (pH 7.4). Viable cell counts were obtained by spotting 10 µl dilutions on MHA and incubating in microaerobic condition (Thermo Forma tri-gas incubator) as described previously18.
Survival assays using S. aureus cells
Campylobacter strains were grown for 48 h in MHA supplemented with 5% laked horse blood and the following antibiotics: cefoperazone, 20 µg/ml; vancomycin, 20 µg/ml; trimethoprim, 20 µg/ml; and amphotericin B, 10 µg/ml {(Bolton Selective Supplement FD231, Himedia, Mumbai, India)}. Campylobacter cells were then subcultured in 25 ml MHB at 110 rpm, 42 °C with microaerobic conditions for 24 h. S. aureus strains were grown in MSA from stock cultures for 24 h and subcultured in 25 ml MHB for 18 h at 37 °C with aerobic conditions. Campylobacter and S. aureus cells were pelleted and resuspended in MHB to OD600 = 0.1. For Campylobacter, cell suspensions were mixed in MHB preincubated at 4 °C to create a 1:5 dilution, and this was used for both mono and mixed cultures. A 1:10 dilution of S. aureus cell suspension was added to Campylobacter cells in MHB for the mixed culture experiment. Triplicate samples (5 ml) of Campylobacter monocultures or mixed cultures (S. aureus + Campylobacter) were incubated at 4 °C in polystyrene culture tubes. Viable counts for Campylobacter were obtained on MHA amended with Bolton Selective Supplement FD231 at 42 °C in microaerobic conditions (Oxoid CampyGen 3.5 L sachet, Thermo Scientific). Meanwhile, S. aureus counts were obtained on MSA at 37 °C with aerobic incubation. Viable counts were recorded up to 7 days; this data point was selected because Campylobacter monocultures could only produce colonies on MHA (with antibiotics) up to 5 days of incubation.
Campylobacter aerotolerance assays
Aerotolerance assays using cell-free extracts from S. aureus
Aerotolerance of Campylobacter strains was assessed as described previously with minor modifications18,21. MHB and cell-free extracts from S. aureus B4-59C-4, B4-59C-25, B4-59C-37, B6-55A-4, B6-55A-25, and B6-55A-37 were used in the experiment. Campylobacter strains were subcultured in freshly-prepared MHB and incubated to an OD600 = 0.5; a 500 µl aliquot was then added to 5 ml of media (MHB and S. aureus cell-free extracts). Triplicate sets (1.5 ml each) were transferred to 50 ml Falcon tubes with vented (cracked open) caps to simulate aerobic conditions and incubated at 42 °C, 200 rpm for 12–24 h. Samples (100 µl) were taken at 0, 6, 12, and 24 h, and viable counts were obtained on MHA (Thermo Forma tri-gas incubator). Each experiment was repeated at least twice.
Aerotolerance assays using S. aureus cells
Campylobacter and S. aureus strains were subcultured in 25 ml freshly-prepared MHB. Suspensions were centrifuged at 10,000 rpm for 5 min, and pellets were resuspended in MHB at OD600 = 0.5 for Campylobacter and OD600 = 0.1 for S. aureus. A tenfold dilution of each Campylobacter strain was prepared in 30 ml freshly-prepared MHB and divided equally into triplicate, 10 ml aliquots. One set of suspensions was used as a control and contained only Campylobacter cells. In another set of suspensions, mixed populations of S. aureus and Campylobacter were prepared by combining S. aureus B4-59C or B6-55A (1 ml, OD600 = 0.1) with a 10 ml suspension of Campylobacter cells. Campylobacter monocultures were prepared as described above for survival assays, and S. aureus monocultures were prepared for both strains by adding 1 ml of S. aureus cell suspension to 10 ml freshly-prepared MHB. Triplicate aliquots (1 ml) of inoculated media (Campylobacter and S. aureus mixed cultures and monocultures of the two organisms) were incubated in 24-well culture plates at 25 °C or 42 °C in aerobic conditions at 200 rpm. To ensure proper aeration, lid of 24-well culture plate was lifted higher using adhesive tapes. Viable counts for Campylobacter were obtained at 0, 6, 12 and 24 h in MHA supplemented with antibiotics (Bolton Selective Supplement FD231); cultures were incubated at 42 °C in microaerobic conditions (Oxoid CampyGen 3.5 L sachet, Thermo Scientific) for at least 48 h. S. aureus cell counts were obtained at 0, 6, 12 and 24 h on MSA plates that were incubated at 37 °C for 24 h.
Biofilm assays
Biofilm formation using cell free extracts and < 30 kDa media fractions
Campylobacter strains and cell-free extracts from S. aureus B4-59C-4, B4-59C-25, B4-59C-37, B655A-4, B6-55A-25 and B6-55A-37 were prepared as described above in aerotolerance and survival assays, respectively. Campylobacter monocultures and cells amended with S. aureus cell-free extracts or the ≤ 30 kDa flow-through fraction were analyzed for biofilm formation as described previously18. The ≥ 30 kDa fraction was excluded due to insufficient volume. Suspensions were diluted tenfold, and four replicate samples (150 µl) were incubated (static) in 96-well polystyrene flat-bottomed plates at 42 °C in microaerobic environment (Thermo Forma tri-gas incubator) or aerobic condition (normal atmospheric condition) for 72 h. The lid of 96 well plate was raised higher using adhesive tape to ensure proper ventilation as mentioned earlier when describing the aerotolerance assay. After 72 h, cell suspensions were then discarded, and plates were rinsed with demineralized water. Plates were dried at 60 °C for 30 min and crystal violet (200 µl; 1%) was added to each well; plates were then gently rocked for 30 min at ambient temperature. Plates were thoroughly washed with demineralized water and allowed to dry at 37 °C. Biofilms were solubilized by adding 180 µl of 20% acetone and 80% ethanol to each well. Bound crystal violet was measured at A595 with an Appliskan Multimode Microplate Reader (Thermo Scientific, Waltham, MA).
Biofilm assays with S. aureus cells
Bacterial suspensions of mono and mixed cultures were prepared as described above for aerotolerance assays with S. aureus cells. Four replicate aliquots (150 µl) of mono and mixed cultures were dispensed into 96-well polystyrene plates and incubated (static) for 48 h at 42 °C or 25 °C in aerobic conditions (normal atmospheric environment) as mentioned previously. After incubation, biofilms were stained with crystal violet staining as described previously38. Each experiment was conducted at least twice.
Statistical analyses
Results were analyzed using Two-way ANOVA (Tukey multiple comparison test), One-way ANOVA (Brown-Forsythe and Welch ANOVA tests) and unpaired t-test as per need in GraphPad (Prism 9), and illustrations were created in GraphPad and Microsoft Excel.
Results
Campylobacter survival at low temperature
Prolonged survival of Campylobacter at 4 °C was found when cells were co-cultivated with S. aureus B6-55A or B4-59C as compared to cells consisting of C. jejuni and C. coli monoculture (Fig. 1A, Supplementary Fig. S1). Likewise, cell-free extracts from S. aureus B4-59C-4 and B6-55A-4 increased survival of most Campylobacter strains at 4 °C when added to the media as compared to the non-amended control (MHB) (Fig. 1B–G). Cell-free extracts from S. aureus cultured at 25 °C and 37 °C (B4-59C-25, B4-59C-37, B6-55A-25 and B6-55A-37) also enhanced prolonged survival of C. jejuni and C. coli at 4 °C as compared to MHB (Fig. 1B–G). Among most of the Campylobacter strains (both C. jejuni and C. coli strains), effects of growth medium (MHB vs cell free extracts from S. aureus cultures), time, and time X growth medium interaction were found significant on survival at lower temperature (Supplementary Table S2.1–S2.3). Significant effects of growth condition (monoculture vs polymicrobial culture), time, and interaction of time X growth condition on survival at lower temperature were found for C. jejuni (NCTC11168, OD2-67) and C. coli HC2-48 strains (Supplementary Table S2.1–S2.3). Viability of S. aureus cells showed a tenfold (1 log) reduction after 120 h at 4 °C (Supplementary Table S3). No significant variability was observed for survival of S. aureus in mixed culture with the six different strains of Campylobacter.
Survival of Campylobacter strains at low temperature (4 °C) in mono or mixed cultures containing S. aureus strains or cell-free extracts. (A) Survival of Campylobacter strains (all C. jejuni and C. coli strains) monocultures and mixed cultures containing S. aureus B4-59C or B6-55A. All individual viable count readings from Campylobacter monocultures or mixed cultures are represented in figure (A). Survival of (B) C. jejuni NCTC11168, (C) C. coli WA3-33, (D) C. jejuni T1-21, (E) C. coli HC2-48, (F) C. jejuni OD2-67 and (G) C. coli ZV1-224 in media containing cell-free extracts of S. aureus at 4 °C. The cell-free extracts were obtained from S. aureus B4-59C grown at 4, 25, and 37 °C (B4-59C-4, B4-59C-25, B4-59C-37) and S. aureus B6-55A grown at 4, 25, and 37 °C (B6-55A-4, B6-55A-25 and B6-55A-37). Campylobacter cells grown in non-amended MHB served as a control. Each bar in figures (B–G) represents the mean of colony counts with standard deviation (SD) error bar. Horizontal dotted lines in the figures represent the limit of detection for colony counts (LOD, ~ 33 CFU/ml) in these assays. All data points plotted on the LOD line in figure (A) and all the bars not exceeding LOD line in figures (B–G) represent the readings for the respective time points with no detectable CFU counts. Tukey multiple comparison tests (Two-way ANOVA with repeated measures) was conducted in GraphPad Prism 9: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Campylobacter survival during aerobic conditions
During aerobic incubation at 25 °C and 42 °C, Campylobacter survival was enhanced throughout the incubation period when cells were co-incubated with S. aureus (Fig. 2A–D, Supplementary Fig. S2); however, Campylobacter monocultures did not survive beyond 6–12 h without extracts from S. aureus. There were differences in the aerotolerance of C. jejuni and C. coli strains when incubated with cell-free extracts of S. aureus strains grown at different temperatures. For example, cells of C. jejuni NCTC11168 were still viable at the 12-h time point when incubated with media extracts from S. aureus B4-59C-25, B4-59C-37, B6-55A-4, B6-55A-25 and B6-55A-37 (Fig. 2E). C. jejuni OD2-67 cells were still viable after 12 h of aerobic incubation with extracts from B6-55A-37 (Fig. 2F), and C. coli WA3-33 cells were viable after 24 h of aerobic incubation with extracts from B6-55A-4 and B6-55A-25 (Fig. 2G). For C. jejuni T1-21, C. coli HC2-48 and ZV1-224, CFU counts were below the limit of detection (< 102 CFU/ml) after 6 h of aerobic incubation in all used media. When statistical analysis was performed, effects of growth condition (monoculture vs polymicrobial culture), time, and time × growth condition interaction were all significant (p < 0.0001) on survival of Campylobacter in aerobic incubation at both temperatures (25 °C and 42 °C) (Supplementary Tables S2.4, S2.5).
Aerotolerance of Campylobacter in mono or mixed cultures containing S. aureus strains or cell-free extracts. (A–D), Survival of C. jejuni T1-21 and C. coli HC2-48 in mono and mixed cultures of S. aureus B4-59C and B6-55A incubated at 25 °C and 42 °C with aerobic conditions. (E) Survival of C. jejuni NCTC11168, (F) C. jejuni OD2-67, and (G) C. coli WA3-33 in media containing cell-free extracts of S. aureus at 42 °C with aerobic conditions. The S. aureus cell-free extracts listed in the Fig. 1 legend were used in this experiment. Campylobacter cells grown in non-amended MHB served as a control. Mean values of CFU counts with SD error bars are represented in figures. Horizontal dotted lines in the figures represent the limit of detection for colony counts (LOD, ~ 33 CFU/ml) in these assays. All data points plotted on the LOD line in figure (A–D) and all the bars not exceeding LOD line in figures (E–G) represent the readings for the respective time points with no detectable CFU counts. Two-way ANOVA with repeated measures (Tukey multiple comparison tests) was conducted in GraphPad Prism 9: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Biofilm formation
Mixed populations of Campylobacter and S. aureus cells produced significantly larger biofilms than Campylobacter monocultures at both 25 °C and 42 °C (Fig. 3A,B, Supplementary Fig. S3). Biofilm formation was also greater with mixed populations of Campylobacter and S. aureus B4-59C than S. aureus monocultures at both temperatures. However, biofilm formation by S. aureus B6-55A monocultures was higher at 42 °C than with mixed cultures (Fig. 3B). Significant effect of growth condition (polymicrobial culture or monoculture) (p < 0.0001) was found at both temperatures (25 °C and 42 °C) on Campylobacter biofilm formation with S. aureus cells in two-way ANOVA analysis (Supplementary Table S2.7). Variable results were obtained when Campylobacter and cell-free extracts from S. aureus media were incubated in microaerobic and aerobic conditions at 42 °C (Fig. 3C–H). B4-59C-37 was able to enhance biofilm formation of C. coli WA3-33 and C. jejuni NCTC11168 strains in both microaerobic and aerobic condition when compared to control MHB. Higher biofilm formation was also found in B6-55A-37 for C. jejuni NCTC11168 (in aerobic environment) and C. coli HC2-48 (in microaerobic condition). Otherwise, most of the Campylobacter strains produced similar or lower amount of biofilm in cell free extracts from S. aureus media than control MHB in both aerobic and microaerobic environment (Fig. 3C–H). In MHB, all strains except C. coli ZV1-224 produced comparatively higher biofilm in aerobic condition than microaerobic condition. Significantly higher biofilm formation was seen in aerobic condition than microaerobic condition in most tested media except B4-59C-25 for C. coli HC2-48 (Fig. 3G) and likewise result for C. jejuni OD2-67 except in B4-59C-37 (Fig. 3E). Meanwhile, biofilm production was lower in aerobic condition than microaerobic condition for C. coli ZV1-224 in all tested media (Fig. 3H). Although none of the cell-free media significantly enhanced biofilm formation for all Campylobacter strains, growth medium factor (MHB control or cell free extract from S. aureus medium) had significant effect on Campylobacter biofilm formation in both aerobic and microaerobic incubation which was confirmed by statistical analysis with biofilm data from all Campylobacter strains (both C. jejuni and C. coli strains) (Supplementary Table S2.8). No consistent enhancement of biofilm production was observed when Campylobacter strains were incubated with the ≤ 30 kDa fraction of S. aureus media extracts (Supplementary Fig. S3C,D). However, biofilm results for C. jejuni OD2-67 in B4-59C-37 (≤ 30 kDa) was found to be significantly higher (p < 0.0001) among all tested media in microaerobic condition. The ≥ 30 kDa fraction could not be analyzed for its impact on biofilm production media due to the insignificant amount of concentrate obtained.
Biofilms produced by Campylobacter in mono and mixed cultures containing S. aureus strains or cell-free extracts. Biofilms adhering to polystyrene plates were measured at A595. (A,B) Biofilms formed by Campylobacter strains (all C. jejuni/coli strains) at 25 °C and 42 °C in mono and mixed cultures containing S. aureus strains B4-59C and B6-55A in aerobic condition. The production of biofilms by S. aureus B4-59C and B6-55A monocultures are included. (C–H) Biofilm formation of Campylobacter strains at 42 °C in cell-free filtrates from S. aureus media in microaerobic (m) and aerobic environment (a). Each bar in figures represents the mean value with SD error bar. Two-way ANOVA (Tukey multiple comparison tests) and Brown-Forsythe and Welch ANOVA tests (One-way ANOVA) were conducted in GraphPad Prism 9: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
In most studies of foodborne pathogens, only a few targeted microorganisms are isolated and characterized2,4. However, polymicrobial colonization of retail meat products and meat processing environments is common16,17,26, and these polymicrobial conditions could have antagonistic or synergistic effects on foodborne pathogens39. S. aureus is ubiquitous and colonizes the skin, nose and hair of humans and other animals; consequently, the contamination of retail meat products with S. aureus strains occurs despite food safety measures. Most S. aureus strains in retail meat products originated from the respective animal3,4,22,34. Although the virulence of the S. aureus strains used in this study has not been tested, multiple virulence factors were identified in their genomes, including the gene encoding toxic shock syndrome34.
Prolonged survival of the six Campylobacter strains when cultivated with S. aureus cells or cell-free exudates indicates that S. aureus improves the survival of Campylobacter at lower temperatures. Previous studies regarding the transcriptional landscape of Campylobacter during survival at low temperature indicated that genes involved in quorum sensing and the acquisition of cryoprotectant molecules were differentially expressed40,41,42. Furthermore, Campylobacter was shown to utilize exogenous siderophores produced by other microorganisms43,44; these are used for iron acquisition during growth and survival and might also contribute to virulence. Our results showing improved survival of Campylobacter in S. aureus cell-free exudates indicates that metabolites produced by S. aureus improve the survival of Campylobacter. It is also important to note that Campylobacter cells undergo a transition to the VBNC stage during cold stress and remain viable for prolonged periods of time14. Hence, the presence of S. aureus cells or cell-free extracts might provide a protective environment for Campylobacter and facilitate viability for prolonged periods at lower temperatures. In this study, we counted culturable Campylobacter cells after incubation at 4 °C; however, this study did not address the impact of S. aureus on the VBNC condition of Campylobacter cells. A previous report documented the detrimental effects of pre-established, organismal biofilms from poultry on the survival of C. jejuni at 10 °C26. Organisms identified in biofilms from poultry included Pseudomonas spp., Staphylococcus spp., E. coli, Bacillus spp., and Flavobacterium spp.26. During cold stress, a percentage of the S. aureus population undergoes a transition to a small colony variant (SCV), which showed alterations in amino acids as compared with normal cells45. It is also important to note that extracellular protein excretion by different S. aureus strains differs among strains, growth conditions and growth stage46. Various extracellular proteins including phosphoglycerate kinase, succinyl-CoA ligase, peroxiredoxin, superoxide dismutase, transmembrane sulfatase, and chaperonin were differentially expressed in S. aureus during growth at 37 °C at 12, 24 and 48 h46. In this study, SDS-PAGE analysis of S. aureus media extracts revealed changes in protein concentration and banding patterns at different pre-incubation temperatures (Supplementary Fig. S4, Supplementary Table S1). Further work is needed to determine the influence of amino acids and metabolites from S. aureus on Campylobacter survival, especially at lower temperatures.
The reduced levels of dissolved oxygen in mixed bacterial populations is another factor that contributes to the survival and growth of Campylobacter47,48. It is important to note that S. aureus can survive and multiply at refrigeration temperatures for prolonged periods of time45,49; this would lead to a consumption of available oxygen from media and the creation of an environment favorable for Campylobacter. Furthermore, Campylobacter was shown to tolerate aerobic conditions by metabolic commensalism with Pseudomonas spp.50, and a similar relationship might exist between Campylobacter spp. and S. aureus.
Campylobacter is a poor initiator of biofilm production in monoculture but is a well-established secondary colonizer of biofilms produced by other bacterial pathogens26,51,52. Variability in biofilm formation among Campylobacter strains in monoculture has been reported27,29; in general, Campylobacter biofilm formation is greater in aerobic than microaerobic conditions30,38. However, lower biofilm production in aerobic environment than microaerobic environment for some strains of C. jejuni has also been reported53. Nutrient-rich environments like chicken and liver juice were shown to enhance attachment, adhesion and biofilm formation by Campylobacter strains18,19,29. Significantly higher levels of biofilm were formed when Campylobacter strains were co-cultivated with S. aureus B4-59C at both 25 °C and 42 °C and suggested a mutually beneficial effect (Fig. 3A,B). In contrast, cumulative biofilm levels by Campylobacter and S. aureus B6-55A were significantly lower than biofilms produced by S. aureus monocultures at 42 °C (Fig. 3A). Similar interactions with mixed biofilm communities have been previously reported between Campylobacter and other organisms and may indicate antimicrobial activities or interspecies competition within the biofilm27,54. Meanwhile, previous studies reported better survival of Campylobacter strains in mixed biofilms during aerobic conditions than in monoculture28,29. Although flagella and luxS-mediated quorum sensing were suggested to be important for biofilm formation in Campylobacter55, the possibility of both flagellum-dependent and flagellum- independent biofilm mechanisms in Campylobacter has been suggested38. A previous study found no evidence for the role of interspecies cell signaling via autoinducer-2 among mixed populations, but instead suggested physical contact as the sole mechanism for biofilm formation when Campylobacter was a secondary colonizer26. Our results failed to find a specific, consistent influence for S. aureus growth media in Campylobacter biofilm formation, which may suggest that physical contact is needed to stimulate biofilm production in mixed populations. The ratio of the two bacterial pathogens might influence survival, aerotolerance and biofilm formation due to the availability of nutrients and quorum sensing. However, it is important to mention that the cell densities of Campylobacter and S. aureus would be far lower in retail meat environments as compared to the densities used in this study.
In this study, we show that S. aureus frequently occurs as a co-contaminant with Campylobacter in retail meat products. S. aureus enhances the survival of C. jejuni and C. coli strains at low temperatures and during aerobic conditions. Select strains of S. aureus potentially enhance biofilm formation by Campylobacter in aerobic conditions (normal atmospheric environment). Extracellular metabolites and proteins produced by S. aureus at multiple temperatures enhance the survival of Campylobacter strains at low temperature. The extracts produced by S. aureus in media improved the survival of some Campylobacter strains when compared to monocultures in MHB. However, S. aureus media extracts did not foster biofilm formation by Campylobacter strains in both aerobic and microaerobic environments.
In summary, it is well-established that the contamination of retail food products by S. aureus increases the risk of food poisoning. This study shows that contamination of retail meats by S. aureus also enhances the survival of Campylobacter during harsh environmental conditions. Hence, food safety measures are still needed to facilitate improved identification and reduced contamination of foodborne pathogens in mixed populations in retail meat and food products.
References
Noormohamed, A. & Fakhr, M. K. A higher prevalence rate of Campylobacter in retail beef livers compared to other beef and pork meat cuts. Int. J. Environ. Res. Public Health 10, 2058–2068 (2013).
Noormohamed, A. & Fakhr, M. K. Incidence and antimicrobial resistance profiling of Campylobacter in retail chicken livers and gizzards. Foodborne Pathog. Dis. 9, 617–624 (2012).
Abdalrahman, L., Wells, H. & Fakhr, M. Staphylococcus aureus is more prevalent in retail beef livers than in pork and other beef cuts. Pathogens 4, 182–198 (2015).
Abdalrahman, L. S., Stanley, A., Wells, H. & Fakhr, M. K. Isolation, virulence, and antimicrobial resistance of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains from Oklahoma retail poultry meats. Int. J. Environ. Res. Public Health 12, 6148–6161 (2015).
Hennekinne, J. A., De Buyser, M. L. & Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 36, 815–836 (2012).
Lin, J. et al. Non-hospital environment contamination with Staphylococcus aureus and methicillin-resistant Staphylococcus aureus: Proportion meta-analysis and features of antibiotic resistance and molecular genetics. Environ. Res. 150, 528–540 (2016).
Tompkins, B. et al. Multistate outbreak of Campylobacter jejuni infections associated with undercooked chicken livers—Northeastern United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 62, 874–876 (2013).
Kaakoush, N. O., Castaño-Rodríguez, N., Mitchell, H. M. & Man, S. M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 28, 687–720 (2015).
Tack, D. M. et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. sites, 2015–2018. Morb. Mortal. Wkly. Rep. 68, 369–373 (2019).
Parkhill, J. et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403, 665–668 (2000).
Marasini, D. & Fakhr, M. K. Complete genome sequences of the plasmid-bearing Campylobacter coli strains HC2-48, CF2-75, and CO2-160 isolated from retail beef liver. Genome Announc. 4, e01004-e1016 (2016).
Marasini, D. & Fakhr, M. K. Complete genome sequences of Campylobacter jejuni strains isolated from retail chicken and chicken gizzards. Genome Announc. 5, e01351-e1417 (2017).
Noormohamed, A. & Fakhr, M. K. Prevalence and antimicrobial susceptibility of Campylobacter spp. in Oklahoma conventional and organic retail poultry. Open Microbiol. J. 8, 130–137 (2014).
Bolton, D. J. Campylobacter virulence and survival factors. Food Microbiol. 48, 99–108 (2015).
Bronowski, C., James, C. E. & Winstanley, C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 356, 8–19 (2014).
Røder, H. L. et al. Interspecies interactions result in enhanced biofilm formation by co-cultures of bacteria isolated from a food processing environment. Food Microbiol. 51, 18–24 (2015).
Wagner, E. M. et al. Identification of biofilm hotspots in a meat processing environment: Detection of spoilage bacteria in multi-species biofilms. Int. J. Food Microbiol. 328, 108668 (2020).
Karki, A. B., Wells, H. & Fakhr, M. K. Retail liver juices enhance the survivability of Campylobacter jejuni and Campylobacter coli at low temperatures. Sci. Rep. 9, 2733 (2019).
Brown, H. L. et al. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 80, 7053–7060 (2014).
Oh, E., McMullen, L. & Jeon, B. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front. Microbiol. 6, 1–8 (2015).
Karki, A. B., Marasini, D., Oakey, C. K., Mar, K. & Fakhr, M. K. Campylobacter coli from retail liver and meat products is more aerotolerant than Campylobacter jejuni. Front. Microbiol. 9, 2951 (2018).
Abdalrahman, L. & Fakhr, M. Incidence, antimicrobial susceptibility, and toxin genes possession screening of Staphylococcus aureus in retail chicken livers and gizzards. Foods 4, 115–129 (2015).
Kapondorah, T. L. & Sebunya, T. K. Occurrence of Salmonella species in raw chicken livers purchased from retail shops in Gaborone, Botswana. J. Anim. Vet. Adv. 6, 87–89 (2007).
von Altrock, A., Roesler, U., Merle, R. & Waldmann, K.-H. Prevalence of pathogenic Yersinia enterocolitica strains on liver surfaces of pigs and their antimicrobial susceptibility. J. Food Prot. 73, 1680–1683 (2010).
Wilhelm, B. et al. Survey of Canadian retail pork chops and pork livers for detection of hepatitis E virus, norovirus, and rotavirus using real time RT-PCR. Int. J. Food Microbiol. 185, 33–40 (2014).
Hanning, I., Jarquin, R. & Slavik, M. Campylobacter jejuni as a secondary colonizer of poultry biofilms. J. Appl. Microbiol. 105, 1199–1208 (2008).
Teh, K. H., Flint, S. & French, N. Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int. J. Food Microbiol. 143, 118–124 (2010).
Feng, J. et al. Chemical, physical and morphological properties of bacterial biofilms affect survival of encased Campylobacter jejuni F38011 under aerobic stress. Int. J. Food Microbiol. 238, 172–182 (2016).
Melo, R. T. et al. Intrinsic and extrinsic aspects on Campylobacter jejuni Biofilms. Front. Microbiol. 8, 1332 (2017).
Zhong, X. et al. Campylobacter jejuni biofilm formation under aerobic conditions and inhibition by ZnO nanoparticles. Front. Microbiol. 11, 207 (2020).
Schmitt, M., Schuler-Schmid, U. & Schmidt-Lorenz, W. Temperature limits of growth, TNase and enterotoxin production of Staphylococcus aureus strains isolated from foods. Int. J. Food Microbiol. 11, 1–19 (1990).
Marasini, D. & Fakhr, M. K. Complete genome sequences of plasmid-bearing Campylobacter coli and Campylobacter jejuni strains isolated from retail chicken liver. Genome Announc. 5, e01350-17 (2017).
Marasini, D. & Fakhr, M. K. Complete genome sequences of plasmid-bearing multidrug-resistant Campylobacter jejuni and Campylobacter coli strains with type VI secretion systems, isolated from retail turkey and pork. Genome Announc. 5, e01360-17 (2017).
Karki, A. B., Neyaz, L. & Fakhr, M. K. Comparative genomics of plasmid-bearing Staphylococcus aureus strains isolated from various retail meats. Front. Microbiol. 11, 574923 (2020).
Noormohamed, A. & Fakhr, M. Molecular typing of Campylobacter jejuni and Campylobacter coli isolated from various retail meats by MLST and PFGE. Foods 3, 82–93 (2014).
Marasini, D. & Fakhr, M. K. Complete genome sequences of Campylobacter jejuni strains OD267 and WP2202 isolated from retail chicken livers and gizzards reveal the presence of novel 116-kilobase and 119-kilobase megaplasmids with type VI secretion systems. Genome Announc. 4, e01060-e1116 (2016).
Marasini, D. & Fakhr, M. K. Whole-genome sequencing of a Campylobacter jejuni strain isolated from retail chicken meat reveals the presence of a megaplasmid with Mu-like prophage and multidrug resistance genes. Genome Announc. 4, e00460-e516 (2016).
Reuter, M., Mallett, A., Pearson, B. M. & Van Vliet, A. H. M. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 76, 2122–2128 (2010).
Giaouris, E. et al. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol. 6, 841 (2015).
Stintzi, A. & Whitworth, L. Investigation of the Campylobacter jejuni cold-shock response by global transcript profiling. Genome Lett. 2, 18–27 (2003).
Stintzi, A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 185, 2009–2016 (2003).
Ligowska, M., Cohn, M. T., Stabler, R. A., Wren, B. W. & Brøndsted, L. Effect of chicken meat environment on gene expression of Campylobacter jejuni and its relevance to survival in food. Int. J. Food Microbiol. 145, S111–S115 (2011).
Field, L. H., Headley, V. L., Payne, S. M. & Berry, L. J. Influence of Iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect. Immun. 54, 126–132 (1986).
Baig, B. H., Wachsmuth, I. K. & Morris, G. K. Utilization of exogenous siderophores by Campylobacter species. J. Clin. Microbiol. 23, 431–433 (1986).
Onyango, L. A., Dunstan, R. H., Gottfries, J., von Eiff, C. & Roberts, T. K. Effect of low temperature on growth and ultra-structure of Staphylococcus spp. PLoS One 7, e29031 (2012).
Atshan, S. S. et al. Comparative proteomic analysis of extracellular proteins expressed by various clonal types of Staphylococcus aureus and during planktonic growth and biofilm development. Front. Microbiol. 6, 524 (2015).
Bui, X. T. et al. Survival of Campylobacter jejuni in co-culture with Acanthamoeba castellanii: Role of amoeba-mediated depletion of dissolved oxygen. Environ. Microbiol. 14, 2034–2047 (2012).
Ica, T. et al. Characterization of mono- and mixed-culture Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 78, 1033–1038 (2012).
Onyango, L. A. & Alreshidi, M. M. Adaptive metabolism in staphylococci: Survival and persistence in environmental and clinical Settings. J. Pathog. 2018, 1–11 (2018).
Hilbert, F., Scherwitzel, M., Paulsen, P. & Szostak, M. P. Survival of Campylobacter jejuni under conditions of atmospheric oxygen tension with the support of Pseudomonas spp. Appl. Environ. Microbiol. 76, 5911–5917 (2010).
Teh, A. H. T., Lee, S. M. & Dykes, G. A. Association of some Campylobacter jejuni with Pseudomonas aeruginosa biofilms increases attachment under conditions mimicking those in the environment. PLoS ONE 14, e0215275 (2019).
Teh, A. H. T., Lee, S. M. & Dykes, G. A. Does Campylobacter jejuni form biofilms in food-related environments?. Appl. Environ. Microbiol. 80, 5154–5160 (2014).
Teh, A. H. T., Lee, S. M. & Dykes, G. A. The influence of dissolved oxygen level and medium on biofilm formation by Campylobacter jejuni. Food Microbiol. 61, 120–125 (2017).
Donlan, R. M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 8, 881–890 (2002).
Reeser, R. J., Medler, R. T., Billington, S. J., Jost, B. H. & Joens, L. A. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 73, 1908–1913 (2007).
Acknowledgements
The authors would like to acknowledge financial support from the Research Office of The University of Tulsa (Tulsa, OK, USA) and from the Beta Beta Beta Research Foundation for granting KB and CH undergraduate student research grants.
Author information
Authors and Affiliations
Contributions
Research design: A.B.K. and M.K.F. Experimental procedures: A.B.K., K.B., C.H. and R.J.S. Manuscript preparation: A.B.K. and M.K.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karki, A.B., Ballard, K., Harper, C. et al. Staphylococcus aureus enhances biofilm formation, aerotolerance, and survival of Campylobacter strains isolated from retail meats. Sci Rep 11, 13837 (2021). https://doi.org/10.1038/s41598-021-91743-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91743-w
- Springer Nature Limited