Abstract
In patients with coronary artery disease (CAD), further increasing the level of high-density lipoprotein (HDL) cholesterol (HDL-C) as an add-on to statins cannot reduce cardiovascular risk. And it has been reported that HDL functional metric—cholesterol efflux capacity (CEC) may be a better predictor of CAD risk than HDL-C. CEC measurement is time-consuming and not applicable in clinical settings. Thus, it is meaningful to explore an easily acquired index for evaluating CEC. Thirty-six CAD patients and sixty-one non-CAD controls were enrolled in this cross-sectional study. All CAD patients had acute coronary syndrome (ACS). CEC was measured using a [3H] cholesterol loading Raw 264.7 cell model with apolipoprotein B-depleted plasma (a surrogate for HDL). Proton nuclear magnetic resonance (NMR) spectroscopy was used to assess HDL components and subclass distribution. CEC was significantly impaired in CAD patients (11.9 ± 2.3%) compared to controls (13.0 ± 2.2%, p = 0.022). In control group, CEC was positively correlated with enzymatically measured HDL-C levels (r = 0.358, p = 0.006) or with NMR-determined HDL-C levels (NMR-HDL-C, r = 0.416, p = 0.001). However, in CAD group, there was no significant correlation between CEC and HDL-C (r = 0.216, p = 0.206) or NMR-HDL-C (r = 0.065, p = 0.708). Instead, we found that the level of high-sensitivity C-reactive protein (hsCRP) was inversely associated with CEC (r = − 0.351, p = 0.036). Multiple regression analysis showed that the hsCRP level was associated with CEC after adjusting other cardiovascular risk factors and HDL-C, although the association would not reach significance if adjusting for multiple testing. NMR spectroscopy showed that HDL particles shifted to larger ones in patients with high hsCRP levels, and this phenomenon was accompanied by decreased CEC. In patients with CAD, the level of HDL-C cannot reflect HDL function. The impaired correlation between HDL-C and CEC is possibly due to an inflammation-induced HDL subclass remodeling. These hypothesis-generating data suggest that hsCRP levels, a marker of acute inflammation, may associate with HDL dysfunction in ACS subjects. Due to the design limited to be correlative in nature, not permitting causal inference and a larger, strictly designed study is still needed.
Similar content being viewed by others
Introduction
Over the last few decades, epidemiological studies have confirmed a strong and inverse relationship between the level of high-density lipoprotein (HDL) cholesterol (HDL-C) and the risk of coronary artery disease (CAD)1. However, the role of HDL-C has been challenged by the failure of HDL-C raising trials using niacin or cholesteryl ester transfer protein (CETP) inhibitors2,3. In addition, genetically increased HDL-C does not necessarily translate to a decreased risk of myocardial infarction4. Even worse, higher HDL-C levels secondary to SCARB1 gene mutations lead to an increased risk of CAD5. A recent epidemiological study has also revealed that extremely high HDL-C levels are associated with increased CAD mortality6. These results highlight the potential limitations of using HDL-C levels, a static mass-based parameter, to assess the risk of CAD, and call for investigations on more robust HDL functional markers for evaluating cardiovascular risks.
HDL exerts favorable effects against atherosclerosis, primarily by reverse cholesterol transport (RCT)7. Cholesterol efflux capacity (CEC), a metric reflecting the ability of HDL as a cellular cholesterol acceptor, has been demonstrated to be inversely associated with subclinical atherosclerosis8,9, the incidence of cardiovascular events10,11,12, and prognosis of CAD13,14, and is now considered a reliable CAD marker10.
The measurement of CEC requires radiolabeled cholesterol and cultured cells, which is time-consuming and not applicable in clinical settings. It has been observed that CEC was positively correlated with HDL-C levels in healthy populations8,11,12,15. However, in patients with CAD, this relationship was inconsistent in different studies. Data from Khera et al.8 and Shao et al.16 showed that the correlation coefficients between HDL-C and CEC were 0.51 (p < 0.0001) and 0.31 (p < 0.05), respectively. In contrast, two other studies showed that the correlation was weak17 or even inexistent13.
The unstable relationship between HDL-C and CEC may be subjected to dynamic changes in the components of HDL subclasses. Inflammation has been a well-established factor that affects HDL components and subclass distribution18,19, and patients with inflammatory connective tissue disease always present a decreased CEC20, suggesting inflammatory markers may serve as surrogate parameters for HDL dysfunction.
Proton nuclear magnetic resonance (NMR) spectroscopy is an emerging technique that can provide a fine-grained snapshot of a person’s lipid metabolism. Using NMR-determined HDL subclasses can improve the mortality risk discrimination in the cardiac catheterization cohort21 and predict the prognosis of patients with pulmonary arterial hypertension22. This study used NMR spectroscopy to provide more detailed information about the components of HDL and HDL subclasses. By simultaneously examining the level of high-sensitivity C-reactive protein (hsCRP), a sensitive marker of systemic inflammation, and HDL functional marker CEC, we intended to clarify the relationship between HDL-C and HDL subclasses and CEC as well as between hsCRP and CEC in patients with CAD.
Subjects and methods
Study population
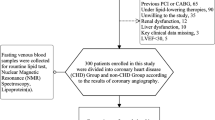
We used samples and data from a trial registered at Chinese Clinical Trial Registry as ChiCTR1900020873. The object of the trial was to characterize lipid profiles by NMR spectroscopy at fasting and non-fasting states in CAD and non-CAD subjects, which has been reported23. From June 2018 to December 2018, we consecutively recruited 50 CAD patients, who were 18–80 years old, presented with myocardial ischemia symptoms and suspected with CAD in the Department of Cardiovascular Medicine of the Second Xiangya Hospital of Central South University. Patients during the gestational phase, under hormone therapy, taking anti-inflammatory drugs were not included. All these patients underwent coronary angiography, and the diagnosis was confirmed by a coronary angiography showing ≥ 50% stenosis in at least one main coronary artery. Forty-two patients were diagnosed with CAD. Patients with significant hematologic disorders, infectious or inflammatory disease, various tumors, severe liver and/ or renal insufficiency, severe uncontrolled diabetes or hypertension, and alcohol use or intensive exercise in the week before enrollment were excluded, and 36 CAD patients were finally enrolled. We also enrolled eighty apparently healthy subjects in the same period, and they were free from atherosclerotic disorders as confirmed by coronary angiography or coronary computed tomography angiography. The excluding criteria was the same as CAD patients, and 61 non-CAD subjects were finally enrolled. Written informed consent was obtained from all the individuals. The research related to human use complied with all the relevant national regulations, institutional policies and followed the tenets of the Helsinki Declaration, and has been approved by the Medical Ethics Committee of the Second Xiangya Hospital of Central South University. This trial was registered at the Chinese Clinical Trial Registry as ChiCTR1900020873.
Clinical and biochemical measurements
Demographic information, such as height, weight, blood pressure, and heart rate were measured and recorded. Fasting blood samples were collected the morning after admission. Routine blood, urine, and lipid profiles, including triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and HDL-C, were analyzed via the enzymatic method. hsCRP was measured with a latex particle, enhanced immunoturbidimetric assay. Cardiac troponin T was measured using a high-sensitivity assay. For the subsequent experiments, fresh plasma was aliquoted and stored at − 80 °C.
ApoB-depleted plasma preparation
The plasma samples were thawed in a refrigerator at 4 °C before the experiment. According to the protocol of the previous experiment24, 540 µL of heparin sodium solution (280 mg/mL, Aladdin, China) and 10 mL of a manganese chloride solution (1.06 mol/L, Aladdin, China) were mixed. Plasma was incubated for 30 min at 4 °C with a mixed solution (10:1 vol/vol) and then centrifuged at 1500 × g for 30 min. The supernatant was collected, and if it was still turbid (especially samples with a high concentration of triglycerides), plasma was centrifuged again at 12,000 × g for 10 min, and the lower liquid fraction was recovered for the next procedure. Compared to conventional ultracentrifugation, this precipitation method is simpler and more efficient for HDL isolation8. Previous studies have revealed that heparin sodium/manganese chloride precipitation had a minor effect on HDL distribution25.
Measurement of Cholesterol efflux capacity
The cholesterol efflux assay was performed according to established procedures8,12. In brief, murine Raw 264.7 macrophages (ATCC, USA) were grown in the Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA), supplemented with 10% fetal bovine serum (Gibco, USA). Macrophages were plated on 48-well plates (300,000 cells/well). Subsequently, cells were loaded with 25 µg/mL acetylated LDL (Peking Union-Biology Co., Ltd, China) and 1 μCi/mL [3H] cholesterol (PerkinElmer, USA) for 24 h. The macrophages were then washed with PBS (Gibco, USA). To upregulate the expression of ATP-binding cassette transporter A1 (ABCA1), cells were stimulated for 24 h with serum-free DMEM containing 0.3 mmol/L 8-Bromoadenosine 3',5'-cyclic monophosphate (Sigma, USA), then washed with PBS again and incubated with 2.8% (vol/vol) apoB-depleted plasma diluted in the medium for 6 h. All steps were performed in the presence of 2 μg/mL of the acyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibitor Sandoz 58–035 (Sigma, USA). The supernatant was collected and centrifuged to remove cellular debris. Cells on the plate were washed with PBS again and then incubated with a 0.1 mol/L NaOH solution for 30 min for cell lysis. The radioactivity within the supernatant and cells was determined by using liquid scintillation counting (PerkinElmer, USA). CEC was calculated using the following equation: CEC (%) = 3H media/ (3H media + 3H cells) × 100. All efflux experiments were performed in triplicate for each sample. Each plate contained blank control and positive control (50 μg/mL HDL, purchased from Peking Union-Biology Co. Ltd). A standard sample (pooled plasma from 20 individuals) was used to correct the inter-assay error.
Nuclear magnetic resonance spectroscopy
The total plasma apolipoprotein A-I (apoA-I)-rich lipoprotein and 30 discrete HDL-related lipoproteins were measured by NMR spectroscopy at ProteinT Biotechnology Co., Ltd (Tianjin, China) by Bruker 600 MHz NMR spectrometer. Details for the NMR experimental condition were provided in Supplementary Table 6. The spectra were normalized to the same quantitative scale using Bruker’s QuantRef manager within TopSpin which is based on the PULCON method; hence, the spectral intensity is normalized to proton concentration in units of millimoles per liter26. The lipoprotein-distribution-prediction method selected for the analysis was the commercial Bruker IVDr Lipoprotein Subclass Analysis (B.I.-LISA) method as previously described22,27, which used a PLS-2 regression model as the algorithm for spectral deconvolution28. HDL-related lipoproteins were classified into four subclasses, labeled numerically according to decreasing size and increasing density. The HDL1 subclass was the largest, while the HDL4 subclass was the smallest. This method showed exceptional reproducibility with inter coefficient variation of 1.39–2.62%. In the context, HDL-C determined by NMR was described as NMR-HDL-C.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 25.0 (IBM, USA, https://www.ibm.com/analytics/spss-statistics-software). All images were made by GraphPad Prism version 8.0 (GraphPad Prism Inc, USA, https://www.graphpad.com/scientific-software/prism/). Normally distributed continuous data have been expressed as mean ± standard deviation. Skewed distributed continuous data have been described as medians with interquartile ranges and were logarithmically transformed when necessary. Comparisons between categorical data were performed with the chi-square test, while continuous variables were assessed by t-test (for normal distribution) or nonparametric tests (for skewed distribution). The Pearson’s correlation analysis was used to evaluate the associations between variables. Multiple linear regression analysis was performed to determine the variables with independent association with CEC. In the correlation and regression analysis, a two-tailed p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
All CAD patients had acute coronary syndrome (ACS), including 4 ST-segment elevated myocardial infarction (MI), 14 non-ST-segment elevated MI, and 18 unstable angina. The demographic and biochemical characteristics of the subjects have been shown in Table 1. The subjects in the CAD group were older, with a higher percentage of the male sex, diabetes, hypertension, statin use, and current smoking than subjects in the non-CAD group. Concentrations of TC, HDL-C, and LDL-C were lower, but serum hsCRP was significantly higher in CAD patients than in the non-CAD controls (1.76 [0.88–4.05] vs. 0.91 [0.32–1.87], p = 0.004). The median hsTnT level in CAD patients was 0.0178 µg/L (0.0086–0.1667). Other parameters showed no statistically significant differences between the two groups. The interval from symptoms onset to admission for CAD patients has been presented in Supplementary Table 1. Twenty-nine (80.6%) of patients with CAD were admitted to the hospital more than seven days after event onset, and 15 (41.7%) patients were admitted after more than one month of event onset.
Correlation between cholesterol efflux capacity and HDL-C levels
The CEC of the standard sample on each 48-well plate has been shown in Supplementary Fig. 1. The intra- and inter-assay coefficients of variation were 5.7% and 4.8%, respectively, which was comparable to previous studies8,29. CEC in CAD group was significantly lower compared to the non-CAD group (11.9 ± 2.3% vs. 13.0 ± 2.2%, p = 0.022, Fig. 1). Correlation analysis showed that HDL-C was positively correlated with CEC in the non-CAD group (r = 0.358, p = 0.006, Fig. 2A), while there was no significant correlation in the CAD group (r = 0.216, p = 0.206, Fig. 2B).
Comparison of CEC between non-CAD controls (n = 61) and CAD patients (n = 36). CEC, cholesterol efflux capacity; CAD, coronary artery disease; *p < 0.05. Data analysis was performed using SPSS version 25.0 (IBM, USA, https://www.ibm.com/analytics/spss-statistics-software) and the figure was made by GraphPad Prism version 8.0 (GraphPad Prism Inc, USA, https://www.graphpad.com/scientific-software/prism/).
Correlation between CEC and HDL-C in (A) non-CAD controls and (B) CAD patients. Correlation between CEC and NMR-HDL-C in (C) non-CAD controls and (D) CAD patients. (E) Correlation between hsCRP levels (log-transformed) and CEC in CAD patients (n = 36); (F) Comparison of CEC between hsCRP-low CAD patients (hsCRP < 1.75 mg/L, n = 18) and hsCRP-high CAD patients (hsCRP ≥ 1.75 mg/L, n = 18). CEC, cholesterol efflux capacity; CAD, coronary artery disease; NMR-HDL-C, NMR measured high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; CEC, cholesterol efflux capacity; CAD, coronary artery disease; * p < 0.05. Statistical analysis was performed using SPSS version 25.0 (IBM, USA, https://www.ibm.com/analytics/spss-statistics-software) and the figure was made by GraphPad Prism version 8.0 (GraphPad Prism Inc, USA, https://www.graphpad.com/scientific-software/prism/).
We also measured the level of HDL-C and other HDL-related lipoproteins using NMR spectroscopy (Supplementary Table 2). Consistent with the results of enzymatic methods, NMR-HDL-C was positively correlated with CEC in non-CAD controls (r = 0.416, p = 0.001, Fig. 2C). However, in CAD patients, there was no correlation between NMR-HDL-C and CEC (r = 0.065, p = 0.708, Fig. 2D).
In the univariate analysis, as shown in Table 2, CEC was positively correlated with the levels of total plasma apolipoprotein A-I (r = 0.369, p = 0.004), HDL-phospholipids (r = 0.338, p = 0.009), HDL-free cholesterol (r = 0.282, p = 0.032), HDL-apoA-I (r = 0.400, p = 0.002), and HDL-apoA-II (r = 0.340, p = 0.009) in the non-CAD group. In the CAD group, however, these parameters showed no correlation with CEC.
In the CAD group, cholesterol efflux capacity was negatively correlated with the hsCRP level
To investigate which factor determined HDL-mediated CEC in CAD patients, univariate analysis was performed. There was no correlation between CEC and age, body mass index, severity of coronary stenosis (expressed as the Gensini score), hsTnT, or the serum levels of TG, TC, and LDL-C (see Supplementary Table 3). However, we found that CEC was negatively correlated with the hsCRP level (r = − 0.351, p = 0.036, Fig. 2E). Then, we divided 36 CAD patients into hsCRP-low (n = 18) and hsCRP-high (n = 18) groups by using the median hsCRP level value (1.75 mg/L) as the criterion (the values of hsCRP in all patients have been shown in Supplementary Fig. 2). Except for hsTnT, the baseline characteristics, the concentrations of total HDL lipids and apolipoproteins were comparable in two groups (see Supplementary Table 4). Nonetheless, CEC was significantly lower in the hsCRP-high group than in the hsCRP-low group (11.3 ± 2.2% vs. 12.6 ± 2.1%, p = 0.038, Fig. 2F).
In CAD patients, HDL particles underwent extensive remodeling with a high level of hsCRP
To explore why CEC reduced despite total HDL lipids and major apolipoproteins remained unchanged between the hsCRP-high and the hsCRP-low group, we compared HDL subclass distribution in the two groups. In the hsCRP-high group, the concentrations of lipids and major apolipoproteins in the largest HDL subclass (HDL1) were significantly higher, while those in the smallest HDL subclass (HDL4) were significantly lower than those in the hsCRP-low group (Fig. 3). Further analysis showed that the levels of lipids and major apolipoproteins in HDL1 were positively correlated with the level of hsCRP, while in HDL4, there was a negative correlation (see Supplementary Table 5).
Comparison of 24 HDL-subclass related lipoproteins between hsCRP-low CAD patients (hsCRP < 1.75 mg/L, n = 18) and hsCRP-high CAD patients (hsCRP ≥ 1.75 mg/L, n = 18). HDL subclasses were labeled numerically according to decreasing size and increasing density. HDL, high-density lipoprotein; apoA-I, apolipoprotein A-I; apoA-II, apolipoprotein A-II. Data have been expressed as mean ± SD; *p < 0.05, **p < 0.01. Statistical analysis was performed using SPSS version 25.0 (IBM, USA, https://www.ibm.com/analytics/spss-statistics-software) and the figure was made by GraphPad Prism version 8.0 (GraphPad Prism Inc, USA, https://www.graphpad.com/scientific-software/prism/).
Multiple linear regression analysis of cholesterol efflux capacity in patients with CAD
To further investigate the relationship between hsCRP and CEC, multiple linear regression analysis was performed. Cardiovascular risk factors including age, sex, LDL-C, diabetes, current smoking, body mass index, TG (log-transformed) and hsTnT (log-transformed) were included as covariates. Adjustments were made for HDL-C, NMR-HDL-C, HDL1-cholesterol, and HDL4-cholesterol. Results showed that hsCRP was associated with HDL-mediated CEC (without Bonferroni correction to adjust the p-value.), regardless of conventional CAD risk factors, HDL-C, and HDL subclasses (Table 3).
Discussion
HDL has been recognized as a traditional protective factor against atherosclerosis1. However, subsequent attempts for drug therapies that aim to raise HDL-C levels with niacin3 or CETP inhibitor2 have both been in vain. HDL-mediated RCT is the key protective function of HDL. CEC, a metric defined by ex vivo experiments that reflects the first and rate-limiting step of RCT, has been demonstrated to be more valuable than HDL-C in predicting CAD risks8,9,10,11,13,14. However, CEC is susceptible to impairment in many disease states.
Our results showed that compared to non-CAD controls, CEC was lower in CAD patients and was not significantly related to HDL-C. The reduced CEC in CAD patients has already been well described in a high-quality study8, but the correlation between HDL-C and CEC was inconsistent in previous reports. For example, the correlation coefficient between HDL-C and CEC was 0.51 (p < 0.0001) in the study by Khera et al.8 (combining 442 CAD patients and 351 controls) but it was − 0.09 in the study by Zhang et al. (313 CAD patients)13. The discordance could result from the different study populations, as most of the patients in Zhang’s study and our study had ACS, while Khera’s study excluded ACS patients. In addition to CAD, the CEC of HDL is also impaired in other diseases, such as acute inflammation18, end-stage renal disease9, type 130 and type 2 diabetes11 or other autoimmune diseases20.
We observed that accompanied by decreased CEC, the hsCRP level was inversely correlated with CEC in CAD patients, although the correlation would not reach significance after adjusting for multiple testing. This phenomenon was quite similar to Vaisar’s results18. Vaisar et al. found that humans with acute inflammation induced by endotoxin had impaired CEC. HDL proteomic analyses revealed that under that circumstance, the content of serum amyloid A1(SAA1) and SAA2 in HDL was significantly increased, and CEC was inversely correlated with HDL SAA1/SAA2 levels. Although not fully elucidated, in vitro experiments and the saa1/2 knock-out mice model demonstrated that SAA enrichment in HDL per se can impair CEC. As CRP is a known acute phase reaction reactant released by the liver along with SAA31, the impaired CEC and its relevance to hsCRP in ACS patients, as shown in our study, could possibly result from an increase of SAA content in HDL. ApoA-I modification could be another reason for decreased CEC. Our previous experimental study showed that myeloperoxidase (MPO)-mediated tryptophan oxidation of apoA-I was harmful to HDL-mediated cholesterol efflux32.
It has been widely reported that hsCRP is a valuable marker of CAD. For example, CAD patients with higher hsCRP baseline level (usually ≥ 2 mg/L) have a significantly higher risk of major adverse cardiovascular events (MACE) compared to those with lower hsCRP baseline level (usually < 2 mg/L)33,34. In the CANTOS trial, participants with canakinumab, a monoclonal antibody targeting interleukin-1β, who achieved an on-treatment hsCRP level < 2 mg/L had a 25% reduction in the risk of MACE, while no significant benefit was observed in participants whose on-treatment hsCRP concentration was 2 mg/L or above35. On the other hand, treatment with another anti-inflammation medicine, methotrexate, did not reduce the risk of MACE, as there was no decrease in hsCRP levels following treatment36. Based on these findings and the results of our study, the reason why hsCRP serve as a marker of CAD can be explained, at least partially, by its ability to reflect HDL dysfunction.
We also found that CAD patients with higher hsCRP levels had more large HDL particles (HDL1) and less small HDL particles (HDL4), consistent with the previous study, which analyzed HDL subclasses by electrophoresis19,37. In our study, the exact mechanism by which inflammation affects HDL remodeling remains to be further elucidated.
In conclusion, we found that HDL-C levels could not reflect HDL's functional status in patients with CAD, and the impaired correlation between HDL-C levels and CEC was possibly due to inflammation-induced HDL-subclass remodeling. We also found that the level of hsCRP was inversely associated with CEC, although the association would not reach significance after adjusting for multiple testing. These hypothesis-generating data suggested that hsCRP levels, a marker of acute inflammation, may associate with HDL dysfunction in ACS subjects. As a relatively small group of patients was taken into account and most of our MI patients were beyond the super-acute-phase (96 h), further studies with larger cohorts, including stable CAD patients, patients with super-acute-phase MI will need to be undertaken to further verify these conclusions. Besides, it is noteworthy that this is a hypothesis generating paper, from a study not designed specifically to test the correlation between hsCRP and CEC, and our conclusions still warrant further investigations.
Abbreviations
- HDL:
-
High-density lipoprotein
- HDL-C:
-
High density lipoprotein cholesterol
- CETP:
-
Cholesteryl ester transfer protein
- RCT:
-
Reverse cholesterol transport
- CEC:
-
Cholesterol efflux capacity
- CAD:
-
Coronary artery disease
- hsCRP:
-
High-sensitivity C-reactive protein
- hsTnT:
-
High-sensitivity Troponin T
- MI:
-
Myocardial infarction
- HIV:
-
Human immunodeficiency virus
- NMR:
-
Nuclear magnetic resonance
- ACS:
-
Acute coronary syndrome
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- DMEM:
-
Dulbecco's Modified Eagle's Medium
- ABCA1:
-
ATP binding cassette transporter A1
- ACAT:
-
Acyl-coenzyme A: cholesterol acyltransferase
- NMR-HDL-C:
-
High-density lipoprotein cholesterol measured by NMR spectroscopy
- SPSS:
-
Statistical Package for Social Sciences
- HDL1:
-
The largest HDL subclass defined by NMR spectroscopy
- HDL4:
-
The smallest HDL subclass defined by NMR spectroscopy
- MPO:
-
Myeloperoxidase
- ApoA-I:
-
Apolipoprotein A-I
- ApoA-II:
-
Apolipoprotein A-II
- ApoB:
-
Apolipoprotein B
- ApoC-III:
-
Apolipoprotein C-III
- SAA:
-
Serum amyloid A
- MACE:
-
Major adverse cardiovascular events
References
Gordon, D. J. et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79(1), 8–15 (1989).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357(21), 2109–2122 (2007).
Investigators, A.-H. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365(24), 2255–2267 (2011).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380(9841), 572–580 (2012).
Zanoni, P. et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 351(6278), 1166–1171 (2016).
Hirata, A. et al. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J. Clin. Lipidol. 12(3), 674–684.e5 (2018).
He, Y., Kothari, V. & Bornfeldt, K. E. High-density lipoprotein function in cardiovascular disease and diabetes mellitus. Arterioscler Thromb Vasc Biol. 38(2), e10–e16 (2018).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364(2), 127–135 (2011).
Wu, S. et al. Impaired cholesterol efflux capacity is related to increased carotid intima media thickness in patients with end-stage renal disease. Int. J. Cardiol. 187, 456–458 (2015).
Rohatgi, A. et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371(25), 2383–2393 (2014).
Saleheen, D. et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 3(7), 507–513 (2015).
Ebtehaj, S., Gruppen, E. G., Bakker, S. J. L., Dullaart, R. P. F. & Tietge, U. J. F. HDL (high-density lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arterioscler. Thromb. Vasc. Biol. 39(9), 1874–1883 (2019).
Zhang, J. et al. Prognostic usefulness of serum cholesterol efflux capacity in patients with coronary artery disease. Am. J. Cardiol. 117(4), 508–514 (2016).
Guerin, M. et al. Association of serum cholesterol efflux capacity with mortality in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 72(25), 3259–3269 (2018).
Koekemoer, A. L. et al. Large-scale analysis of determinants, stability, and heritability of high-density lipoprotein cholesterol efflux capacity. Arterioscler. Thromb. Vasc. Biol. 37(10), 1956–1962 (2017).
Shao, B. et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 114(11), 1733–1742 (2014).
Hafiane, A., Jabor, B., Ruel, I., Ling, J. & Genest, J. High-density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am. J. Cardiol. 113(2), 249–255 (2014).
Vaisar, T. et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J. Lipid. Res. 56(8), 1519–1530 (2015).
Gomaraschi, M. et al. The plasma concentration of Lpa-I:A-II particles as a predictor of the inflammatory response in patients with ST-elevation myocardial infarction. Atherosclerosis 202(1), 304–311 (2009).
Smith, C. K. et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 66(9), 2532–2544 (2014).
McGarrah, R. W. et al. High-density lipoprotein subclass measurements improve mortality risk prediction, discrimination and reclassification in a cardiac catheterization cohort. Atherosclerosis 246, 229–235 (2016).
Harbaum, L. et al. Reduced plasma levels of small HDL particles transporting fibrinolytic proteins in pulmonary arterial hypertension. Thorax 74(4), 380–389 (2019).
Chen, J. et al. Comparison of calculated remnant lipoprotein cholesterol levels with levels directly measured by nuclear magnetic resonance. Lipids Health Dis. 19(1), 132 (2020).
Warnick, G. R. & Albers, J. J. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 19(1), 65–76 (1978).
Davidson, W. S. et al. The effects of apolipoprotein B depletion on HDL subspecies composition and function. J. Lipid. Res. 57(4), 674–686 (2016).
Wider, G. & Dreier, L. Measuring protein concentrations by NMR spectroscopy. J. Am. Chem. Soc. 128(8), 2571–2576 (2006).
Jimenez, B. et al. Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by (1)H NMR spectroscopy in a multilaboratory trial. Anal. Chem. 90(20), 11962–11971 (2018).
Okazaki, M. et al. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc. Biol. 25(3), 578–584 (2005).
Luo, M. et al. ApoCIII enrichment in HDL impairs HDL-mediated cholesterol efflux capacity. Sci. Rep. 7(1), 2312 (2017).
Gourgari, E. et al. Low cholesterol efflux capacity and abnormal lipoprotein particles in youth with type 1 diabetes: a case control study. Cardiovasc. Diabetol. 17(1), 158 (2018).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352(16), 1685–1695 (2005).
Peng, D. Q. et al. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler Thromb Vasc. Biol. 28(11), 2063–2070 (2008).
Bohula, E. A. et al. Inflammatory and cholesterol risk in the FOURIER trial. Circulation 138(2), 131–140 (2018).
Guedeney, P. et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 73(19), 2401–2409 (2019).
Ridker, P. M. et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391(10118), 319–328 (2018).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380(8), 752–762 (2019).
Gomaraschi, M. et al. Inflammation impairs eNOS activation by HDL in patients with acute coronary syndrome. Cardiovasc. Res. 100(1), 36–43 (2013).
Acknowledgements
The authors want to thank the National Natural Science Foundation of China, Natural Science Foundation of Hunan Province, and the Hunan Provincial Innovation Foundation for their financial support. The authors also appreciate the nursing staff of the Department of Cardiovascular Medicine of The Second Hospital of Central South University, for their everyday help and support.
Funding
This project was supported by the National Natural Science Foundation of China (No. 81670426, 81870336 to Peng D; 81670420 to Yu B); Natural Science Foundation of Hunan Province of China (2018JJ1045 to Yu B); Chinese Cardiovascular Association-Access fund (2020-CCA-ACCESS-070 to Yu B); Hunan Provincial Innovation Foundation for Postgraduate (CX20190072 to Tang X).
Author information
Authors and Affiliations
Contributions
Among the authors, D.P., B.Y., X.T. conceived the hypotheses and analyses. L.M., X.T., J.C., T.Z., X.G., J.K. collected samples and data. X.T. and S.W. conducted the experiments. X.T. performed statistical analysis and drafted the paper. D.P., B.Y., T.Z. refined interpretation and the final manuscript. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, X., Mao, L., Chen, J. et al. High-sensitivity CRP may be a marker of HDL dysfunction and remodeling in patients with acute coronary syndrome. Sci Rep 11, 11444 (2021). https://doi.org/10.1038/s41598-021-90638-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90638-0
- Springer Nature Limited