Abstract
International guidelines do not recommend surgery for the first episode of primary spontaneous pneumothorax (PSP), except in cases of persistent air leak, hemopneumothorax, bilateral pneumothorax, or occupations at risk. However, these recommendations have been challenged because of a significant reduction in the recurrence rate in emerging studies. We evaluated the rationale of recommendations by systematically reviewing RCTs and observational studies by using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system. We searched articles in PubMed, EMBASE, and Cochrane databases up to August 15, 2020. The primary outcomes were the recurrence rate and complication rate. The secondary outcomes were hospital stay and drainage duration. Nine eligible studies with 1121 patients were retrieved and analyzed. The recurrence rate was lower in the VATS than in conservative treatment with moderate evidence (OR 0.13, 95% CI 0.09 to 0.19, P < 0.001, I2 = 0%). We did not find significant differences in complication rate (Peto OR 1.17, 95% CI 0.33 to 4.12, P = 0.80), hospital stay duration (MD − 0.48 days, 95% CI − 2.84 to 1.87, P = 0.69, very low evidence), and in drainage duration (MD − 3.99 days, 95% CI − 9.06 to 1.08, P = 0.12, very low evidence) between the two groups. Our results would suggest VATS treatment as a weak recommendation for patients with the first episode of PSP, based on our systematic review of the current evidence by using the GRADE system, indicating that different treatments will be appropriate for different patients and that patients’ values and preferences should be incorporated through shared decision making.
Trial REGISTRY: PROSPERO; No.: CRD42020162267.
Similar content being viewed by others
Introduction
Primary spontaneous pneumothorax (PSP) is a significant global health concern that commonly affects young people who do not have clinically apparent lung disease. The annual incidence rates of PSP are 18–28 per 100,000 in men and 1.2–6 per 100,000 in women1. PSP typically results from the rupture of subpleural blebs or bullae2. According to international guidelines, conservative treatments, such as observation, simple aspiration, and chest tube treatment, are established as the first-line treatments for the first episode of PSP in patients3,4. However, the most substantial problem of PSP under conservative management is the variable recurrence rate ranging from 14 to 50% within one to 5 years in patients4,5,6,7.
With advancements in surgical technology, video-assisted thoracoscopic surgery (VATS) has become the mainstream avenue of thoracic surgery. Compared with traditional open thoracotomy, VATS has the advantages of smaller operative wound size and less hospitalization time. Therefore, studies have investigated the effectiveness and safety of performing VATS for the first episode of PSP8,9 despite international guidelines not recommending surgery for initial pneumothorax except in cases of persistent air leak, hemopneumothorax, bilateral pneumothorax, or for people with at-risk occupations3,4,10. The optimal management of patients during their first episode of PSP has remained debatable.
Clinical guidelines can help practitioners and patients make decisions in specific contexts. The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system has offered a transparent and comprehensive framework for assessing the quality of evidence and the strength of recommendations for systematic reviews and guidelines11. The importance of assessing the quality of evidence is to reflect whether the confidence in an estimate of the effect is adequate to support recommendations12. Therefore, we conducted a systematic review and meta-analysis by using the GRADE system to evaluate the outcomes of the recurrence rate, complication rate, hospital stay, and drainage duration between VATS operation and conservative treatment during the first episode in patients with PSP.
Material and methods
We included randomized controlled trials (RCTs) and observational studies following the PICO framework. (1) Population: the first episode of PSP; (2) Intervention: VATS techniques; (3) Comparison: conservative treatment that included observation, intercostal drainage, pigtail drainage, and chest tube drainage; (4) Outcomes: the recurrence rate, complications, hospital stay, and drainage duration. We excluded studies that met at least one of the following criteria: (1) did not directly evaluate treatment outcomes, (2) investigated recurrent, secondary, traumatic, or iatrogenic pneumothorax, (3) did not report both outcomes of VATS and conservative treatment, and (4) involved the duplicate reporting of patient cohorts.
We systematically searched PubMed, EMBASE, Cochrane library, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov registry electronic databases. The following terms and Boolean operators were used in MeSH and free-text searches: ("pneumothorax"[MeSH Terms] OR "pneumothorax"[All Fields]) AND ("thoracic surgery, video-assisted"[MeSH Terms] OR ("thoracic"[All Fields] AND "surgery"[All Fields] AND "video-assisted"[All Fields]) OR "video-assisted thoracic surgery"[All Fields] OR "vats"[All Fields]). The “related articles” facility in PubMed was used to broaden the search. The search terms and Boolean operators were similar in all databases. Our searches were performed by two experienced reviewers (HYC, YCL) and validated by a certified librarian. No language restrictions were applied. A comprehensive search was performed on August 15, 2020. We searched the reference sections of relevant papers and contacted experts in the field to identify additional studies. We contacted authors if required data were unpublished.
Two researchers independently extracted the data pertaining to participants, inclusion and exclusion criteria, VATS techniques, types of conservative treatment, treatment outcomes, complications, duration of hospital stay, and duration of pleural drainage from the included studies. The independent recorded decisions of the two reviewers were compared, and any disagreements were resolved based on the evaluation of another reviewer.
Two reviewers independently assessed the quality and the risk of bias of the included studies, and the consensus was reached by discussing with other reviewers. We used the Revised Cochrane Risk of Bias tool 2.0 to evaluate the random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other bias in the included RCTs13. The Newcastle-Ottawa Quality Assessment Scale was used as the tool to appraise the representativeness, comparability, outcome, and follow-up length of cohort studies14.
We assessed each outcome for the quality of evidence by using GRADEpro (GRADEproGDT, http://www.gradepro.org). Factors downgrading the quality included risk of bias, inconsistency, indirectness, imprecision, and publication bias, whereas factors upgrading the quality included large effect, plausible confounding, and dose–response. We classified the quality of evidence as “very low,” “low,” “moderate,” or “high.” We obtained recommendations according to the level of evidence, balancing both desirable and undesirable effects as well as cost-effectiveness and patient preferences11. The quality of evidence was assessed by a multidisciplinary team of health care professionals and researchers.
The primary outcomes were the recurrence rate and complications, such as persistent air leaks and surgical complications. Secondary outcomes were the duration of hospital stay and the duration of pleural drainage. We performed subgroup analyses regarding study design, surgical techniques, and types of conservative treatment in the outcome of the recurrence rate and types of complications in the outcome of the complication rate.
We performed statistical analyses by using the Review Manager software version 5.3 (Cochrane Collaboration, Oxford, England, UK) and used Jamovi version 0.9 (free software, https://www.jamovi.org). We performed the meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines15. The protocol of the study was registered online in the International Prospective Register of Systematic Reviews (PROSPERO: No. CRD42020162267). The effect sizes of dichotomous outcomes were reported as odds ratios (ORs) and Peto odds ratios (ORs), and continuous outcomes were reported as mean differences (MD) with standard deviations (SDs). We used Peto ORs in the meta-analysis with no event in one arm16. The precision of effect sizes was reported as a 95% confidence interval (CI). Statistical significance was defined as two-sided P values of less than 0.05. We used the random-effects model to calculate the pooled estimate of ORs and mean differences. The random-effect model provides relatively wide CIs and an appropriate estimate of the average treatment effect for statistically heterogeneous studies, contributing a conservative statistical claim16. We only pooled data with adequate clinical and methodological similarity. We assessed statistical heterogeneity by using the I2 test, which quantified the proportion of the total outcome variability and the variability among studies. To address moderate to high heterogeneity, we also conducted the sensitivity test for meta-analysis results excluding outlying studies16. Furthermore, we used the Jamovi software to analyze the funnel plot, Egger’s test, and Begg’s test for the detection of publication bias in the meta-analysis17.
Results
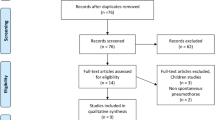
The flowchart in Fig. 1 illustrates the screening and selection process of the study. Our initial search yielded 3703 studies. After excluding duplicates (n = 789), 2914 studies remained. The titles and abstracts of the 2914 studies were screened, and 2072 ineligible studies were excluded. The full text of 842 articles was assessed to determine their eligibility. We excluded 833 citation records for the following reasons: 273 investigated different subgroups of pneumothorax, 349 did not include a comparison group of pneumothorax, 194 investigated a different comparison in patients with pneumothorax, 14 did not investigate the first episode of PSP, two discussed different outcomes in the first episode of pneumothorax, and one trial was terminated on the basis of a poor accrual rate. The remaining 9 eligible studies were included in our meta-analysis18,19,20,21,22,23,24,25,26. The characteristics of eligible studies are presented in Table 1.
As listed in Table 1, the 9 studies were published between 2005 and 2018 and included a total of 1121 patients (ranging from 41 to 268 patients). Four studies were from Asia, four from Europe, and one from North America. Of the 9 studies, 2 were RCTs (222 patients)18,22, one was a prospective cohort study (122 patients)19, and 6 were retrospective cohort studies (777 patients)20,21,23,24,25,26. Regarding the conservative treatment, 5 studies compared pure chest tube with VATS18,19,20,21,22, whereas 4 studies compared observation or chest tube with VATS23,24,25,26. Surgical techniques used for VATS varied little. Three studies performed VATS with pleurodesis in all surgical patients22,25,26, 3 studies conducted VATS with pleurectomy in all surgical patients18,19,20, and 3 studies conducted VATS without regularly performing pleurodesis or pleurectomy21,23,24.
Supplementary Table S1 presents the quality assessment of observational studies with the Newcastle-Ottawa Scale for nonrandomized studies. Some studies were determined to have moderate19,20,23,25,26 or low21,24 methodological quality. Supplementary Table S2 presents the assessment of the risk of bias of 2 RCTs evaluated using ROB 2.0. The methodological quality of the study conducted by Olesen et al.22 was of some concern. The study conducted by AI-Mourgi and Alshehri18 was evaluated to be of low methodological quality.
All the studies compared the PSP recurrence rate between conservative treatment and VATS groups. A significant difference was observed in the recurrence rate between groups (OR: 0.13, 95% CI, 0.09 to 0.19, P < 0.001) with low heterogeneity (I2 = 0%, P = 0.43). We determined that the recurrence rate was significantly lower in VATS groups than in conservative treatment groups, both in the subgroup in RCTs (OR: 0.16, 95% CI, 0.03 to 0.95, P = 0.04; I2 = 44% with P = 0.18) and in observation studies (OR: 0.10, 95% CI, 0.06 to 0.16, P < 0.001; I2 = 0%) (Fig. 2). We performed subgroup analyses regarding types of surgical techniques and types of conservative treatment (Supplementary Fig. S1 and S2). We found that compared with conservative treatment including observation and pleural drainage, recurrence rates were lower for VATS regardless of different types of surgical techniques, such as VATS with pleurodesis in all patients (OR 0.24, 95% CI 0.12–0.46, P < 0.001, I2 = 0%), VATS with pleurectomy in all patients (OR 0.09, 95% CI 0.04 to 0.23, P < 0.001, I2 = 0%), or VATS with or without pleurodesis or pleurectomy (OR 0.09, 95% CI 0.05 to 0.16, P < 0.001, I2 = 0%) (Supplementary Fig. S1). Similarly, recurrence rates were lower for VATS compared with different subgroups of conservative treatment, including the comparisons with conservative treatment with chest tube (OR 0.17, 95% CI 0.09 to 0.29, P < 0.001, I2 = 3%) or with conservative treatment mixed with observation and chest tube insertion (OR 0.09, 95% CI 0.05 to 0.17, P < 0.001, I2 = 0%) (Supplementary Fig. S2).
The incidence of complications between conservative treatment and VATS was reported in 3 studies19,22,25. No significant difference in the complication rate was observed (Peto OR 1.17, 95% CI 0.33 to 4.12, P = 0.80) between the 2 groups, albeit, heterogeneity was high (I2 = 83%, P = 0.002) (Fig. 3). Figure 3 showed a subgroup analysis by types of complications. After excluding the study19 with the complication of prolonged air leak, we observed that complication rates were significantly higher (Peto OR 10.50, 95% CI 1.79–61.52, P = 0.009) in the VATS group than in the conservative treatment group, with low heterogeneity (I2 = 0%).
Three studies investigated the duration of hospital stay between conservative treatment and VATS groups18,22,25. As illustrated in Fig. 4, there was no considerable difference in the duration of hospital stay (MD − 0.48 days, 95% CI − 2.84 to 1.87, P = 0.69) between the 2 groups. However, significant heterogeneity (I2 = 95%, P < 0.001) across studies was observed. To identify the source of heterogeneity, we performed the sensitivity test (Supplementary Fig. S3. After excluding Al-Mourgi’s study18, our result suggested that hospital stay did not differ between conservative treatment and VATS groups (MD 0.63 days, 95% CI − 0.06 to 1.33, P = 0.08; I2 = 0%).
As displayed in Fig. 5, the duration of pleural drainage was reported in 3 studies18,19,22. There was no difference in the duration of pleural drainage between conservative treatment with pleural drainage and VATS groups, with a mean difference of − 3.99 days (95% CI − 9.06 to 1.08, P = 0.12). However, heterogeneity across studies was high (I2 = 100%, P < 0.001).
Finally, based on the asymmetry of the funnel plot for the recurrence rate, we did not find significant unbalance in this meta-analysis (Supplementary Fig. S4). Results were similar by using Egger’s (P = 0.218) and Begg’s (P = 0.919) tests, which suggested that there was no significant publication bias in our meta-analysis.
Table 2 presents the GRADE approach for rating the quality of evidence. The recurrence rate and bleeding complication were judged as critical outcomes, and complications involving prolonged air leak, hospital stay, and drainage duration were considered as important outcomes, which were discussed by our team group. The process of rating the quality of evidence began with the study design (RCT trial or observational studies)27,28. Thus, we stratified the analysis according to RCTs and observation studies for each outcome (recurrence rate, hospital stay, and drainage duration). The quality of evidence of the recurrence rate in both RCTs and observational studies was rated as moderate. The RCTs were rated down from high to moderate for the recurrence rate outcome because of the high risk of bias, whereas the observational studies were rated up from low to moderate owing to very large effects (OR 0.10, 95% CI 0.06 to 0.16, P < 0.001; I2 = 0%) across studies. The evidence of the bleeding complication was rated as moderate owing to imprecision, which indicated that the clinical decision would differ because of the upper or lower boundaries of the CI (OR 8, 95% CI 0.82 to 78). The evidence of complications of prolonged air leak was rated from low to moderate owing to a very large effect size (OR 0.13, 95% CI 0.02 to 0.75, P = 0.02). The quality of evidence in the individual outcomes of hospital stay and drainage duration were both judged as very low because of the high risk of bias, inconsistency, and imprecision. An overall rating of confidence in the estimates of effect is based on the critical outcome that provides the lowest confidence29. Hence, we made an overall rating of confidence in effect estimates as moderate based on critical outcomes (recurrence rate and bleeding complication).
Discussion
Although international guidelines do not advocate surgery for patients with the first episode of pneumothorax, except in cases of complicated pneumothorax or for occupations at risk3,4,10. The optimal management of these patients has remained debatable30,31. Therefore, we conducted a systematic review and meta-analysis to facilitate the integration of information from current trials and to assist clinicians in making treatment decisions. We employed the GRADE system for a more transparent and efficient rating of the quality of evidence and the strength of recommendations. We included two RCTs and seven observational studies in the meta-analysis18,19,20,21,22,23,24,25,26. Our results demonstrated that patients with the first episode of PSP would have a significant reduction of the ipsilateral recurrence rate when treated with VATS than when treated with conservative treatment. However, the VATS group also had a higher rate of bleeding complications than conservative treatment, despite not being statistically significant. In addition, we found no difference regarding hospital stay and drainage duration between VATS and conservative treatment groups, but the quality of current evidence for these two outcomes was very low.
We used the GRADE system to evaluate the quality of evidence because it evaluates more than just the risk of bias, which refers to an appraisal of the internal validity of an individual study28. The quality of evidence according to the GRADE system also reflects the degree of confidence of an estimate of effect for systematic reviews12. Therefore, we regard the strength of recommendation of performing VATS surgery for the first episode of PSP as weak because the evidence is of moderate (recurrence rate and bleeding complication) to very low quality (hospital stay and drainage duration) in individual outcomes, and the desirable effect (recurrence rate) does not outweigh undesirable effects (bleeding complication, total costs, hospital stay, and drainage duration). Furthermore, we suggest sharing the decision-making for patients with the first episode of PSP in order to reach the optimal personal decision, which incorporates patient preferences and values, balances both desirable and undesirable effects, and cost-effectiveness. It is crucial for clinicians to recognize that different treatment options will be suitable for different patients and thus help patients reach a decision according to their preferences and values. Therefore, we offer a weak recommendation for these circumstances.
Daemen et al. conducted a systematic review and meta-analysis comparing chest tube drainage versus VATS for the first episode of PSP by analyzing two randomized trials and two observational studies32. They found that VATS could significantly reduce the recurrence rate and duration of hospital stay. In our study, we incorporated whole conservative treatments, which typically comprised observation, aspiration, thoracentesis, and chest tube drainage, to have a broader application in clinical settings. We also performed subgroup analysis by stratifying the analysis according to methods of conservative treatment (Supplementary Fig. S2). We searched thoroughly without language limitation and included nine studies, but we did not find any studies comparing sole observation or sole aspiration with VATS. Our results showed that the recurrence rate in the VATS group was significantly lower than in the subgroup with chest tube only and the subgroup with observation and chest tube. Furthermore, we employed the GRADE system to investigate the quality of evidence to inform recommendations, which is practicable and essential for clinical practice.
Our findings showed that the recurrence rate was significantly lower after VATS than after conservative treatment. The quality of evidence was moderate; thus, further research will probably have a considerable effect on our confidence in the estimate of effect and may also change the estimate12. The number needed to treat (NNT) in our study was 3.1, which indicates that for every three patients that undergo VATS operations, one recurrence is avoided. Although the results suggested VATS was associated with lower recurrence rates, not all patients with the first episode of PSP should undergo VATS operations, because there is a concern of overtreatment for two-thirds of patients. Hoffman et al. reported that nearly 50% of patients healed without recurrence with conservative treatments, and the overtreatment rate of pneumothorax patients was nearly 60%20. Although VATS is associated with a very low operative risk, surgical trauma, and complication rate, surgeons still need to explore safe and effective adhesion therapy, which is a mild and spotty adhesion that can prevent the collapse of the lungs. Recurrence rates can be significantly reduced by strong and tight adhesion. However, it is important for clinicians to consider that excessively tight adhesion might increase risks associated with future chest surgeries in people who have undergone pleurodesis or pleurectomy31.
Although no significant difference was observed in the pooled data of complication rates between conservative and VATS groups, the heterogeneity was high. Therefore, we separated subgroups to prolonged air leaks and other complications, which effectively reduced the heterogeneity. Prolonged air leak has been reported as one of the most common complications of pleural drainage in pneumothorax patients19,33,34. We also found that VATS groups have higher rates of complications than conservative groups. Olsen et al. reported that three surgical patients underwent reintervention because of bleeding from intercostal arteries22. Although the complication of bleeding was not significantly different between groups, our clinical decision would differ because the upper CI represented the surgical bleeding. The other complications reported included re-expansion edema in the pleural drainage group and postoperative complications, one with fever and one with phlebitis, in the VATS group25. The risk of complications from VATS should not be overlooked despite it being less invasive than open surgery. It is important that patients understand the possibility of complications from different treatments before making a decision.
We found no significant differences in the duration of hospital stay and the drainage duration between conservative and VATS groups. Only three studies were included in outcomes of hospital stay and drainage duration. Furthermore, we rated those outcomes as having high heterogeneity and very low quality of evidence by using the GRADE system, which indicates that the estimate of effect is very uncertain. The drainage duration can be influenced both by external factors (e.g., physicians’ decisions, and hospitals’ routine practices) and internal factors (e.g., lung conditions and initial severity of pneumothorax at diagnosis). Thus far, neither CHEST10 nor the British Thoracic Society4 has provided explicit recommendations concerning the timing of removal of intercostal drains, which may result in different standards on when to remove these drains and the timing of discharge. To minimize the effect of external factors (e.g., physicians’ decisions and hospitals’ practices), we need clear guidelines and more studies with large patient numbers to investigate these issues.
The economic outcome plays a critical role in robust clinical guidelines and recommendations. However, only one included study19 reported that the cost of VATS was more advantageous than that of pleural drainage (2423 vs 4855 Euros). Other studies have reported that the costs of VATS were higher than those of conservative treatment for patients with pneumothorax6,33,35 and the contrary36. Those studies did not meet our inclusion criteria, such as populations of combined PSP and SSP or recurrent pneumothorax. Therefore, further research of economic evaluations such as costs, resource use, and affordability are warranted.
There are some limitations to our study. First, because we did not have individual patient data (IPD), we could not perform stratified analyses according to age, sex, BMI, smoking status, initial severity of pneumothorax, or radiographic findings that might indicate the risk of recurrence. Second, we only included two RCTs and seven observational studies in our meta-analysis. The outcomes of complications, hospital stay, and drainage duration were not assessed in all nine studies, and the quality of evidence in those outcomes was very low. Furthermore, only one included study19 reported on economic evaluation, which was insufficient for meta-analysis. To assess potential bias due to the various qualities of the included studies and heterogeneity, we incorporated the quality assessment of included studies (Supplementary Table S1 and S2) and the quality of evidence by using the GRADE system (Table 2). We also conducted subgroup and sensitivity analyses (Supplementary e-Figure S1, S2 and S3). Third, we did not include the outcomes of quality of life or pain score because studies regarding those evaluations were few and unstandardized19,37,38. Fourth, as the case for most meta-analyses, publication bias is a potential concern, which can affect the validity and generalizability of the findings. Nonetheless, we conducted a comprehensive search of available literature to minimize the possibility of publication bias. We also conducted the funnel plot (Supplementary e-Figure S4) and the corresponding statistics of Egger’s and Begg’s tests, which suggested no significant publication bias. However, given the limited studies, we encouraged an updated analysis once several other studies become available. Further research is needed for the grading of recommendation, as it includes the continuum of quality of evidence. Outcomes such as complications, hospital stay, drainage duration, economic evaluation, quality of life, and pain score between conservative and VATS groups will necessitate clarification to facilitate decision-making in clinical settings.
We offer a weak recommendation regarding VATS as the treatment for the first episode of PSP according to our systematic review of the current evidence using the GRADE system. Weak recommendation indicates that clinicians should recognize that different choices will be appropriate for different patients and that they must help each patient reach a treatment decision consistent with their values and preferences through shared decision making.
Abbreviations
- PSP:
-
Primary spontaneous pneumothorax
- SSP:
-
Secondary spontaneous pneumothorax
- PTX:
-
Pneumothorax
- VATS:
-
Video-assisted thoracoscopic surgery
- GRADE system:
-
Grading of Recommendations, Assessment, Development, and Evaluations system
- RCTs:
-
Randomized controlled trials
- ROB:
-
Risk of Bias
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- MD:
-
Mean difference
- ORs:
-
Odds ratios
- SDs:
-
Standard deviations
- CI:
-
Confidence interval
- NNT:
-
Number needed to treat
- BMI:
-
Body mass index
- MeSH:
-
Medical Subject Headings
References
Sahn, S. A. & Heffner, J. E. Spontaneous pneumothorax. N. Engl. J. Med. 342, 868–874. https://doi.org/10.1056/nejm200003233421207 (2000).
Lesur, O., Delorme, N., Fromaget, J. M., Bernadac, P. & Polu, J. M. Computed tomography in the etiologic assessment of idiopathic spontaneous pneumothorax. Chest 98, 341–347. https://doi.org/10.1378/chest.98.2.341 (1990).
Tschopp, J. M. et al. ERS task force statement: Diagnosis and treatment of primary spontaneous pneumothorax. Eur. Respir. J. 46, 321–335. https://doi.org/10.1183/09031936.00219214 (2015).
MacDuff, A., Arnold, A. & Harvey, J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65(Suppl 2), ii18–ii31. https://doi.org/10.1136/thx.2010.136986 (2010).
Cardillo, G. et al. Primary spontaneous pneumothorax: A cohort study of VATS with talc poudrage. Thorax 71, 847–853. https://doi.org/10.1136/thoraxjnl-2015-207976 (2016).
Chen, J. S. et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: An open-label, parallel-group, prospective, randomised, controlled trial. Lancet 381, 1277–1282. https://doi.org/10.1016/s0140-6736(12)62170-9 (2013).
Ouanes-Besbes, L. et al. Prediction of recurrent spontaneous pneumothorax: CT scan findings versus management features. Respir. Med. 101, 230–236. https://doi.org/10.1016/j.rmed.2006.05.016 (2007).
Bintcliffe, O. J. et al. Spontaneous pneumothorax: Time to rethink management?. Lancet Respir. Med. 3, 578–588. https://doi.org/10.1016/s2213-2600(15)00220-9 (2015).
Chou, S. H., Cheng, Y. J. & Kao, E. L. Is video-assisted thoracic surgery indicated in the first episode primary spontaneous pneumothorax?. Interact. Cardiovasc. Thorac. Surg. 2, 552–554. https://doi.org/10.1016/s1569-9293(03)00143-9 (2003).
Bauman, M. H. et al. Management of spontaneous pneumothorax: An American College of Chest Physicians Delphi Consensus Statement. Chest 119, 590–602 (2001).
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. https://doi.org/10.1136/bmj.39489.470347.AD (2008).
Guyatt, G. H. et al. What is “quality of evidence” and why is it important to clinicians?. BMJ 336, 995–998. https://doi.org/10.1136/bmj.39490.551019.BE (2008).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. https://doi.org/10.1007/s10654-010-9491-z (2010).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6, e1000097. https://doi.org/10.1371/journal.pmed.1000097 (2009).
Higgins, J. P. T. et al. Cochrane Handbook for Systematic Reviews of Interventions 2nd edn. (Wiley, 2019).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. J. B. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Al-Mourgi, M. & Alshehri, F. Video-assisted thoracoscopic surgery for the treatment of first-time spontaneous pneumothorax versus conservative treatment. Int. J. Health Sci. (Qassim) 9, 428–432 (2015).
Divisi, D., Di Leonardo, G. & Crisci, R. Video-assisted thoracic surgery versus pleural drainage in the management of the first episode of primary spontaneous pneumothorax. Am. J. Surg. 210, 68–73. https://doi.org/10.1016/j.amjsurg.2014.10.025 (2015).
Hofmann, H. S. et al. Burden between undersupply and overtreatment in the care of primary spontaneous pneumothorax. Thorac. Cardiovasc. Surg. 66, 575–582. https://doi.org/10.1055/s-0037-1609011 (2018).
Iablonskii, P. K., Atiukov, M. A., Pishchik, V. G. & El’kina, E. A. Diagnostic and treatment strategy for patients with the first episode of primary spontaneous pneumothorax. Vestn. Khir. Im. I. I. Grek. 164, 11–14 (2005).
Olesen, W. H. et al. Surgical treatment versus conventional chest tube drainage in primary spontaneous pneumothorax: A randomized controlled trial. Eur. J. Cardiothorac. Surg. 54, 113–121. https://doi.org/10.1093/ejcts/ezy003 (2018).
Primavesi, F. et al. First episode of spontaneous pneumothorax: CT-based scoring to select patients for early surgery. World J. Surg. 40, 1112–1120. https://doi.org/10.1007/s00268-015-3371-3 (2016).
Sawada, S., Watanabe, Y. & Moriyama, S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: Evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 127, 2226–2230. https://doi.org/10.1378/chest.127.6.2226 (2005).
Seguier-Lipszyc, E., Elizur, A., Klin, B., Vaiman, M. & Lotan, G. Management of primary spontaneous pneumothorax in children. Clin. Pediatr. (Phila.) 50, 797–802. https://doi.org/10.1177/0009922811404699 (2011).
Soler, L. M., Raymond, S. L., Larson, S. D., Taylor, J. A. & Islam, S. Initial primary spontaneous pneumothorax in children and adolescents: Operate or wait?. J. Pediatr. Surg. 53, 1960–1963. https://doi.org/10.1016/j.jpedsurg.2017.12.014 (2018).
Guyatt, G. et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64, 383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026 (2011).
Balshem, H. et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406. https://doi.org/10.1016/j.jclinepi.2010.07.015 (2011).
Guyatt, G. et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 66, 151–157. https://doi.org/10.1016/j.jclinepi.2012.01.006 (2013).
Cardillo, G., Ricciardi, S., Rahman, N., Walker, S. & Maskell, N. A. Primary spontaneous pneumothorax: Time for surgery at first episode?. J. Thorac. Dis. 11, S1393–S1397. https://doi.org/10.21037/jtd.2019.03.22 (2019).
Goto, T. Is surgery the choice for treatment for first presentation of pneumothorax?. J. Thorac. Dis. 11, S1398–S1401. https://doi.org/10.21037/jtd.2019.03.16 (2019).
Daemen, J. H. T. et al. Chest tube drainage versus video-assisted thoracoscopic surgery for a first episode of primary spontaneous pneumothorax: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 56, 819–829. https://doi.org/10.1093/ejcts/ezz116 (2019).
Abdala, O. A. et al. Advantages of video assisted thoracic surgery in the treatment of spontaneous pneumothorax. Medicina (B. Aires) 61, 157–160 (2001).
Torresini, G., Vaccarili, M., Divisi, D. & Crisci, R. Is video-assisted thoracic surgery justified at first spontaneous pneumothorax?. Eur. J. Cardiothorac. Surg. 20, 42–45 (2001).
Lopez, M. E. et al. Management of the pediatric spontaneous pneumothorax: Is primary surgery the treatment of choice?. Am. J. Surg. 208, 571–576. https://doi.org/10.1016/j.amjsurg.2014.06.009 (2014).
Schramel, F. M., Sutedja, T. G., Braber, J. C., van Mourik, J. C. & Postmus, P. E. Cost-effectiveness of video-assisted thoracoscopic surgery versus conservative treatment for first time or recurrent spontaneous pneumothorax. Eur. Respir. J. 9, 1821–1825 (1996).
Morimoto, T., Fukui, T., Koyama, H., Noguchi, Y. & Shimbo, T. Optimal strategy for the first episode of primary spontaneous pneumothorax in young men: A decision analysis. J. Gen. Intern. Med. 17, 193–202. https://doi.org/10.1046/j.1525-1497.2002.10636.x (2002).
Morimoto, T. et al. Effects of timing of thoracoscopic surgery for primary spontaneous pneumothorax on prognosis and costs. Am. J. Surg. 187, 767–774. https://doi.org/10.1016/j.amjsurg.2003.07.032 (2004).
Acknowledgements
The authors would like to thank Li-Ting Chien, the certified librarian of Taipei Medical University, for her assistance and the validation of the search strategy. The authors would like to acknowledge the Laboratory Animal Center at Taipei Medical University for language editing support.
Author information
Authors and Affiliations
Contributions
H.Y.C.: takes responsibility for the integrity of the data and the accuracy of the data analysis. H.Y.C., Y.C.H., P.C.Y., and C.M.C. contributed to the conception of the study. H.Y.C., Y.C.H., P.C.Y., C.M.C., C.C.C., W.C.W., Y.C.L., C.Y.C., and Y.C.W contributed to the design of the study. H.Y.C., Y.C.H., P.C.Y., C.M.C., Y.C.L., and C.Y.C. contributed to the literature search and data extraction. H.Y.C., Y.C.H., P.C.Y., and C.M.C. contributed to data analysis and interpretation. H.Y.C., C.C.C., W.C.W., Y.C.L., C.Y.C., and Y.C.W. conducted the quality assessment. H.Y.C., Y.C.H., P.C.Y., and C.M.C. contributed to writing the manuscript. H.Y.C., C.C.C., W.C.W., Y.C.L., C.Y.C., and Y.C.W. contributed to the critical revision of the manuscript. H.Y.C., Y.C.H., P.C.Y., C.M.C., C.C.C., W.C.W., Y.C.L., C.Y.C., and Y.C.W. approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiu, HY., Ho, YC., Yang, PC. et al. Recommendation for management of patients with their first episode of primary spontaneous pneumothorax, using video-assisted thoracoscopic surgery or conservative treatment. Sci Rep 11, 10874 (2021). https://doi.org/10.1038/s41598-021-90113-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90113-w
- Springer Nature Limited