Abstract
The use of sentinel node biopsy (SNB) following neoadjuvant chemotherapy (NAC) for patients with cN1 breast cancer is controversial. Improvements of negative predictive value (NPV) by axillary ultrasound (AUS), which corresponds to the accurate prediction rate of node-negative status after NAC, would lead to decreased FNR of SNB following NAC. In this study, we retrospectively investigated the accurate prediction rate of NPV by AUS after NAC in patients with cytologically node-positive breast cancer treated between January 2012 and December 2016. Of 279 eligible patients, the NPV was 49.2% in all patients, but varied significantly by tumor subtype (p < 0.001) and tumor response determined by magnetic resonance imaging (MRI) (p = 0.0003). Of the 23 patients with clinically node negative (ycN0) by AUS and clinical complete response in primary lesion by MRI, the NPV was 100% in patients with HR±/HER2+ or HR−/HER2− breast cancer. In conclusion, regarding FNR reduction post-NAC, it will be of clinical value to take tumor subtype and primary tumor response using MRI into account to identify patients for SNB after NAC.
Similar content being viewed by others
Introduction
Neoadjuvant chemotherapy (NAC) is widely used as a perioperative systemic therapy for operable breast cancer to reduce the tumor volume and nodal stage, to evaluate chemosensitivity and to facilitate translational research1. A pathological complete response (pCR) occurs in 40% to 50% of patients with human epidermal growth factor receptor-2 (HER2)-positive and triple-negative breast cancer2. Although axillary dissection is currently considered standard for patients with clinically node-positive breast cancer, a more conservative approach may be applicable if patients achieve pCR in the axilla after NAC. However, the use of sentinel lymph node biopsy (SNB) following NAC for patients with cN1 breast cancer is controversial even if nodal status becomes clinically negative after NAC. Following NAC, lymphatic drainage from the breast can be impaired and tumor regression in the axilla can take a nonuniform pattern, leading to an increased false-negative rate (FNR)3. Three major prospective trials previously evaluated the use of SNB in patients with clinically node-positive breast cancer receiving NAC: American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, SENTINA trial, and SN FNAC trial. These three trials reported respective FNRs of 12.6%, 14.2%, and 13.4%, all of which were above the currently accepted cut off of 10%3,4,5. In the ACOSOG Z1071 trial, patients underwent SNB regardless of nodal response to NAC, but when patients with normal ultrasound (US) findings in the axilla after NAC were selected, FNR was reduced from 12.6 to 9.8%6. However, although 70.4% of patients had normal lymph node-US findings after NAC, nodal pCR was only 39.0%, which suggests that normal-appearing lymph nodes on US does not preclude residual disease within lymph nodes on final pathology6. Although preoperative axillary imaging assessment may help to decide axillary surgery procedure after NAC, AUS is not commonly used to assess axillary response to NAC.
It is conceivable that improvements of negative predictive value (NPV) by AUS, which corresponds to the accurate prediction rate of node-negative status after NAC, would lead to decreased FNR of SNB following NAC. Therefore, in this study, we retrospectively investigated the accurate prediction rate of NPV by AUS after NAC in patients with cytologically node-positive breast cancer and investigated factors related to the rate.
Materials and methods
Patients
From January 2012 through December 2016, 298 patients with clinical stage T1–T4, N1–N2, M0 primary breast cancer with cytologically proven axillary metastasis, who underwent surgery including axillary lymph node dissection (ALND) following NAC, were retrospectively reviewed. All the patients received fine needle aspiration in axillary lymph node before NAC, which proved cytologically positive axillary node metastasis. Patients received anthracycline- and/or taxane-based regimens. Patients with HER2-positive breast cancer received anti-HER2 therapy. HER2 amplification status was determined by fluorescence in situ hybridization (FISH) and/or positive immunohistochemistry findings. Patient exclusion criteria included no nodal assessment by AUS at our institution and two or fewer cycles of chemotherapy for NAC.
The protocol has been approved by the institutional ethical committee (Cancer Institute Hospital of Japanese Foundation for Cancer Research) (No.2018-1100). The present study was carried out in accordance with the relevant guidelines and regulations/ethical principles of the Declaration of Helsinki.
Imaging diagnosis
Clinical staging was determined by mammogram, breast ultrasonography, AUS, abdominal ultrasonography, magnetic resonance imaging (MRI) and bone scintigraphy, or positron emission tomography-computed tomography in all patients with node-positive disease. We evaluated NAC efficacy by breast ultrasonography, AUS, and contrast-enhanced MRI before NAC, at changes in chemotherapy regimen, and after NAC.
If there were any concerns regarding disease progression, imaging studies were conducted. Tumor response assessment by MRI was performed by measuring the maximum tumor diameter before and after NAC based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
AUS and MRI imaging
AUS was conducted by an experienced breast sonographer. A clinically positive lymph node was defined as having a concentric cortical thickness > 3 mm, absent fatty hilum, or irregular morphology (Fig. 1). To standardize evaluation of nodal status after NAC, lymph node status was assessed by both breast radiologists and breast surgeons. All breast MRI images were evaluated by two experienced radiologists. Tumor extent, morphology, and relative enhancement were assessed during initial and late enhancements at baseline and during NAC using MRI images. The extent of each tumor was assessed by its largest diameter in three reformatted planes (sagittal, axial, and coronal) at initial and late enhancements. Radiological complete response in breast MRI was defined as the absence of dynamic contrast-enhancement on T1-weighted MRI series.
Representative ultrasound images. Patient 1. Before NAC, oval shaped lymph node was detected, which was cytologically confirmed as metastatic node (A). After NAC, lymph node shrunk, which was pathologically confirmed as negative node (B). Patient 2. Before NAC, ultrasound image of an axillary lymph node on fine needle aspiration showed irregular shaped lymph node, which was cytologically confirmed as metastatic node (C). After NAC, irregular shaped lymph node remained, which was pathologically confirmed as positive node (D).
Axillary surgery
All patients received ALND regardless of clinical response. A dissection of Levels I and II was conducted for all patients. For suspected Level II lymph nodes identified by palpation during surgery, intraoperative frozen section analysis was performed. In cases of nodes positive for Level II, a Level III dissection was performed.
Pathological evaluation
Estrogen receptor (ER), progesterone receptor (PgR), and HER2 status were determined on core biopsy samples obtained prior to NAC. ER- and PgR-positive status (hormone receptor positive, HR +) were defined as ≥ 10% ER- and PgR-positive cancer cells by immunohistochemistry, and HER2 positive was defined either by an immunohistochemical HER2 score of 3+ or a score of 2+ with evidence of gene amplification by FISH. Lymph nodes were cut in half, and interior surfaces were stained using hematoxylin and eosin. Axillary pCR was defined as no evidence of metastatic carcinoma, including absence of isolated tumor cell clusters in lymph node.
Statistical analysis
Chi-square test was used to compare tumor characteristics between pathological and AUS nodal assessments, and p-values < 0.05 were considered statistically significant. All analyses were performed using JMP Pro 15 (SAS Institute Inc., Cary, NC, USA).
Ethical approval
The protocol has been approved by the institutional ethical committee (Cancer Institute Hospital of Japanese Foundation for Cancer Research) (No. 2018-1100). Due to the retrospective nature of this study the need for informed consent was waived.
Results
Patient characteristics
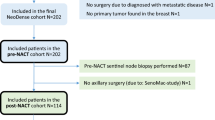
Our initial cohort consisted of 298 patients with clinical stage T1–T4, N1–N2, M0 primary breast cancer and cytologically proven axillary metastasis who underwent surgery with ALND following NAC. Eligibility criteria were met by 279 patients, and 19 patients were excluded because 11 had no nodal assessment by AUS at our institution and 8 had two or fewer cycles of chemotherapy (Fig. 2).
Patient characteristics and treatment are presented in Table 1 by pathological and clinical nodal status after NAC. The majority of chemotherapy regimens included both anthracycline and taxane (96.8%), and all patients with HER2+ disease received an anti-HER2+ regimen. Clinical tumor responses in the breast assessed by MRI were complete response (CR) in 38 patients (13.6%), partial response (PR) in 193 (69.2%), stable disease (SD) in 40 (14.3%), and progressive disease (PD) in 8 (2.9%). The median number of nodes removed at surgery was 16 (range 8–37). The median number of pathologically positive residual nodes was 2 (range 1–16) in the whole cohort of patients with ypN+ . Of the 61 patients with ycN0ypN+ , 26 (42.6%) had 1 positive lymph node on pathological review, 9 (14.8%) had 2 positive lymph nodes, 7 (11.5%) had 3 positive lymph nodes, and 19 (31.1%) had more than 3 positive lymph nodes (Table 1).
Axillary pCR rate by tumor subtype
Of our 279 patients, 101 patients (36.2%) were pathologically confirmed axillary node-negative (ypN0). The rate of ypN0 was 20.2% (37/183) in HR+ /HER2−, 71.9% (23/32) in HR+ /HER2+ , 83.3% (20/24) in HR−/HER2+ , and 52.5% (21/40) in HR−/HER2− patients (Fig. 3).
Sensitivity and specificity of AUS
Of our 279 patients, 124 patients (44.4%) were clinically node negative by AUS after NAC (ycN0), of whom 63 patients (50.8%) were pathologically node negative at surgery (ypN0). Of 155 patients with clinically node positive by AUS after NAC (ycN +), only 38 patients (24.5%) had nodal pCR (Table 1, Fig. 2). The sensitivity and specificity of AUS after NAC were 65.7% and 62.3%, respectively (Table 2). We also determined that the negative predictive value (NPV), defined as the accurate prediction rate of node-negative status after NAC by AUS, was 50.8% in our total cohort and 29.7% in HR+ /HER2−, 89.5% in HR+ /HER2+ , 86.7% in HR−/HER2+ , and 68.8% in HR−/HER2− patients (Table 3). NPV was highest in patients with HER2+ breast cancer and lowest in patients with HR+ /HER2− breast cancer. The accuracy of AUS, which is calculated as (true positive; TP+ true negative; TN)/(true positive; TP+ true negative; TN+ false positive; FP+ false negative; FN), is 63.4% in HR+ /HER2−, 75% in HR+ /HER2+ , 62.5% in HR−/HER2+ , and 62.5% in HR−/HER2−.
Factors related to NPV
Comparison of tumor characteristics between patients with ycN0ypN0 and patients with ycN0ypN+ are presented in Table 4. We found that the NPV by AUS varied significantly by tumor subtype (p < 0.001) and tumor response as assessed by MRI after NAC completion (p < 0.0003); however, there was no significant difference between the two groups regarding tumor category at diagnosis, tumor histology, and the number of positive nodes before NAC as assessed by AUS. Of the 23 patients who achieved ycN0 by AUS and breast cCR by MRI, we found that the accurate prediction rate of ypN0 was 100% in patients with HR±/HER2+ or HR−/HER2− breast cancer, and only 57% in HR+ /HER2− breast cancer.
Discussion
The use of SNB following NAC for patients with cN1 breast cancer is controversial because clinical trials have shown that the FNR exceeds the prespecified threshold of 10%3,4,5. To decrease FNR, dual-agent mapping, examining more than two sentinel lymph nodes (SLNs)3,4,5,7, targeted axillary dissection (TAD) placement including MARI, and tattooing procedure have been suggested8,9,10,11,12. When limited to patients with more than two SLNs removed, the FNR was 7.3% and 9.1% in the SENTINA and ACOSOG Z1071 trails, respectively3,4. However, in these two trials, these were unplanned analyses and only 56% and 34% of patients had more than two SLNs removed, respectively13. In addition, in SENTINA, lymph node status was not confirmed pathologically but suspected radiologically. Caudle et al. reported that the FNR decreased to as low as 2% using TAD8; however, TAD has limitations in that it requires extra procedures, such as clip placement at the time of biopsy and seed placement before surgery14. In addition, the response to NAC may differ among metastatic nodes in patients with multiple metastatic nodes15.
Another promising strategy to reduce FNR is preoperative axillary assessment by imaging. In the ACOSOG Z1071 trial, patients underwent SNB regardless of NAC response4. Following a secondary analysis of the trial, ACOSOG investigators reported that FNR was reduced to 9.8% by considering AUS assessment following NAC6. Similarly, in the SN FNAC trial, FNR was decreased to 2.7% by taking AUS assessment into account16. Although both studies showed that AUS reduced FNR, investigators took the position that US alone is not accurate enough to assess axillary response. Admittedly, the accurate prediction rate of node-negative status after NAC by AUS varies ranging from 46.2 to 89.6% in a number of studies17,18,19. Similarly, we found in our cohort that the NPV by AUS after NAC was 50.8%. However, it is conceivable that improvements of NPV by AUS would lead to decreased FNR of SNB following NAC. In this context, we demonstrated that the NPV by AUS after NAC was improved by addition of subtype information and breast tumor response to NAC by MRI. In fact, we found that the NPV was over 85% in HER2-positive cases in our cohort. These results suggest that the FNR of SNB after NAC can be reduced in patients with HER2-positive breast cancer under AUS determination of no lymph node metastasis after NAC. Moreover, we showed that all patients with HER2+ or HR−/HER2− disease and identified as ycN0 by AUS and cCR in primary tumor by MRI showed ypN0, indicating that these patients may be good candidates not requiring axillary surgery in the future.
Peppe et al. reported that there were notable variations in NPV and PPV by AUS according to tumor subtype, with HER2+ cases having a higher NPV, which is concordant with our results. However, other related factors were not investigated19. Several studies have reported factors associated with pathological response in nodes following NAC. Barrio et al. revealed that patients with HR+ breast cancer or those without breast pCR were less likely to have nodal pCR (p = 0.05); there was no evidence for other associative factors20. Tadros et al. reported that patients without breast pCR had a relative risk of 7.4 (95% confidence interval, 3.7–14.8; p < 0.001) to remaining positive for nodal metastases compared with those with breast pCR2. These results support our findings that accurate prediction of nodal pCR by AUS depends on subtype and breast tumor response as determined by MRI.
There are several limitations of our study. First, our sample size was modest. Particularly, in the subgroup analysis with subtypes and tumor response, the sample size in each subgroup was small. Thus, this study cannot make a solid conclusion. Because it is important to determine patients suitable for SNB after NAC, a larger study is necessary to ensure a sufficient sample size for subgroup analysis considering subtypes and breast tumor response. Second, this was a single institutional study so caution is warranted when considering the results in other institutions. However, it is worth noting that imaging diagnosis, such as AUS and breast MRI, was determined by consistent criteria in the single institution.
In conclusion, we found that the accurate prediction rate of node-negative status by AUS after NAC was subtype-dependent and highest in patients with HER2+ breast cancer. We also found that this increased by combining US assessment with breast tumor response determined by MRI. Regarding FNR reduction post-NAC, it will be of clinical value to take tumor subtype and primary tumor response using MRI into account to identify patients for SNB after NAC. In the future, it would be possible that patients with HER2+ or HR−/HER2− breast cancer who achieve ycN0 by AUS and cCR in primary tumor by MRI may be spared from axillary surgery. However, to better clarify optimal axillary management for patients with clinically node-positive breast cancer that converts to node negative after NAC, further prospective data considering clinical stage, molecular subtype, and clinical response in both breast and LNs is needed.
References
Mieog, J. S., van der Hage, J. A. & van de Velde, C. J. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005002.pub2 (2007).
Tadros, A. B. et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 152(7), 665–670. https://doi.org/10.1001/jamasurg.2017.0562 (2017).
Kuehn, T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicenter cohort study. Lancet Oncol. 14(7), 609–618. https://doi.org/10.1016/S1470-2045(13)70166-9 (2013).
Boughey, J. C. et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14), 1455–1461. https://doi.org/10.1001/jama.2013.278932 (2013).
Boileau, J. F. et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J. Clin. Oncol. 33(3), 258–264. https://doi.org/10.1200/JCO.2014.55.7827 (2015).
Boughey, J. C. et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J. Clin. Oncol. 33(30), 3386–3393. https://doi.org/10.1200/JCO.2014.57.8401 (2015).
Zetterlund, L. H. et al. Swedish prospective multicenter trial evaluating sentinel lymph node biopsy after neoadjuvant systemic therapy in clinically node-positive breast cancer. Breast Cancer Res. Treat. 163, 103–110. https://doi.org/10.1007/s10549-017-4164-1 (2017).
Caudle, A. S. et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J. Clin. Oncol. 34(10), 1072–1078. https://doi.org/10.1200/JCO.2015.64.0094 (2016).
Funes, D. F. et al. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surg. Oncol. 30, 52–57. https://doi.org/10.1016/j.suronc.2019.05.019 (2019).
Nijnatten, T. J. A. et al. A novel less-invasive approach for axillary staging after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer by combining radioactive iodine seed localization in the axilla with the sentinel node procedure (RISAS): a Dutch prospective multicenter validation study. Clin. Breast Cancer 17(5), 399–402. https://doi.org/10.1016/j.clbc.2017.04.006 (2017).
Donker, M. et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann. Surg. 261(2), 378–382. https://doi.org/10.1097/SLA.0000000000000558 (2015).
Park, S. et al. Feasibility of charcoal tatooing of cytology-proven metastatic axillary lymph node at diagnosis and sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. Cancer Res. Treat. 50(3), 801–812. https://doi.org/10.4143/crt.2017.210 (2018).
Pilewskie, M. & Morrow, M. Axillary nodal management following neoadjuvant. Chemotherapy: a review. JAMA Oncol. 3(4), 549–555. https://doi.org/10.1001/jamaoncol.2016.4163 (2017).
Racz, J. M. & Caudle, A. S. Sentinel node lymph node surgery after neoadjuvant. Therapy: principles and techniques. Ann. Surg. Oncol. 26, 3040–3045. https://doi.org/10.1245/s10434-019-07591-6 (2019).
Kim, W. H. et al. Ultrasound-guided restaging and localization of axillary lymph nodes after neoadjuvant chemotherapy for guidance of axillary surgery in breast cancer patients: experience with activated charcoal. Ann. Surg. Oncol. 25, 494–500. https://doi.org/10.1245/s10434-017-6250-3 (2018).
Morency, D. et al. Axillary lymph node ultrasound following neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: results from the SN FNAC Study. Ann. Surg. Oncol. 26(13), 4337–4345. https://doi.org/10.1245/s10434-019-07809-7 (2019).
Schwentner, L. et al. Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy-results from the multi-center SENTINA trial. Breast 31, 202–207. https://doi.org/10.1016/j.breast.2016.11.012 (2017).
Morgan, C. et al. Neoadjuvant chemotherapy in patients with breast cancer: Does response in the breast predict axillary node response?. Eur. J. Surg. Oncol. 46(4), 522–526. https://doi.org/10.1016/j.ejso.2019.11.498 (2020).
Peppe, A., Wilson, R., Pope, R., Downey, K. & Rusby, J. The use of ultrasound in the clinical re-staging of the axilla after neoadjuvant chemotherapy (NACT). Breast 35, 104–108. https://doi.org/10.1016/j.breast.2017.05.015 (2017).
Barrio, A. V. et al. How often is treatment effect identified in axillary nodes with a pathologic complete response after neoadjuvant chemotherapy. Ann. Surg. Oncol. 23(11), 3475–3480. https://doi.org/10.1245/s10434-016-5463-1 (2016).
Acknowledgements
We would like to thank all member of breast oncology center in our institution. We would also like to extend our thanks to Ms. Gokan, data manager of the Cancer Institute Hospital of Japanese Foundation for Cancer Research, for her help in collecting data.
Author information
Authors and Affiliations
Contributions
Y.Ma., T.S. and T.U. wrote the manuscript. Y.M. and A.O. collected the data. Y.Ma., T.S., A.O., Y.T., Y.Mi., Y.I., S.T., S.O., and T.U. provided the treatments to the patients. Y.Ma., A.O. and Y.K. reviewed the radiological images. T.O. made the pathological diagnosis. All authors contributed to the final approved version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maeshima, Y., Sakai, T., Ogiya, A. et al. Assessment of axillary node status by ultrasound after neoadjuvant chemotherapy in patients with clinically node-positive breast cancer according to breast cancer subtype. Sci Rep 11, 10858 (2021). https://doi.org/10.1038/s41598-021-89738-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89738-8
- Springer Nature Limited
This article is cited by
-
Axillary ultrasound for predicting response to neoadjuvant treatment in breast cancer patients—a single institution experience
World Journal of Surgical Oncology (2023)
-
Improved false-negative rates using a novel patient selection flowchart in initially biopsy-proven node-positive breast cancer undergoing blue-dye alone guided sentinel lymph node biopsy after neoadjuvant chemotherapy
Breast Cancer Research and Treatment (2022)
-
Combining conventional ultrasound and sonoelastography to predict axillary status after neoadjuvant chemotherapy for breast cancer
European Radiology (2022)