Abstract
Although a considerable volume of data supporting induction or aggravation of psoriasis because of angiotensin-converting enzyme (ACE) inhibitor use exists, it remains insufficient for definitive conclusions. Therefore, we aimed to evaluate the association between ACE inhibitor use and psoriasis incidence through a systematic literature review and meta-analysis. We searched for qualifying studies across PubMed, Web of Science, and Embase. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the association between ACE inhibitor use and psoriasis incidence. Eight studies with a total of 54,509 patients with a psoriasis diagnosis were included in this meta-analysis. The pooled OR for psoriasis incidence among ACE inhibitor users was 1.52 (95% CI, 1.16–2.00) compared to that among non-users. From subgroup analysis by continent, the OR for ACE inhibitor users versus non-users was 2.37 (95% CI 1.28–4.37) in Asia. Per the subgroup analysis by climate, the OR for ACE inhibitor users vs non-users in dry climate was 3.45 (95% CI: 2.05–5.79) vs 1.32 (95% CI 1.01–1.73) in temperate climate. Our results reveal a significant association between ACE inhibitor use and psoriasis incidence.
Similar content being viewed by others
Introduction
Angiotensin-converting enzyme (ACE) inhibitors are widely used medications for treatment of hypertension and congestive heart failure and prevention of kidney diseases in patients with hypertension or diabetes1. Although ACE inhibitors are generally well-tolerated, several adverse events have been reported, including dry cough, angioedema, rash, and hyperkalemia2. In addition, psoriasis has been reported as a rare adverse event. Several case reports have implicated the use ACE inhibitors, particularly captopril and enalapril, in the incidence and exacerbation of psoriasis1,3,4,5,6,7,8,9. Moreover, psoriasiform eruptions and exacerbation of pre-existing psoriasis were reported in patients receiving other ACE inhibitors such as ramipril and lisinopril3,5.
Therapeutic agents which may induce or aggravate psoriasis may be classified into three categories depending on the strength of the association. First group of drugs have a strong association with psoriasis incidence or aggravation and include lithium, β-blockers, and antimalarials. Second group of drugs have considerable yet insufficient data supporting incidence or aggravation of psoriasis, such as ACE inhibitors, interferons, and terbinafine. Third group of drugs have been occasionally reported to be associated with incidence or aggravation of psoriasis and include a large group of miscellaneous drugs such as clonidine, digoxin, and amiodarone10.
Although studies reporting the possible association between ACE inhibitor use and psoriasis exist, the results have been inconsistent. Therefore, we investigated the association between ACE inhibitor use and psoriasis incidence through a systematic literature review and meta-analysis. In addition, because environmental factors may affect the association between ACE inhibitor use and psoriasis, subgroup analyses by study design, continent and climate were also performed.
Results
Identification and characteristics of the included studies
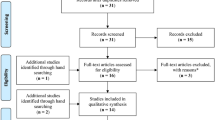
The study selection process is summarized in Fig. 1. In total, 636 records were identified from the three databases (PubMed n = 201, Web of Science n = 60, Embase n = 375), and 128 duplicates were excluded. After removing 464 studies during title and abstract screening, 44 studies were selected for full-text review, and 36 studies were excluded for the following reasons: not having appropriate data (n = 15), not original articles (n = 14), not case–control or cohort studies (n = 4), not confirmed whether drugs were taken before diagnosis (n = 2), and not medically classified as psoriasis (n = 1). Finally, eight studies were included for meta-analysis11,12,13,14,15,16,17,18.
The characteristics of the included studies are shown in Table 1. Of the eight studies, four were cohort studies15,16,17,18 and four were case–control studies11,12,13,14. Four studies were performed in Asia11,14,17,18, three in Europe12,13,16, and one in the USA15. The number of patients in each study ranged from 625 to 256,356. Regarding study quality using the Newcastle–Ottawa Scale (NOS) score, each study received ≥ 6 points out of 9 (Table 1).
Quantitative data synthesis
For the association of ACE inhibitor use with psoriasis incidence, eight studies with a total of 54,509 patients with a psoriasis diagnosis were included in the meta-analysis. As shown in Fig. 2, ACE inhibitor user group had a higher association with psoriasis incidence compared to the non-user group (Odds ratio (OR) 1.52, 95% confidence interval (CI) 1.16–2.00). We observed heterogeneity between these studies (I2 = 86%; p < 0.00001); hence, the random-effects model was used to calculate effect size. As there were four studies each in case–control and cohort study, we performed a subgroup analysis by study design. The ORs were 2.20 (95% CI 1.08–4.48) for case–control studies and 1.24 (95% CI 0.87–1.75) for cohort studies.
For a subgroup analysis by continent, we divided into two groups; four and three studies in Asia11,14,17,18 and Europe12,13,16, respectively. As shown in Fig. 3, the analysis of the Asia subgroup showed that the ACE inhibitor user group had a higher risk of psoriasis incidence than the non-user group (OR 2.37, 95% CI 1.28–4.37), whereas the analysis of the Europe subgroup failed to show statistical significance. OR of the analysis of Asia was higher than that of all 8 studies (2.37 vs 1.52). Further analysis using subgroups of the West Asia and the East Asia showed that higher association between ACE inhibitor use and psoriasis incidence was found in studies conducted in West Asia (OR 3.45, 95% CI 2.05–5.79) compared to those in East Asia (OR 1.47, 95% CI 1.25–1.73).
For the subgroup analysis by climate, since there were five and two were conducted in temperate climate12,13,16,17,18 and dry climates11,14, respectively. As shown in Fig. 4, the ACE inhibitor users within the dry climate subgroup showed a considerably higher risk of psoriasis incidence compared to the non-users (OR 3.45, 95% CI 2.05–5.79), whereas the analysis of the temperate climate subgroup showed marginal significance (p = 0.05).
Sensitivity analysis and publication bias
Neither the Egger’s test (z = 0.68, p = 0.50) nor the Begg’s test (t = 1.06, p = 0.40) showed significant publication bias for these studies. Sensitivity analysis was performed by sequentially excluding each study. There were no significant effects on ORs for the association of ACE inhibitor use with psoriasis and heterogeneity (OR 1.36–1.74, I2 range 84%-88%).
Discussion
The results of our meta-analysis indicate a significant association between ACE inhibitor use and psoriasis incidence. The sensitivity analyses yielded similar results, demonstrating the robustness of the results. In the subgroup analyses by continent and climate, risk of psoriasis related to ACE inhibitor use was higher in Asia and in a dry climate. Although statistical significance was obtained in the subgroup analysis using case–control studies, we failed to get a statistical significance in cohort studies. This was possibly due to the Jacob et al. study tending to be contrary to other studies.
A hypothesis for the association between ACE inhibitor use and psoriasis is related to bradykinin. ACE has two natural substrates and two catalytic domains: cleaving angiotensin I and inactivating bradykinin19. An in vitro study showed that ACE inhibitors had higher affinity to the bradykinin than the angiotensin I binding sites, indicating that these agents primarily inhibit bradykinin degradation20. The increased bradykinin levels because of ACE inhibitor use can cause vascular relaxation through the release of endothelial relaxation factors including prostacyclin, nitric oxide (NO), and epoxyeicosatrienoic acids. In contrast, inhibition of bradykinin degradation by ACE inhibitors alters the kinin-kallikrein arachidonic acid system leading to increased concentrations of inflammatory metabolites. This kinin-kallikrein arachidonic acid system is an important pathway in the pathogenesis of psoriasis20,21,22,23.
Angiotensin II is known to have a proinflammatory activity, which is mediated by the activation of macrophages, T-cells, mesangial cells, dendritic cells, and vascular smooth muscle cells24. Moreover, angiotensin II stimulates the production of specific cytokines such as interleukin-12, potent monocyte chemoattractant protein-1, NO, and tumor necrosis factor-α, thereby causing infiltration of inflammatory cells into tissues24,25. A previous in vivo study observed that angiotensin II enhances vascular inflammation by activating nuclear factor-kappa B (NF-κB)-mediated pro-inflammatory genes. Angiotensin II also downregulates peroxisome proliferator-activated receptors (PPARs) alpha and gamma, resulting in decrease of the anti-inflammatory potential of PPARs26.
The increased risk of psoriasis by the use of ACE inhibitors, although angiotensin II has aforementioned proinflammatory properties, is partially explained by the various activities of angiotensin II receptors. Angiotensin II has two major G protein-coupled receptor subtypes, the angiotensin II type 1 receptor (AT1R) and the angiotensin II type 2 receptor (AT2R)27. Binding of angiotensin II to AT1R increases inflammatory response whereas binding to AT2R decreases it. A recent study demonstrated that AT2R stimulation reduced expression of proinflammatory cytokine genes and activation of NF-κ in rheumatoid synovitis28. The use of ACE inhibitors prevents angiotensin II from binding to both receptors. Therefore, psoriasis due to ACE inhibitor use could involve more complex processes. A further study of this pathway is needed.
A previous study showed that Chinese patients had a notably higher risk of ACE inhibitor-related cough than Caucasians29. In a meta-analysis, the incidence of cough due to ACE inhibitor use has been reported to be 2.7-fold higher in East Asian populations compared to that in Caucasian populations30. These findings suggest the possible association between race and ACE inhibitor-related cough. Because bradykinin is seemingly the main mechanism of both ACE inhibitor-induced psoriasis and cough, the higher incidence of ACE inhibitor-related cough in Asians could explain the high risk of psoriasis in Asians.
Psoriasis is a multifactorial disease with both genetic and environmental contributing factors to its clinical manifestation31. In general, psoriasis is more common in colder northern regions than in tropical regions. Incidence among African blacks from the hot, wet west-central countries is less than that among African blacks from the milder, dry east-central nations31. Consistent with this finding, the climate subgroup analysis in our study showed a stronger association between ACE inhibitor use and psoriasis in dry climates.
This meta-analysis has some limitations that should be considered when interpreting the results. First, considerable heterogeneity was observed. Second, some confounding factors, which could affect the risk of psoriasis, could not be adjusted for. For example, most patients using ACE inhibitors for hypertension or other indications generally have more than one commitment medications, which could affect study results. Besides, within-study variation remained even after subgroup analysis due to the lack of adjusted odds ratio data in the included studies. Third, the duration and dosage of the drug were not specified. Fourth, the power of Begg's and Egger's test was low due to the number of included studies was small (less than 10). Therefore, the results of Begg's and Egger's test need to be interpreted with caution. Although we conducted subgroup analyses to look into the between-study variations, they still have within-study variations.
Nevertheless, this study is valuable in that it is the first systematic review and meta-analysis to evaluate the association between ACE inhibitor use and psoriasis. By combining the statistically insignificant findings of several studies, this study revealed significant associations between ACE inhibitor use and psoriasis. More prominent association between ACEI inhibitor use and psoriasis incidence was found in studies conducted in Asia or dry climate area. Based on our results, ACE inhibitor users should be carefully screened for cutaneous adverse events, particularly psoriasis.
Methods
Literature search strategy
The literature search included querying PubMed, Embase, and Web of Science for studies up to August 6, 2020, on the association between ACE inhibitor use and psoriasis incidence. The search included the following keywords: ((psoriasis OR psoria* OR psoriatic) AND ((ACEI OR ACE inhibitor* OR angiotensin converting enzyme inhibitor*) OR (antihypertensive agent* OR antihypertensive drug* OR antihypertensive medication*)). After removing duplicates, two researchers independently screened the titles and abstracts of all records to identify potentially eligible studies. Then, a full-text review was performed to determine final inclusion according to the eligibility criteria. In cases of disagreement, a consensus was reached by discussion.
Inclusion and exclusion criteria
The following criteria were used to identify eligible studies: (1) selected participants with psoriasis diagnosed using specific criteria and controls without psoriasis; (2) used case–control or cohort study designs; (3) provided sufficient information to calculate OR or hazard ratio (HR) and a 95% CI; and (4) published in English. Exclusion criteria included (1) conference or meeting abstracts, summaries, reviews, comments, letters, news, case reports, case series, and editorials; (2) studies without appropriate data; (3) studies that did not confirm whether drugs were taken before diagnosis; (4) studies not medically classified as psoriasis; or (5) repeated study population.
Data extraction
The following information was extracted from each study: name of the first author, publication year, country, study design, definition of patients with psoriasis, the number of patients with psoriasis (male %), the population size (male %), OR or HR with 95% CI, and comorbidities when available.
Quality assessment
NOS was used to assess the quality of selected studies32. The scoring system of this scale was based on three components: subject selection (0–4 points); comparability of study groups (0–2 points); and exposure (case–control study) or outcome (cohort study; 0–3 points). The highest NOS score available for each publication was 9 points.
Statistical analysis
We used ORs with 95% CIs as measures of effect size of ACE inhibitors for psoriasis. Statistical significance was analyzed using the z test, and a p value < 0.05 was considered statistically significant. It was assumed that the OR calculated from case–control studies approximates the HR in cohort studies. Heterogeneity was assessed using the Q test and I2 test33. Based on the heterogeneity results, either the fixed-effects model (Mantel–Haenszel method) or the random-effects model (when heterogeneity existed [p < 0.1, I2 > 50%]; DerSimonian-Laird method) was used to calculate effect size34,35.
The Egger regression test of the funnel plot was performed to identify publication bias36. A p value < 0.05 was considered statistically significant. Sensitivity analysis using the leave-one-out method was performed to assess the stability of results. Subgroup analyses by study design, continent and climate were also conducted if there were at least two studies in each subgroup. All statistical analyses were performed using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark) and R software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria). The paper was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines37.
References
Stavropoulos, P. G., Kostakis, P. G., Papakonstantinou, A. M., Panagiotopoulos, A. & Petridis, A. D. Coexistence of psoriasis and pemphigus after enalapril intake. Dermatology 207, 336–337 (2003).
Izzo, J. L. Jr. & Weir, M. R. Angiotensin-converting enzyme inhibitors. J. Clin. Hypertens. (Greenwich). 13, 667–675 (2011).
Thakor, P. et al. Ramipril-induced generalized pustular psoriasis: case report and literature review. Am. J. Ther. 17, 92–95 (2010).
Wolf, R., Tamir, A. & Brenner, S. Psoriasis related to angiotensin-converting enzyme inhibitors. Dermatologica 181, 51–53 (1990).
Gilleaudeau, P., Vallat, V. P., Carter, D. M. & Gottlieb, A. B. Angiotensin-converting enzyme inhibitors as possible exacerbating drugs in psoriasis. J. Am. Acad. Dermatol. 28, 490–492 (1993).
Hamlet, N. W., Keefe, M. & Kerr, R. E. Does captopril exacerbate psoriasis?. Br. Med. J. Clin. Res. Ed. 295, 1352 (1987).
Hauschild, T. T., Bauer, R. & Kreysel, H. W. Erstmanifestation einer eruptiv-exanthematischen Psoriasis vulgaris unter Captoprilmedikation [Initial manifestation of eruptive exanthematous psoriasis vulgaris caused by captopril medication]. Hautarzt 37, 274–277 (1986).
Wolf, R., Dorfman, B. & Krakowski, A. Psoriasiform eruption induced by captopril and chlorthalidone. Cutis 40, 162–164 (1987).
Antono, D., Grozdev, I., Pehlivanov, G. & Tsankov, N. Psoriatic erythroderma associated with enalapril. Skinmed 5, 90–92 (2006).
Tsankov, N., Angelova, I. & Kazandjieva, J. Drug-induced psoriasis. Recognition and management. Am. J. Clin. Dermatol. 1, 159–165 (2000).
Cohen, A. D. et al. Drug exposure and psoriasis vulgaris: case-control and case-crossover studies. Acta. Derm. Venereol. 85, 299–303 (2005).
Gerdes, S., Zahl, V. A., Knopf, H., Weichenthal, M. & Mrowietz, U. Comedication related to comorbidities: a study in 1203 hospitalized patients with severe psoriasis. Br. J. Dermatol. 159, 1116–1123 (2008).
Brauchli, Y. B., Jick, S. S., Curtin, F. & Meier, C. R. Association between beta-blockers, other antihypertensive drugs and psoriasis: population-based case-control study. Br. J. Dermatol. 158, 1299–1307 (2008).
Al-Mutairi, N., Al-Farag, S., Al-Mutairi, A. & Al-Shiltawy, M. Comorbidities associated with psoriasis: an experience from the Middle East. J. Dermatol. 37, 146–155 (2010).
Wu, S., Han, J., Li, W. Q. & Qureshi, A. A. Hypertension, antihypertensive medication use, and risk of psoriasis. JAMA Dermatol. 150, 957–963 (2014).
Jacob, L. & Kostev, K. Psoriasis risk in patients with type 2 diabetes in German primary care practices. Prim. Care. Diabetes. 11, 52–56 (2017).
Kim, H. N., Han, K., Song, S. W. & Lee, J. H. Hypertension and risk of psoriasis incidence: an 11-year nationwide population-based cohort study. PLoS ONE 13, e0202854 (2018).
Liu, J. M., Lin, C. Y., Chuang, H. C. & Hsu, R. J. No increased risk of psoriasis in patients receiving androgen deprivation therapy for prostate cancer: a 17-year population-based study. Ther. Clin. Risk Manag. 14, 1831–1837 (2018).
Ceconi, C. et al. Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE. Eur. J. Pharmacol. 577, 1–6 (2007).
Eriksen, J. G., Christiansen, J. J. & Asmussen, I. Pustulosis palmoplantaris udløst af angiotensinkonverterende enzymhaemmere [Postulosis palmoplantaris caused by angiotensin-converting enzyme inhibitors]. Ugeskr. Laeger. 157, 3335–3336 (1995).
Gauthier, K. M., Cepura, C. J. & Campbell, W. B. ACE inhibition enhances bradykinin relaxations through nitric oxide and B1 receptor activation in bovine coronary arteries. Biol. Chem. 394, 1205–1212 (2013).
Taddei, S. & Bortolotto, L. Unraveling the pivotal role of bradykinin in ACE inhibitor activity. Am. J. Cardiovasc. Drugs. 16, 309–321 (2016).
Wolf, R., Machtey, I., Feverman, E. J. & Creter, D. The kallikrein-kinin pathway in psoriasis. Preliminary observations. Biomedicine 35, 77–78 (1981).
Benigni, A., Cassis, P. & Remuzzi, G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol. Med. 2, 247–257 (2010).
Gonzalez-Villalobos, R. A. et al. Rediscovering ACE: novel insights into the many roles of the angiotensin-converting enzyme. J. Mol. Med. (Berl) 91, 1143–1154 (2013).
Tham, D. M. et al. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol. Genom. 11, 21–30 (2002).
Chang, Y. & Wei, W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 179, 137–145 (2015).
Terenzi, R. et al. Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci. Rep. 7, 13293 (2017).
Woo, K. S., Norris, R. M. & Nicholls, G. Racial difference in incidence of cough with angiotensin-converting enzyme inhibitors (a tale of two cities). Am. J. Cardiol. 75, 967–968 (1995).
McDowell, S. E., Coleman, J. J. & Ferner, R. E. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 332, 1177–1181 (2006).
Farber, E. M. & Nall, L. Psoriasis in the tropics. Epidemiologic, genetic, clinical, and therapeutic aspects. Dermatol. Clin. 12, 805–816 (1994).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 (2010).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Mantel, N. & Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer. Inst. 22, 719–748 (1959).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 45, 139–145 (2015).
Egger, M., Davey, S. G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
All the authors have made substantial contributions to the conception of the study. G.S., J.Y.K. and H.S.G. contributed to designing the study. G.S. and H.Y.Y. contributed to acquisition and analysis of data. J.Y.K., J.Y. and H.S.G. contributed to interpretation of data. G.S., J.Y.K and H.Y.Y contributed to drafting of the manuscript. J.Y. and H.S.G. contributed to critical revision of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, G., Kim, J.Y., Yoon, H.Y. et al. A systematic review and meta-analysis of angiotensin-converting enzyme inhibitor use and psoriasis incidence. Sci Rep 11, 10037 (2021). https://doi.org/10.1038/s41598-021-89490-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89490-z
- Springer Nature Limited