Abstract
Spondyloarthritis (SpA) is characterized by chronic inflammation and structural damage involving spine and peripheral joints. Monocytes, as part of innate immune system, following migration into affected tissue, may play a role in the pathogenesis of SpA. Here, potential associations between osteogenesis-linked gene expression profile in particular monocyte subpopulations and clinical signs of SpA were investigated. The 20 patients with axial and 16 with peripheral SpA were enrolled in the study. Monocyte subpopulations (classical—CD14++CD16−, intermediate—CD14++CD16+ and non-classical—CD14+CD16++) were isolated from blood using flow cytometry and gene expression analysis was performed using real-time PCR method and TaqMan Array, Human Osteogenesis, Fast 96-well plates. Next, the characteristic clinical features shared by axial and peripheral SpA were analyzed in the context of the expression of selected genes in the three subpopulations of monocytes. We demonstrated that expression of VEGFA in classical and MSX2 in non-classical monocytes were associated with the number of swollen and painful peripheral joints of SpA patients. We conclude that monocytes may contribute to the development of peripheral arthritis in SpA patients. This might be possible through subpopulation specific effects, linking number of inflamed joints with expression of VEGFA in classical monocytes and MSX2 in non-classical monocytes.
Similar content being viewed by others
Introduction
Spondyloarthritis (SpA) represents a group of second most prevalent inflammatory rheumatic disorders (ca. 0.2–1.6% depending on geographic area)1 characterized by chronic inflammation and structural damage involving axial and peripheral skeleton. Recent research in SpA has been focused on the phenotypic presentations and pathophysiology of SpA subgroups (ie. non-radiographic axial spondyloarthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis, arthritis in inflammatory bowel diseases and undifferentiated spondyloarthritis) exploring, whether SpA is a single disease with various clinical expression or rather a group of distinct clinical entities sharing common signs and symptoms2.
Genetic, immunopathologic, and clinical evidence indicate that despite common downstream pathways, mediated e.g. by macrophage-derived TNFα, inflammation in SpA is driven and maintained by different cellular and molecular mediators3,4. Moreover, it has been proposed that SpA is an autoinflammatory disease driven rather by innate immune cells, than a genuine autoimmune disease triggered by self-reactive T and/or B lymphocytes5. The phenotypic subclassification of SpA is usually based on extraarticular signs (psoriasis and inflammatory bowel disease), pathogenesis (reactive arthritis) or outcomes (ankylosing spondylitis)2. Nevertheless, all phenotypes share similar axial (sacroiliitis, spondylitis, back pain) or peripheral (arthritis, enthesitis, dactylitis) manifestations, and therefore SpA might be classified as one of two subforms with different pathophysiology, with predominant involvement of axial or peripheral skeleton1. In other words, there is a proposal to define SpA by its pathophysiology rather than by its phenotypic presentation, since the emerging data from immunopathology studies and clinical trials suggest that axial (axSpA) and peripheral (pSpA) spondyloarthritis might be driven by different mechanisms and respond differently to treatment, supporting the classification of SpA according to the presence of axial or peripheral disease2. However, considering SpA as a possible single entity the question remains whether shared clinical features of axSpA and pSpA could have common triggers, particularly during the early phase before chronic compensatory and therapy effects occur. Therefore, it is interesting to explore mechanisms leading to SpA manifestations shared by axSpA and pSpA. They are likely to be most variable and at the same time most informative at an early stage of the disease.
In such setting, pathophysiological role of monocyte subpopulations as a source of pro- and anti-inflammatory cytokines, bone remodeling proteins and other biologically active compounds is not fully elucidated. Moreover, there is some evidence that monocytes may be the source of novel bone forming cells (“monoosteophils”)6.
The aim of our study was to link characteristic clinical features seen in axSpA and pSpA with different expression of selected genes in three subpopulations of monocytes isolated from blood of SpA patients. We focused on the manifestations which could be expressed both in axSpA and pSpA—i.e. arthritis, enthesitis, dactylitis and inflammatory back pain. This might help to understand how monocytes and possibly derived from them macrophages and osteoclasts might drive particular pathological processes, which are then interpreted as characteristic clinical signs of both axSpA and pSpA.
Results
Demographic, clinical and laboratory data
Table 1 presents characteristics of patients. Briefly, median age (years, IQR) of axSpA patients was 33.5 (29.7–39.7) and pSpA patients was 35.5 (31–38.5). Median disease duration (years, IQR) was 7 (5–10.7) for axSpA and 3 (2–9.5) for pSpA patients. 95% of axSpA and 38% of pSpA patients were HLA-B27 positive.
Fifteen axSpA patients fulfilled mNY criteria for ankylosing spondylitis.
Selected gene and probe panels are differently expressed among three monocyte subpopulations
To explore the expression of mRNAs tested in our panel across monocyte subpopulations in SpA we utilized microarray expression data generated in a previous study by Metcalf et al.7 (18 individuals, 3 subsets of monocytes per sample). We acknowledged 94 genes (154 probes) which constituted our mRNA SpA panel and then using a Principal Component Analysis we identified 3 clusters of samples—each corresponding to different subpopulation of monocytes as shown in Fig. 1.
The results of PC analysis of microarray expression data in classical (black dots), non-classical (green dots) and intermediate (red dots) monocytes. Only probes in genes whose expression was initially measured (and expressed well on the array) were selected for this analysis. PC1 and PC2 are the first two principal components estimated based on the expression data with the aid of the prcomp function in R49.
Associations between mRNAs and clinical signs
We analyzed whether mRNAs identified in different monocyte subpopulations were correlated with clinical signs of axSpA and pSpA. The following were selected: (1) inflammatory back pain (total back pain, BASDAI question 2, range 1–10), and (2) number of swollen joints (out of 66 total joint count and out of 28 joint count from DAS28 score), (3) number of painful joints (out of 68 total joint count and out of 28 joint count from DAS28 score), (4) presence of enthesitis and (5) presence of dactylitis.
Expression of VEGFA in classical monocytes is associated with the number of swollen and painful joints
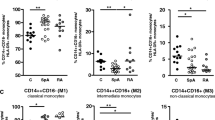
We found Vascular Endothelial Growth Factor A (VEGFA) mRNA in classical monocytes to be positively associated with measurement of joint involvement, i.e. number of swollen joints from total joint count and number of swollen and painful joints from DAS28 score, with False Discovery Rate (FDR) < 0.05 for each feature. The most robust association, with FDR < 0.001 was observed for the number of swollen joints from DAS28 score (Table 2).
Expression of MSX2 in non-classical monocytes is also associated with peripheral arthritis
We identified the Muscle Segment Homeobox 2 (MSX2) mRNA expression in non-classical monocytes as positively associated with measurements of peripheral arthritis, i.e. number of swollen joints from total joint count and number of swollen and painful joints from DAS28 score, under the FDR < 0.08 for each feature (Table 2).
There were no significant associations between the expression of selected genes in the intermediate subset of monocytes with the number of swollen and painful joints. There were either no associations between the expression of VEGFA or MSX2 mRNAs in subsequent monocytes subpopulations with other SpA clinical features, e.g. inflammatory back pain, enthesitis or dactylitis.
Discussion
Here, we provide evidence that monocytes, cells of innate immune system, may contribute to the development of peripheral arthritis in SpA and therefore might be at play when considering chronic inflammation, one of the most significant features of this disease.
There is a substantial evidence concerning the role of the innate immune system in the pathogenesis of a variety of SpA features, including chronic inflammation, repair and new bone formation8,9. It seems that peripheral blood monocytes in SpA are functionally primed and/or functionally reprogrammed by not yet well identified factor(s) and exhibit molecular or cellular features characteristic for SpA10,11,12. Monocytes are classified into three subpopulations—classical (CD14++CD16−), intermediate (CD14++CD16+) and non-classical (CD14+CD16++); the latter two subpopulations being referred to as “proinflammatory”13. The “proinflammatory” subsets form app. 5–15% of circulating monocytes, express and secrete upon stimulation by various factors large amounts of pro-inflammatory cytokines, e.g. TNFα, interleukins (IL)—IL-12 and IL-1, but insignificant amount of anti-inflammatory IL-1014. Contrariwise, classical monocytes produce relatively small amounts of TNFα, but they are a robust source of IL-1015. Under physiological conditions most of classical monocytes (ca. 80–90%) leave the bloodstream after a circulating lifespan of 1 day, whereas remaining fraction further mature into intermediate cells and finally convert (in app. 12 days) to non-classical monocytes before leaving the circulation16. Then extravasated monocytes supplement the population of resting tissue macrophages, but they may differ functionally. These cells may influence inflammatory processes by factors released inside blood vessels or locally, upon their migration17.
Currently, there are only a few studies (based on the analysis of single blood samples) that investigated differences in transcriptome/proteome profile of monocyte subpopulations isolated from healthy individuals [e.g.18,19,20,21,22,23,24,25]. These studies indicate, on molecular basis, the significant differences in genetic profiles between these three monocyte subpopulations, confirming and extending most of the former phenotypic and functional observations. However, there is no data so far comparing gene expression in monocyte subsets in SpA patients exhibiting axial or peripheral signs.
Moreover, we have previously shown, that there were no differences in numbers of classical, intermediate and non-classical monocytes between axSpA and pSpA patients. There were also no differences concerning numbers of intermediate and non-classical monocytes between SpA patients and controls. The only statistically significant difference was seen between SpA patients and controls regarding classical monocytes26.
Vascular endothelial growth factor-A (VEGFA) is one of the most important growth factors engaged in vascular development and angiogenesis. Since bone is a highly vascularized organ and angiogenesis plays an important role in osteogenesis, it has been established that VEGFA also influences skeletal development and postnatal bone repair27. Process of bone remodeling is based on a balance between bone formation and resorption28. Disturbance of this balance may strongly depend on both osteoclastic and osteoblastic activity. Liu et al.29 suggested, that VEGFA and TNFα might directly participate in the differentiation of fibroblasts into osteoblasts, and anti-VEGFA has been suggested as a possible new therapy preventing osteogenesis in SpA patients28.
We have shown that VEGFA-mRNA expression in classical monocytes positively correlated with the number of swollen and painful joints. Our results also confirmed higher VEGFA level in SpA patients’ sera when compared to healthy blood donors (Supplementary Fig. 1) supporting the previous findings by Lin et al.30. This may identify the classical monocyte subpopulation as the main source of VEGFA, which, upon migration to the synovium of peripheral joints, may promote local inflammation in SpA patients. It was already shown that VEGFA serum and synovial fluid levels are elevated in patients with ankylosing spondylitis expressing features of peripheral arthritis31,32. Moreover, VEGFA may be secreted by various cell types, including macrophages, which are present in the synovial membrane and entheses in patients with SpA, but no association with particular monocyte subpopulation (as a source of particular tissue macrophages) has been specified32.
Whereas the role of VEGF in the etiopathogenesis of SpA may be pragmatically interpreted on the ground of former scientific observations and is clinically proven, our second finding referring to the MSX2 is not so easy interpretable. MSX2 is a transcription factor with a homeobox domain, presumably involved in bone development and ectopic calcifications, although its role in these processes is still controversial33,34,35,36,37,38,39,40,41,42. Furuichi et al. examined a total of 45 single nucleotide polymorphisms (SNPs) in 15 genes by sequential screening and reported promising evidence for the association between MSX2 polymorphisms and SpA in Japanese population33. Moreover, in the basic studies involving animal models, the MSX2 knockout mice displayed remarkable decrease in mineralization of the axial skeleton, reduced proliferation of osteoprogenitors defecting skull ossification and abnormal calvarial development34, whereas transgenic mice overexpressing MSX2 showed enhanced proliferation of calvarial cells35,36. A loss-of-function mutation of MSX2 in humans, which reduces DNA binding activity, causes defect in skull ossification37. These observations contrast with a gain-of-function mutation of MSX2, which results in an autosomal dominant disorder, Boston-type craniosynostosis38,39. These findings demonstrate that MSX2 expression is critical for human skull development and suggest its positive ossific role in bone development. However, MSX2 protein suppresses the expression of bone marker genes, including RUNX2 (a master regulator of osteoblast differentiation) and osteocalcin, and negatively regulates bone development and ectopic calcification40,41,42. The roles of MSX2 may vary depending on cell type and/or cell differentiation stage.
We have demonstrated that MSX2-mRNA overexpression in non-classical monocytes is positively associated with the number of swollen and painful joints, and therefore question the canonical role of this protein expressed preferentially in non-classical subset of monocytes. It was shown that TNFα may induce MSX2 expression and MSX2 mediates the inhibitory action of TNFα in osteoblast differentiation43,44. It may not be excluded that MSX2 expression in non-classical monocytes is secondary to their proinflammatory functions related to TNFα auto- or paracrine action. Moreover, upon migration into soft tissue, non-classical monocytes may contribute to local inhibition of the BMP2-regulated osteoblast differentiation within inflamed peripheral joints44 and therefore being possibly involved in new bone formation and joint remodeling. These concepts must be verified applying the appropriate mouse model (e.g. SKG mice) of SpA associated peripheral arthritis, which is currently underway.
Our study has some limitations. Obviously, the number of patients is small, but it is our belief that this pilot observation is interesting although requires further investigation. Also, this is a cross-sectional study and we do not know whether the discovered findings are durable enough to be attributed to chronic pain and synovitis characteristic for pSpA. Finally, we were exploring the peripheral blood monocytes only, having no matching data considering the local environment of synovial tissue.
Conclusions
Our data suggest that monocytes may contribute to the development of peripheral arthritis in SpA patients. This might be due to the subpopulation specific effects, linking the number of swollen and painful joints with the expression of VEGFA in classical monocytes and MSX2 in non-classical monocytes. We argue for the first time that overexpression of both proteins in classical and non-classical subsets of monocytes may be linked with the inflammatory process within peripheral joints in SpA patients.
Methods
Patients
Thirty-six patients with SpA (20 axSpA and 16 pSpA) according to the Assessment of SpondyloArthritis International Society classification criteria45,46 were enrolled in the study. Patients were under 45 years, naive to synthetic, synthetic-targeted or biologic Disease Modifying Anti-Rheumatic Drugs (DMARDs) and without administration of systemic glucocorticosteroids. Patients provided a signed informed consent and the study protocol was approved by the local bioethics committee and all methods were performed in accordance with the relevant guidelines and regulations.
Isolation of monocytes and their subsets
Monocyte subpopulations were isolated from peripheral blood mononuclear cells (PBMC) obtained from SpA patients. PBMC were isolated from EDTA-treated whole peripheral blood by the standard Pancoll human (PAN-Biotech, Aidenbach, Germany) density gradient centrifugation. PBMC were washed in PBS (Sigma-Aldrich, Saint Louis, USA) and then monocyte subsets (classical—CD14++CD16−, intermediate—CD14++CD16+ and non-classical—CD14+CD16++) were isolated using flow cytometry cell sorting. The following monoclonal antibodies (mAbs) were used to stain monocytes: anti-CD14-FITC (clone MφP9, BD Biosciences, San Jose, CA, USA), anti-CD16-PE (clone 3G8, BD Biosciences) and anti-HLA-DR-PerCP (clone L243, BD Biosciences), in 1:25 dilution v/v stained and gated as previously described by us and others47,48. The stained monocytes were then incubated for 30 min at 4 °C after which they were sorted using the FACSAria II cell sorter (BD Biosciences). Sorter was equipped with 488 nm laser for excitation of FITC, PE and PerCP. The following band-pass filters were used for the measurement of fluorescence: 530/30 for FITC, 582/42 for PE and 695/40 for PerCP. After isolation, the cells were washed in PBS, centrifuged for 10 min at 350×g and kept frozen at − 80 °C until RNA isolation.
Gene expression analysis using real-time PCR
Gene expression analysis was performed using real-time PCR method and TaqMan Array, Human Osteogenesis, Fast 96-well plates, # 4418741 (coverage of 92 osteogenesis associated genes and 4 endogenous control genes, Supplementary Table 1) (Thermo Fisher Scientific, Waltham, MA, USA). RNAs were isolated using miRVana microRNA isolation kit (Thermo Fisher Scientific); isolated RNA were transcribed into cDNA using Superscript IV VILO mastermix (Thermo Fisher Scientific). Next, cDNA was used for the assessment of gene expression profile using the TaqMan Array Human Osteogenesis plate and QuantStudio 3 Real-Time PCR instrument (Thermo Fisher Scientific) according to manufacturer’s protocol.
Statistical methodology
All statistical analyses, as well as data pre-processing, normalization and visualization was done in R (version 3.5.2)49. The expression was calculated as the Ct values and filtered according to manufacturer’s instructions (only values with high confidence scores were used for further analyses). Subsequently, data was divided into three panels—each corresponding to a different subpopulation of monocytes: (1) classical, (2) intermediate, and (3) non-classical. The expression values were further normalized using the quantile normalization method as implemented in the package ‘preprocessCore’ (version 1.44.0). For the target statistical analysis, only genes with at least 5 observations in a given panel were used. In return, 88 genes were selected for further analysis in 37 samples of non-classical monocytes, and 38 samples of classical as well as intermediate monocytes. The association between axial or peripheral signs and gene expression in subpopulations of monocytes was tested via a simple linear model (with intercept and one predictor only) with empirical Bayes correction as implemented in the package ‘limma’ (version 3.38.3).
Ethics approval and consent to participate
The study protocol was approved by the local bioethics committee (KBET/252/B/2012, Bioethics Committee of the Jagiellonian University, Podwale 3 Str., 31-118 Krakow, Poland). All included patients gave their informed written consent.
References
Stolwijk, C., van Onna, M., Boonen, A. & van Tubergen, A. Global prevalence of spondyloarthritis: A systematic review and meta-regression analysis. Arthritis Care Res. https://doi.org/10.1002/acr.22831 (2016).
Baeten, D., Breban, M., Lories, R., Schett, G. & Sieper, J. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype?. Arthritis Rheum. https://doi.org/10.1002/art.37829 (2013).
Dougados, M. & Baeten, D. Spondyloarthritis. Lancet https://doi.org/10.1016/S0140-6736(11)60071-8 (2011).
Lories, R. J. U., Luyten, F. P. & de Vlam, K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res. Ther. https://doi.org/10.1186/ar2642 (2009).
Ambarus, C., Yeremenko, N., Tak, P. P. & Baeten, D. Pathogenesis of spondyloarthritis: Autoimmune or autoinflammatory?. Curr. Opin. Rheumatol. https://doi.org/10.1097/BOR.0b013e3283534df4 (2012).
Zhang, Z. & Shively, J. E. Generation of novel bone forming cells (Monoosteophils) from the cathelicidin-derived peptide LL-37 treated monocytes. PLoS ONE https://doi.org/10.1371/journal.pone.0013985 (2010).
Metcalf, T. U. et al. Human monocyte subsets are transcriptionally and functionally altered in aging in response to pattern recognition receptor agonists. J. Immunol. https://doi.org/10.4049/jimmunol.1700148 (2017).
Wright, C. et al. Ankylosing spondylitis monocytes show upregulation of proteins involved in inflammation and the ubiquitin proteasome pathway. Ann. Rheum. Dis. https://doi.org/10.1136/ard.2008.097204 (2009).
Smolen, J. S. et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2017-211734 (2018).
Korkosz, M. et al. Sera of patients with axial spondyloarthritis (axSpA) enhance osteoclastogenic potential of monocytes isolated from healthy individuals. BMC Musculoskelet. Disord. https://doi.org/10.1186/s12891-018-2356-4 (2018).
Zhai, Y. et al. TNFAIP3-DEPTOR complex regulates inflammasome secretion through autophagy in ankylosing spondylitis monocytes. Autophagy https://doi.org/10.1080/15548627.2018.1458804 (2018).
Conrad, K., Wu, P., Sieper, J. & Syrbe, U. In vivo pre-activation of monocytes in patients with axial spondyloarthritis. Arthritis Res. Ther. https://doi.org/10.1186/s13075-015-0694-2 (2015).
Ziegler-Heitbrock, L. et al. Nomenclature of monocytes and dendritic cells in blood. Blood https://doi.org/10.1182/blood-2010-02-258558 (2010).
Skrzeczyńska-Moncznik, J. et al. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand. J. Immunol. https://doi.org/10.1111/j.1365-3083.2007.02051.x (2008).
Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. https://doi.org/10.1189/jlb.0806510 (2007).
Patel, A. A. et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. https://doi.org/10.1084/jem.20170355 (2017).
Ożańska, A., Szymczak, D. & Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. https://doi.org/10.1111/sji.12883 (2020).
Mobley, J. L., Leininger, M., Madore, S., Baginski, T. J. & Renkiewicz, R. Genetic evidence of a functional monocyte dichotomy. Inflammation https://doi.org/10.1007/s10753-007-9036-0 (2007).
Zhao, C. et al. Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. J. Proteome Res. https://doi.org/10.1021/pr900364p (2009).
Zhao, C. et al. The CD14+/lowCD16+ monocyte subset is more susceptible to spontaneous and oxidant-induced apoptosis than the CD14+CD16-subset. Cell Death Dis. https://doi.org/10.1038/cddis.2010.69 (2010).
Ancuta, P. et al. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16− monocyte subsets. BMC Genomics https://doi.org/10.1186/1471-2164-10-403 (2009).
Ingersoll, M. A. et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood https://doi.org/10.1182/blood-2009-07-235028 (2010).
Wong, K. L. et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood https://doi.org/10.1182/blood-2010-12-326355 (2011).
Zawada, A. M. et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood https://doi.org/10.1182/blood-2011-01-326827 (2011).
Frankenberger, M. et al. Transcript profiling of CD16-positive monocytes reveals a unique molecular fingerprint. Eur. J. Immunol. https://doi.org/10.1002/eji.201141907 (2012).
Guła, Z. et al. The absolute number of circulating nonclassical (CD14+CD16++) monocytes negatively correlates with DAS28 and swollen joint count in patients with peripheral spondyloarthritis. Polish Arch. Intern. Med. https://doi.org/10.20452/pamw.4142 (2017).
Hu, K. & Olsen, B. R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. https://doi.org/10.1002/dvdy.24463 (2017).
Lacout, A., Carlier, R. Y., El Hajjam, M. & Marcy, P. Y. VEGF inhibition as possible therapy in spondyloarthritis patients: Targeting bone remodelling. Med. Hypotheses https://doi.org/10.1016/j.mehy.2017.02.009 (2017).
Liu, K. G., He, Q. H., Tan, J. W. & Liao, G. J. Expression of TNF-α, VEGF, and MMP-3 mRNAs in synovial tissues and their roles in fibroblast-mediated osteogenesis in ankylosing spondylitis. Genet. Mol. Res. https://doi.org/10.4238/2015.June.18.28 (2015).
Lin, T. et al. Elevated serum level of IL-27 and VEGF in patients with ankylosing spondylitis and associate with disease activity. Clin. Exp. Med. https://doi.org/10.1007/s10238-014-0281-x (2015).
Przepiera-Będzak, H., Fischer, K. & Brzosko, M. Serum VEGF, EGF, basic FGF, and acidic FGF levels and their association with disease activity and extra-articular symptoms in ankylosing spondylitis. Polish Arch. Intern. Med. https://doi.org/10.20452/pamw.3339 (2019).
Laloux, L. et al. Immunohistological study of entheses in spondyloarthropathies: Comparison in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. https://doi.org/10.1136/ard.60.4.316 (2001).
Furuichi, T. et al. Association of the MSX2 gene polymorphisms with ankylosing spondylitis in Japanese. J. Hum. Genet. https://doi.org/10.1007/s10038-008-0265-3 (2008).
Satokata, I. et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. https://doi.org/10.1038/74231 (2000).
Liu, Y. H. et al. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: A possible mechanism for MSX2-mediated craniosynostosis in humans. Dev. Biol. https://doi.org/10.1006/dbio.1998.9114 (1999).
Cheng, S. L., Shao, J. S., Cai, J., Sierra, O. L. & Towler, D. A. Msx2 exerts bone anabolism via canonical Wnt signaling. J. Biol. Chem. https://doi.org/10.1074/jbc.M800851200 (2008).
Wilkie, A. O. M. et al. Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat. Genet. https://doi.org/10.1038/74224 (2000).
Jabs, E. W. et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell https://doi.org/10.1016/0092-8674(93)90379-5 (1993).
Ma, L., Golden, S., Wu, L. & Maxson, R. The molecular basis of Boston-type craniosynostosis: The Pro148→His mutation in the N-terminal arm of the MSX2 homeodomain stabilizes DNA binding without altering nucleotide sequence preferences. Hum. Mol. Genet. https://doi.org/10.1093/hmg/5.12.1915 (1996).
Shirakabe, K., Terasawa, K., Miyama, K., Shibuya, H. & Nishida, E. Regulation of the activity of the transcription factor Runx2 by two homeobox proteins, Msx2 and Dlx5. Genes Cells https://doi.org/10.1046/j.1365-2443.2001.00466.x (2001).
Hassan, M. Q. et al. Dlx3 transcriptional regulation of osteoblast differentiation: Temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol. Cell. Biol. https://doi.org/10.1128/mcb.24.20.9248-9261.2004 (2004).
Newberry, E. P., Boudreaux, J. M. & Towler, D. A. Stimulus-selective inhibition of rat osteocalcin promoter induction and protein-DNA interactions by the homeodomain repressor Msx2. J. Biol. Chem. https://doi.org/10.1074/jbc.272.47.29607 (1997).
Lee, H. L., Woo, K. M., Ryoo, H. M. & Baek, J. H. Tumor necrosis factor-α increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem. Biophys. Res. Commun. https://doi.org/10.1016/j.bbrc.2009.12.027 (2010).
Lee, H. L. et al. Msx2 mediates the inhibitory action of TNF-α on osteoblast differentiation. Exp. Mol. Med. https://doi.org/10.3858/emm.2010.42.6.045 (2010).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. https://doi.org/10.1136/ard.2009.108233 (2009).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. https://doi.org/10.1136/ard.2010.133645 (2011).
Korkosz, M., Bukowska-Strakova, K., Sadis, S., Grodzicki, T. & Siedlar, M. Monoclonal antibodies against macrophage colony-stimulating factor diminish the number of circulating intermediate and nonclassical (CD14++CD16+/CD14+CD16++) monocytes in rheumatoid arthritis patient. Blood https://doi.org/10.1182/blood-2012-02-412551 (2012).
Heimbeck, I. et al. Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytom. Part A https://doi.org/10.1002/cyto.a.20942 (2010).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018) https://www.R-project.org/.
Funding
This study was supported by the grant from National Science Center (NCN; Poland), No: 2013/09/B/NZ6/02545.
Author information
Authors and Affiliations
Contributions
M.St. performed experiments, collected data and wrote the manuscript; M.Se. analyzed results, performed statistical analysis and wrote the manuscript; M.K. recruited patients, analyzed results and edited the manuscript; Z.G. recruited patients and analyzed results; R.S. collected data and edited the manuscript; K.W. performed experiments and collected data; M.R-Z. performed experiments and collected data; M.L. performed experiments and collected data; M.C. discussed and edited the manuscript; J.C. discussed and edited the manuscript; J.B. discussed and edited the manuscript; A.G. performed experiments; K.W.L. performed experiments; P.W. analyzed results and edited the manuscript; M.Si. developed the scientific concept of the research, supervised the project, analyzed results and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stec, M., Seweryn, M., Korkosz, M. et al. Expression of VEGFA-mRNA in classical and MSX2-mRNA in non-classical monocytes in patients with spondyloarthritis is associated with peripheral arthritis. Sci Rep 11, 9693 (2021). https://doi.org/10.1038/s41598-021-89037-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89037-2
- Springer Nature Limited