Abstract
Excess fructose consumption contributes to development obesity, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD). Uric acid (UA), a metabolite of fructose metabolism, may have a direct role in development of NAFLD, with unclear mechanism. This study aimed to evaluate role of fructose and UA in NAFLD and explore mechanisms of allopurinol (Allo, a UA lowering medication) on NAFLD in Otsuka Long-Evans Tokushima Fatty (OLETF) rats fed a high fructose diet (HFrD), with Long-Evans Tokushima Otsuka (LETO) rats used as a control. There were six groups: LETO, LETO-Allo, OLETF, OLETF-Allo, OLETF-HFrD, and OLETF-HFrD-Allo. HFrD significantly increased body weight, epididymal fat weight, and serum concentrations of UA, cholesterol, triglyceride, HbA1c, hepatic enzymes, HOMA-IR, fasting insulin, and two hour-glucose after intraperitoneal glucose tolerance tests, as well as NAFLD activity score of liver, compared to the OLETF group. Allopurinol treatment significantly reduced hepatic steatosis, epididymal fat, serum UA, HOMA-IR, hepatic enzyme levels, and cholesterol in the OLETF-HFrD-Allo group. Additionally, allopurinol significantly downregulated expression of lipogenic genes, upregulated lipid oxidation genes, downregulated hepatic pro-inflammatory cytokine genes, and decreased ER-stress induced protein expression, in comparison with the OLETF-HFrD group. In conclusion, allopurinol ameliorates HFrD-induced hepatic steatosis through modulation of hepatic lipid metabolism, inflammation, and ER stress pathway. UA may have a direct role in development of fructose-induced hepatic steatosis, and allopurinol could be a candidate for prevention or treatment of NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease that occurs in patients with modest or no alcohol use and no viral hepatitis. It is characterized by the accumulation of excess lipids inside hepatocytes1. NAFLD ranges from simple steatosis, which is a benign condition, to nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatic failure1. NAFLD usually emerges alongside insulin resistance associated medical conditions (i.e., obesity, type 2 diabetes, and dyslipidemia), and is considered a hepatic manifestation of metabolic syndrome2. A dramatic increase in worldwide obesity prevalence has also resulted in increased NAFLD prevalence3. NAFLD prevalence rates in Western and Asian countries during the past 20–30 years have been approximately 20–30% and 25% of the general population, respectively4. Currently, NAFLD is one of the most common liver diseases, and its clinical burden is expected to rise in the future.
Specific dietary factors, including high levels of saturated fats, cholesterol, or fructose, contribute to the development of obesity, metabolic syndrome, and NAFLD5. In particular, consumption of fructose, a dietary monosaccharide, has increased over the last century due to the usage of high-fructose corn syrup as a major sweetener in soft drinks6. Two pathogenic mechanisms have been suggested for the HFrD-induced development of NAFLD. First, fructose is lipogenic during its metabolism in the liver7. Second, uric acid, a metabolite of fructose metabolism, may have an additional role in the development of fatty liver8. Fructose is primarily metabolized in the liver, where it is first phosphorylated by fructokinase. This process requires ATP, which leads to increased intracellular uric acid concentrations, resulting in hyperuricemia9. Uric acid generation is a unique feature of fructose metabolism, distinguishing it from glucose metabolism. Previous research has shown that chronic fructose consumption significantly increases both fasting plasma uric acid and 24 h uric acid concentrations in humans10. Furthermore, it has been shown in cross-sectional human studies that hyperuricemia is independently associated with hepatic steatosis11 and serum uric acid (UA) concentration is an independent risk factor for NAFLD12.
Lifestyle modification to reduce body weight is the primary recommended treatment for NAFLD, similar to metabolic syndrome treatment. Weight loss through caloric restriction and exercise is safe and effective in the management of NAFLD, but it is difficult to reach and maintain weight loss target13. Several pharmacological agents, including ursodeoxycholic acid14, pentoxifylline15, statins16, thiazolidinediones17, and Vitamin E therapy18 have been employed in attempts to improve liver histology. Although many drugs are currently being developed and investigated, there are no pharmacological agents approved for NAFLD treatment19.
Uric acid may directly affect NAFLD development in HFrD-induced hepatic steatosis, and as such, uric acid level lowering treatment may improve NAFLD. However, the pathogenic molecular mechanism of uric acid in the development of NAFLD has not been completely elucidated.
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is a well-known animal model of metabolic syndrome, including insulin resistance, obesity, hypertension, dyslipidemia, hyperglycemia, and hepatic steatosis20. Hyperuricemia and increased urinary UA excretion have been reported in OLETF rats21. However, as hyperglycemia and hyperuricemia in OLETF rats are mild, we treated OLETF rats with a high fructose diet (OLETF-HFrD) to exaggerate hyperuricemia and investigated the effect of allopurinol on metabolic parameters and NAFLD in LETO, OLETF, and OLETF-HFrD rats.
Therefore, this study aimed to evaluate the role of fructose and uric acid on the development of fatty liver disease and explore the effects and mechanisms of allopurinol, a uric acid lowering treatment, on NAFLD using HFrD-fed OLETF rats and OLETF rats.
Results
Effects of HFrD and allopurinol treatment on metabolic parameters
Body weight, organ weights, and serum biochemical parameters were measured after the 16-week study period in the LETO, LETO-Allo, OLETF, OLETF-Allo, OLETF-HFrD, and OLETF-HFrD-Allo groups (Table 1). Body weight, as well as serum UA, cholesterol, triglyceride (TG), HbA1C, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels, were significantly higher in OLETF rats than in LETO rats (P < 0.05). These parameters, in addition to epididymal fat weight, of the OLETF-HFrD group were significantly higher than those of the OLETF group (P < 0.05). There was no significant difference in the body weight and serum levels of lipid, HbA1c, and hepatic enzymes between the LETO and LETO-Allo groups. In addition, in the OLETF-Allo group, these parameters were not significantly different from those in the OLETF group, even though the uric acid level of the OLETF-Allo group was significantly lower than that of the OLETF group (P < 0.05). However, rats in the OLETF-HFrD-Allo group had significantly lower body weight, epididymal fat weight, and serum levels of UA, cholesterol, TG, and ALT, compared to the OLETF-HFrD group (P < 0.05).
Effects of HFrD and allopurinol treatment on glucose metabolism parameters
According to the intraperitoneal glucose tolerance test (IPGTT) results, fasting glucose levels of the OLETF-HFrD and OLETF-HFrD-Allo groups were significantly higher than those of LETO rats (P < 0.05). The glucose levels at 30, 60, and 90 min and AUC (0–120) after intraperitoneal glucose loading in OLETF, OLETF-Allo, OLETF-HFrD and OLETF-HFrD-Allo groups were significantly higher than those of LETO rats (P < 0.05). Glucose levels at 60, 90, and 120 min and AUC (0–120) after intraperitoneal glucose loading in the OLETF-HFrD and OLETF-HFrD-Allo groups were significantly higher than those of OLETF rats (P < 0.05). However, the glucose levels of the allopurinol treatment groups were not significantly different from those of each control group. (LETO vs. LETO-Allo, OLETF vs. OLETF-Allo, OLETF-HFrD vs. OLETF-HFrD-Allo) (Fig. 1A and Table 2).
Effects of HFrD and allopurinol treatment on glucose tolerance test. (A) Glucose concentrations and (B) insulin concentrations in LETO, LETO-Allo, OLETF, OLETF-Allo, OLETF-HFrD, and OLETF-HFrD-Allo group during intraperitoneal glucose tolerance test (IPGTT). IPGTT were conducted. 2 g/kg glucose was intraperitoneally administered to rats after overnight fasting. And blood samples were collected for the measurement of biochemical parameters at 0 min, 30 min, 60 min, 90 min, and 120 min after glucose injection. Data are expressed as mean ± SE. †P < 0.05, versus the LETO group. *P < 0.05, versus OLETF group. LETO, Long-Evans Tohushima Otsuka; LETO-Allo, LETO rats treated with allopurinol; OLETF, Otsuka Long-Evans Tokushima Fatty rats; OLETF-Allo, OLETF rats treated with allopurinol; OLETF-HFrD, OLETF rats fed a high fructose diet; OLETF-HFrD-Allo rats, OLETF rats fed a high fructose diet treated with allopurinol.
Fasting insulin levels in the OLETF-HFrD and OLETF-HFrD-Allo groups were significantly higher than those in LETO rats (P < 0.05). The insulin levels at 30, 60, and 90 min, as well as AUC (0–120) after intraperitoneal glucose loading, IGI30, and HOMA-beta, in the OLETF and OLETF-Allo groups were significantly lower than those of LETO rats (P < 0.05). The insulin levels at 30, 60, 90, and 120 min, and AUC (0–120) after glucose loading, HOMA-beta, and HOMA-IR, of OLETF-HFrD rats were significantly higher than those of OLETF rats. The insulin levels at 120 min and AUC (0–120) of LETO-Allo were significantly decreased compared to those in the LETO group. However, those of OLETF-Allo and OLETF-HFrD-Allo numerically decreased and were not significantly lower than those of OLETF and OLETF-HFrD, respectively. However, the OLETF-HFrD-Allo group had significantly higher HOMA-beta levels and lower HOMA-IR levels than the OLETF-HFrD group (Fig. 1B and Table 2).
Effects of HFrD and allopurinol treatment on hepatic steatosis
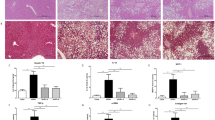
Histological analysis of liver tissue showed pronounced accumulation of lipid droplets in OLETF, OLETF-Allo, OLETF-HFrD, and OLETF-HFrD-Allo group rats compared to LETO rats. However, allopurinol treatment ameliorated HFrD-induced fat accumulation in the OLETF-HFrD-Allo group compared to that in the OLETF-HFrD group (Fig. 2A). Hepatic fibrosis was evaluated using Masson’s trichrome staining and was not observed in any of the six groups (Fig. 2B). The NAFLD activity score (NAS) was significantly higher in the OLETF, OLETF-Allo, OLETF-HFrD, and OLETF-HFrD-Allo group rats than in LETO rats. The NAS was significantly decreased in the OLETF-HFrD-Allo group compared to that in the OLETF-HFrD group (P < 0.05, Fig. 2C).
Liver pathology and NAFLD activity score after 16 weeks of LETO, OLETF, and HFrD-fed OLETF rats with or without allopurinol treatment. Liver morphology was visualized and evaluated using light microscopy (400 × magnification) on tissue sections subjected to hematoxylin and eosin (H&E) and Masson’s trichrome stains. (A). H&E-stained sections from rats in the OLETF and OLETF-HFrD group showed significant hepatic lipid accumulation compared to LETO group. Hepatic steatosis was decreased in the OLETF-HFrD-Allo group. (B). Significant fibrotic changes were not observed in all experimental groups. (C). NAFLD activity score was significantly elevated in the OLETF and OLETF-HFrD group compared to LETO group. Also, it was significantly decreased in OLETF-HFrD-Allo group, compared to OLETF-HFrD group. † P < 0.05 versus LETO group. *P < 0.05 versus OLETF-HFrD group. LETO, Long-Evans Tohushima Otsuka; LETO-Allo, LETO rats treated with allopurinol; OLETF, Otsuka Long-Evans Tokushima Fatty rats; OLETF-Allo, OLETF rats treated with allopurinol; OLETF-HFrD, OLETF rats fed with high fructose diet; OLETF-HFrD-Allo rats, OLETF rats fed with high fructose diet which were treated with allopurinol.
Effects of HFrD and allopurinol treatment on hepatic expressions of lipid metabolism genes
To determine whether HFrD and uric acid-lowering therapy affected the enzymes involved in hepatic lipid metabolism, hepatic expression of genes encoding lipogenic and fat oxidation enzymes was evaluated using real-time PCR. Expression of lipogenic genes, including sterol regulatory element-binding protein 1c (SREBP-1c) and stearoyl-CoA desaturase 1 (SCD-1) was significantly upregulated in the OLETF and OLETF-HFrD groups compared to LETO group. However, expressions of SREBP-1c and SCD-1 genes were significantly downregulated in the OLETF-HFrD-Allo group compared to the OLETF-HFrD group (P < 0.05, Fig. 3A, B). Peroxisome proliferator-activated receptor alpha (PPARα) and carnitine palmitoyltransferase 1 (CPT-1) were significantly downregulated in the OLETF and OLETF-HFrD groups compared to the LETO group. However, allopurinol treatment (OLETF-HFrD-Allo group) ameliorated the downregulation of lipid oxidation genes observed in the OLETF-HFrD group (P < 0.05, Fig. 3C,D). Therefore, the expression of the lipogenic genes of the OLETF-HFrD-Allo group was significantly suppressed compared to that in the OLETF-HFrD group. Additionally, the expression of lipid oxidation genes in the OLETF-HFrD-Allo group was significantly upregulated compared to that in the OLETF-HFrD group.
Hepatic mRNA expression of lipid metabolism genes and pro-inflammatory cytokine genes. Hepatic expression of genes was evaluated using real-time PCR. (A and B): Lipogenic genes expressions (SREBP-1c and SCD-1) were significantly upregulated in the OLETF and OLETF-HFrD groups compared to LETO group and significantly downregulated in the OLETF-HFrD-Allo group compared to the OLETF-HFrD group. (C and D): The expressions of lipid oxidation genes (PPARα and CPT-1) were significantly suppressed in the OLETF and OLETF-HFrD groups compared to the LETO group. However, allopurinol-treatment (OLETF-HFrD-Allo group) ameliorated the downregulation of lipid oxidation genes observed in OLETF-HFrD group. (E and F): Proinflammatory cytokine genes (TNF-α and PAI-1) significantly increased in the OLETF and OLETF-HFrD groups, and this increase was abolished in the OLETF-HFrD-Allo group. Data are expressed as mean ± SE. †P < 0.05 versus LETO group, *P < 0.05 versus OLETF group, #P < 0.05 versus OLETF-HFrD group LETO, Long-Evans Tohushima Otsuka; LETO-Allo, LETO rats treated with allopurinol; OLETF, Otsuka Long-Evans Tokushima Fatty rats; OLETF-Allo, OLETF rats treated with allopurinol; OLETF-HFrD, OLETF rats fed with high fructose diet; OLETF-HFrD-Allo rats, OLETF rats fed with high fructose diet which were treated with allopurinol; SREBP-1c, sterol regulatory element-binding protein 1c; SCD-1, stearoyl-CoA desaturase 1; PPARα, peroxisome proliferator-activated receptor-alpha; CPT-1, carnitine palmitoyltransferase 1; TNF-α, tumor necrosis factor-alpha; PAI-1, plasminogen activator inhibitor 1.
Effects of HFrD and allopurinol treatment on hepatic expression of pro-inflammatory cytokine genes
The gene expression levels of hepatic pro-inflammatory cytokines were evaluated to determine the effect of allopurinol treatment on liver inflammation. Hepatic mRNA expression of tumor necrosis factor-alpha (TNF-α) was significantly increased in the OLETF and OLETF-HFrD groups, and this increase was abolished in the OLETF-HFrD-Allo group (P < 0.05, Fig. 3E). Similarly, mRNA expression of plasminogen activator inhibitor 1 (PAI-1) was significantly elevated in the OLETF and OLETF-HFrD groups, but this increase was abolished by allopurinol treatment (OLETF-HFrD-Allo group) (P < 0.05, Fig. 3F).
Effects of HFrD and allopurinol treatment on the hepatic ER stress pathway
To examine the effect of fructose and allopurinol on the hepatic endoplasmic reticulum (ER) stress pathway activation, BiP, p-IRE1α, and t-IRE1α protein expression levels were determined by Western blot analysis (Fig. 4). Β-actin was used as a loading control. The expression of BiP and p-IRE1α in OLETF and OLETF-HFrD groups significantly increased (P < 0.05) compared to LETO group (Fig. 4). Allopurinol treatment tended to decrease the expression of Bip and p-IRE1α in the LETO-Allo and OLETF-Allo group compared to LETO and OLETF group, respectively. However, allopurinol-treatment significantly attenuated the HFrD-induced increased expression of BiP and p-IRE1 in the OLETF-HFrD-Allo group compared to the OLETF-HFrD group (P < 0.05) (Fig. 4).
Hepatic ER stress pathway. BiP, p-IRE1α, and t-IRE1α protein expression levels were determined by Western blot analysis. Β-actin was used as a loading control. (A) OLETF and OLETF-HFrD groups significantly increased BiP over-expression and phosphorylation of IRE1 compared to LETO group. allopurinol-treatment significantly attenuated the HFrD-induced increased expression of BiP, and p-IRE1 in the OLETF-HFrD-Allo group compared to OLETF-HFrD group. (B,C). Relative protein expression was quantified using densitometry. *P < 0.05 versus NC group; #P < 0.05 versus HFrD group. ‘Full-length blots/gels’ are presented in Supplementary Fig. 1. LETO, Long-Evans Tohushima Otsuka; LETO-Allo, LETO rats treated with allopurinol; OLETF, Otsuka Long-Evans Tokushima Fatty rats; OLETF-Allo, OLETF rats treated with allopurinol; OLETF-HFrD, OLETF rats fed with high fructose diet; OLETF-HFrD-Allo rats, OLETF rats fed with high fructose diet which were treated with allopurinol; BiP, immunoglobulin heavy chain-binding protein; p- and t-IRE1, phosphorylated, and total inositol-requiring enzyme 1.
Discussion
The present study showed that HFrD aggravated the development of hepatic steatosis, obesity, hyperinsulinemia, glucose intolerance, dyslipidemia, and hyperuricemia in OLETF rats with a phenotype of metabolic syndrome compared to LETO rats. These effects, specifically, fatty liver, dyslipidemia, hyperuricemia, and insulin resistance, were significantly ameliorated by the administration of allopurinol (a uric acid-lowering agent) in the HFrD-fed OLETF rats but not in the OLETF rats. Also, allopurinol significantly downregulated expression of lipogenic genes, upregulated lipid oxidation genes, downregulated hepatic pro-inflammatory cytokine genes, and decreased ER-stress induced protein expressions compared with those of OLETF-HFrD group. Therefore, it is suggested that uric acid plays an important role in the development of high fructose-induced hepatic steatosis.
NAFLD is a one of the clinical manifestations of metabolic syndrome, which consists of insulin resistance, obesity, hyperglycemia, hypertension, and dyslipidemia2. Recently, metabolic dysfunction-associated fatty liver disease (MAFLD) became the updated single overarching nomenclature to describe the disease22. MAFLD is commonly observed in clinical practice in association with overweight/obesity22. Some studies have shown that allopurinol does not affect weight change in fructose-fed animals23. The present study showed that fructose diet-induced weight gain and increase in epididymal fat in the OLETF-HFrD group were significantly reduced in the allopurinol-treated OLETF-HFrD-Allo group. We did not assess the amount of dietary intake or metabolic rate. However, Nakagawa et al.24 stated that allopurinol prevented weight gain in HFrD-fed rats, although allopurinol did not have any specific effects on food intake. The mechanism of the weight loss effect of allopurinol should be studied further. In addition, a decrease in epididymal fat weight and increased expression of hepatic lipid oxidation genes suggest that studies on adipose tissue are needed.
There is an intimate association between MAFLD and T2DM: more than 70% of patients with T2DM have MAFLD. Moreover, HFrD induced hepatic steatosis by direct modulation of hepatic lipogenesis and indirect action through uric acid, a metabolite of fructose7,8. This study showed that OLETF-HFrD rats showed higher HOMA-IR, fasting insulin, HOMA-beta, and AUC (0–120) of insulin compared to OLETF rats. Decreased hepatic insulin clearance usually results in increased insulin secretion to compensate for insulin resistance. Chronic hyperinsulinemia stimulates hepatic de novo lipogenesis and hepatic steatosis. Therefore, impaired hepatic insulin clearance can be linked to hepatic insulin resistance and hepatic steatosis25. This suggests that HFrD may impair hepatic insulin clearance, which can also contribute to hepatic steatosis. Further studies, on the effect of HFrD on hepatic insulin clearance, are needed. The present study also showed that allopurinol treatment improves HFrD-induced insulin resistance. The HOMA-IR of OLETF-HFrD-Allo rats was significantly lower than that of OLETF-HFrD rats. Insulin AUC (0–120) also decreased numerically, despite an increase in the insulinogenic index (IGI) to 30. However, hyperglycemia did not improve after allopurinol treatment. This may be due to impaired pancreatic beta cell function in the OLETF rats. In our study, HOMA-beta, insulin 30 min, and AUC (0–120) of insulin after the glucose loading test were significantly lower in OLETF rats than in LETO rats. Thus, allopurinol did not improve hyperglycemia in the OLETF-Allo and OLETF-HFrD-Allo groups. Further studies on the effect of allopurinol on glucose metabolism, in a non-diabetic animal model, are needed. In addition, according to Andrikopoulos et al.26, OGTT was superior to IPGTT in testing glucose metabolism under conditions of insulin resistance. In our study, the IPGTT results did not show a significant difference in glucose metabolism. Therefore, the OGTT would be more appropriate to assess whether the reduction in steatosis improves insulin resistance, with respect to glucose metabolism.
Excessive fructose consumption causes NAFLD through various mechanisms. Fat accumulation in the liver occurs when the input of fatty acids (i.e., via uptake or de novo synthesis) exceeds output (i.e., via degradation or export)14. After fructose enters the liver, it is first converted by the glycolytic pathway to pyruvate, and then metabolized to acetyl-CoA in the hepatic mitochondrial tricarboxylic acid (TCA) cycle. An elevated fructose load leads to the generation of excess acetyl-CoA that exits the mitochondria into the cytosol where it converts to citrate, a substrate for de novo lipogenesis14. The present study showed that the hepatic mRNA expression of SREBP-1c and SCD-1 were significantly increased in OLETF and OLETF-HFrD rats compared to LETO rats. In addition, there was no significant difference in lipogenic gene expression between OLETF and OLETF-HFrD rats. However, other studies have indicated that fructose can upregulate the expression of genes involved in de novo lipogenesis by stimulating the activation of transcription factors, including carbohydrate response element-binding protein (ChREBP) and SREBP-1c27. This discrepancy may be caused by the use of different animal strains and/or species in studies. Previous studies have generally used non-obese and non-diabetic rats, while this study used OLETF rats, an animal model of metabolic syndrome. Lipogenic gene expression is usually elevated in OLETF rats compared with control LETO rats, even in the absence of any specific dietary manipulation28. In the current study, allopurinol treatment significantly downregulated SREBP1C and SCD1 in the OLETF-HFrD-Allo group compared to the OLETF-HFrD group. This suggests that allopurinol treatment could suppress HFrD-induced lipogenesis.
There is a close relationship between lipogenesis and fatty acid oxidation processes in the liver. De novo lipogenesis increases the malonyl-CoA level, which inhibits CPT-1 enzyme mediation of the entry of long-chain acyl-CoA coenzymes into the mitochondria and the initiation of fatty acid breakdown via beta-oxidation29. PPARα, part of the superfamily of ligand-activated nuclear hormone receptors, is also associated with hepatic lipid metabolism, as it regulates mitochondrial beta-oxidation enzymes30. The present study showed that OLETF rats and OLETF-HFrD rats had significantly downregulated PPARα and CPT-1 expressions, leading to fatty liver pathogenesis. These results were consistent with other studies that showed the reduction of hepatic PPARα expression by excessive fructose in rats, resulting in the inhibition of fatty acid oxidation enzymes and suppression of hepatic lipid export31. Furthermore, the present study showed that allopurinol-treatment ameliorated HFrD-induced hepatic steatosis in OLETF rats through the downregulation of lipogenic gene expression and upregulation of lipid oxidation genes expression. Indeed, drugs that can increase PPARα activity, such as fenofibrate or statins, have been shown to prevent NAFLD development in vivo32. As such, allopurinol could improve fatty liver through the modulation of PPARα signaling, and its efficacy serves as evidence that uric acid is directly involved in the development of NAFLD. In this study, we measured only mRNA expression related to lipogenesis and lipid oxidation but did not measure protein expression. The efficiency of translation or regulation of translation might affect the final protein content of specific proteins, depending on many variables33. Further studies are required to understand protein expression related to lipid metabolism.
Lipotoxicity due to excessive accumulation of lipids in hepatocytes can lead to inflammation, fibrosis, and pathological angiogenesis34. Inflammatory responses are caused by oxidative stress, reactive oxygen species, and activation of cytokines such as TNF-α2,14. Previous studies have indicated that hepatic TNF-α expression is significantly increased in mice fed with an HFrD35 and TNF-α has a critical role in the pathogenesis of NAFLD35,36. PAI-1 is an acute-phase protein, and in vitro and in vivo studies have shown that its hepatic expression is regulated by TNF-α37. Plasma PAI-1 levels are associated with hepatic steatosis severity in obese humans and genetically obese mice38. Accordingly, PAI-/- mice are protected from lipid accumulation after alcohol administration37. This study found that HFrD increased the hepatic expression of TNF-α and PAI-1. This was consistent with a recent study that reported increased hepatic PAI-1 expression with fructose administration and the important role PAI-1 plays in fructose-mediated liver injury39. The present study also showed that allopurinol treatment significantly reduced the hepatic gene expression of pro-inflammatory cytokines. This phenomenon may occur via inhibition of xanthine oxidase, which has been shown to reduce liver injury by decreasing reactive oxygen species formation40 and modulating transcription factors that regulate the production of pro-inflammatory cytokines41.
Disruption of ER homeostasis can lead to hepatic steatosis, inflammation, and insulin resistance42. The ER is an intracellular organelle that plays an important role in protein synthesis, folding, modification, and trafficking. When the protein folding or modification processes are disrupted, ER stress is induced, and the unfolded protein response (UPR) is activated43. There are three main branches of the UPR: the protein kinase RNA-like ER kinase (PERK) pathway, the activated transcription factor 6 (ATF6) pathway, and the IRE1 pathway. BiP also referred to as glucose-regulated protein 78, is an intra-luminal chaperone protein that plays a key role in initiating the UPR. Under normal conditions, BiP binds to UPR transducers such as PERK, ATF, and IRE1, and acts to suppress pathway activation. When ER stress is induced, BiP is separated from UPR transducers, facilitating the initiation of signaling transduction in each pathway. The present study found that as BiP expression and IRE1-XBP-1 pathway activation increased, fructose consumption induced hepatic ER stress pathway activation. Previous research showed that fructose-treatment induced fat accumulation in primary hepatocytes via induction of ER stress44 and in vivo45. Uric acid has been demonstrated to increase ER stress in various cell types, including primary hepatocytes27, glomerular mesangial cells46, and endothelial cells47. Of the three UFR pathways, the IRE1-XBP-1 pathway has been reported as a regulator of hepatic metabolism. XBP-1 increases hepatic expression of lipogenic genes such as SCD-148, and the activation of c-Jun N-terminal kinase by IRE1α induces hepatic insulin resistance49. Additionally, the IRE1α-XBP-1 pathway plays a key role in the regulation of hepatic very-low-density lipoprotein assembly and secretion50. Finally, a previous study showed that the hepatic-specific deletion of IRE1α results in the development of severe hepatic steatosis when ER stress is induced51. Therefore, HFrD-induced activation of ER stress mechanisms may contribute to fatty liver development.
Recently, many in vitro studies using hepatocytes have evaluated the direct effects of uric acid on hepatic steatosis. Lanaspa et al. indicated that uric acid can stimulate fat accumulation via the generation of mitochondrial oxidative stress in HepG2 cells and is related to the mechanisms by which fructose induces fatty liver8. Furthermore, they also reported that uric acid can upregulate fructokinase, resulting in increased lipid accumulation via sensitizing hepatocytes to fructose metabolism8. Another study has revealed that uric acid induces fat accumulation in hepatocytes by ER stress-mediated SREBP-1c activation27. Another study has also shown that uric acid induces hepatic steatosis and insulin resistance in vitro and in vivo through the activation of the NOD-like receptor family, pyrin domain-containing protein 3 (NLRP3) inflammasome52. These studies support the hypothesis that uric acid generation from fructose metabolism accelerates fructose-induced NAFLD. The present study is, to the best of my knowledge, the first in vivo study to show that allopurinol inhibited fructose load-induced activation of ER stress pathways, especially the IRE1 related pathway. Therefore, it is suggested that uric acid may be involved in the fructose-induced ER stress pathway activation.
Allopurinol is a xanthine oxidase inhibitor, which has been widely used for the treatment of gout. In rat studies that evaluated the effects of uric acid-lowering therapy on metabolic syndrome, allopurinol was used at a dose range of 5 mg/kg to 30 mg/kg20,53,54. The present study used 10 mg/kg/day of allopurinol that was found to be effective in significantly reducing uric acid levels and had been previously recommended to minimize organ toxicity55. Considering FDA guidance for estimating a human efficient dose, an allopurinol dose of 10 mg/kg/day in rats correspond to 100–300 mg/day in humans, per standard doses used the treatment of hyperuricemia in patients56. Therefore, the dosage used in the present study and the results generated with that dosage may be useful for a future study that evaluates the efficacy of allopurinol treatment in human patients with NAFLD.
Limitations in the present study include the following: First, allopurinol treatment did not improve hyperglycemia despite significant amelioration of hepatic steatosis and insulin resistance index such as HOMA-IR and AUC of insulin. Data were not collected from muscle or adipose tissues which are peripheral tissues that may have contributed to insulin resistance and the pancreas, which are sources of insulin secretion. Further studies are also required to evaluate alterations in the expression of insulin resistance-related genes in muscle or adipose tissues and insulin secretion-related genes in pancreatic tissues. Second, allopurinol treatment reduced HFrD-induced weight gain and epididymal fat weight. We did not measure dietary parameters or metabolic rate and did not estimate the weight loss effect of allopurinol. Therefore, further studies are needed to evaluate the mechanism of the weight loss effect of allopurinol in HFrD-induced obesity animal models. Third, in the present study, allopurinol decreased serum UA levels and improved NAFLD by decreasing de novo lipogenesis, fatty acid oxidation, and ER stress; however, we do not know whether reduction of serum UA level directly influences the improvement of NAFLD. Usually, serum uric acid levels increase in patients with metabolic syndrome due to decreased clearance of uric acid. Hyperuricemia itself may aggravate the progression of NAFLD, which can be partially protected by allopurinol through amelioration of hyperuricemia-induced NAFLD pathogenesis. However, this hypothesis should be evaluated in other in vivo models of hepatic steatosis and in vitro experiments, to examine whether high fructose with or without uric acid influences the progression of NAFLD, even though the in vitro UA treatment is not the same as the UA concentration as a metabolite of fructose.
In conclusion, HFrD induces the development of significant hepatic steatosis, obesity, hyperinsulinemia, glucose intolerance, dyslipidemia, and hyperuricemia. In addition, allopurinol, uric acid lowering agent treatment reduces serum uric acid and ameliorates HFrD-induced hepatic steatosis through the modulation of hepatic lipid metabolism, expression of pro-inflammatory cytokines, and the ER stress pathway, independent of glucose metabolism. Therefore, uric acid may have a direct role in the development of fructose-induced hepatic steatosis, and uric acid lowering therapy could be a candidate for the prevention or treatment of NAFLD.
Methods
Ethics statement
All animal experiments were approved by the Institutional Animal Care and Use Committee of Kyung Hee University Hospital at Gangdong (KHNMC-IACUC-2016–04). Principles of laboratory animal care (NIH publication no. 85–23, revised 1996; http://grants1.nih.gov/grants/olaw/references/phspol.htm) were followed, as well as specific national laws when applicable. All animal facilities were approved by the Use Committee of the Kyung Hee University Hospital at Gangdong. The use of animals was kept to an absolute minimum, as per requirement, to achieve statistical significance for validation. This study was conducted in compliance with the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines.
Animal
Otsuka Long-Evans Tokushima Fatty (OLETF) male rats, which are characterized as insulin resistant, obese, hyperglycemic, and dyslipidemia, were used20. Male LETO rats were used as controls. LETO and OLETF male rats were purchased from Tokushima Research Institute (Otsuka Pharmaceutical, Tokushima, Japan) and maintained on a 12 h light/dark cycle at 22 °C to 26 °C with ad libitum access to food and water.
Experimental design
The 30-week-old LETO and OLETF male rats were randomly divided into six groups of 10 rats as follows: (1) LETO rats fed a normal chow diet (LETO group), (2) LETO rats treated with allopurinol (LETO-Allo group), (3) OLETF rats fed a normal chow diet (OLETF group), (4) OLETF rats treated with allopurinol (OLETF-Allo group), (5) HFrD-fed OLETF rats (OLETF-HFrD group), and (6) HFrD-fed OLETF rats with allopurinol treatment (OLETF-HFrD-Allo group). The HFrD contained 60% fructose (Central Lab Animal Inc., Seoul, Republic of Korea). Allopurinol (Sigma-Aldrich, St. Louis, MO, USA), a xanthine oxidase inhibitor, was used to block the generation of intracellular uric acid and was administered through drinking water at a dose of 10 mg/kg/day. Each intervention was continued for 16 weeks. At the end of the study, body weights were measured, and IPGTTs were conducted. Glucose (2 g/kg) was intraperitoneally administered to rats after overnight fasting. Blood samples were collected for the measurement of biochemical parameters at 0, 30, 60, 90, and 120 min after glucose injection.
Termination of the experiment
After the IPGTT study at the end of the experimental period (16 weeks), rats were sacrificed by an overdose of diethyl ether57. Blood was collected and serum was stored at − 20 °C until used for further analysis. Liver and pancreas were removed and weighed to calculate relative organ weight. A small portion of liver was excised and fixed in in 4% formalin for histopathological examination. The remaining parts of liver were stored at − 70 °C until used for RNA and protein analysis58.
Measurement of serum biochemical parameters
Serum fasting glucose, total cholesterol, triglyceride (TG), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) concentrations were measured using a commercial kit (Asan Pharm. Co., Ltd, Hwaseong-si, Gyeonggi-do, Republic of Korea). Serum fasting insulin concentrations were determined using a rat insulin ELISA kit (EZRMI-13 K, Millipore, Billerica, MA, USA), and uric acid concentrations were measured using a colorimetric assay kit (Abcam, Cambridge, MA, USA). Hemoglobin A1c (HbA1c) concentrations were determined using an automatic analyzer (DCA 2000 System, Bayer, Elkhart, IN, USA).
The IGI, an indicator of beta-cell function, was defined as the ratio of the change in insulin concentration (Δ Insulin) to the change in glucose concentration (Δ Glucose)59. IGI at 30 min and AUC (0–120) of insulin and glucose after glucose administration were calculated from the IPGTT data as follows:
IGI (Δ Insulin 30/Δ Glucose 30) = (insulin at 30 min – insulin at 0 min [μIU/mL])/(glucose at 30 min – glucose at 0 min [mg/dL]). AUC (0–120) = Area under the curve from 0 to 120 min for glucose and insulin each.
The homeostasis model assessment of beta-cell function (HOMA-beta) and insulin resistance (HOMA-IR)60 were calculated using fasting glucose and insulin concentrations as follows:
HOMA-beta = 360 × fasting insulin (μIU/mL) / (fasting glucose [mg/dL] – 63); HOMA-IR = fasting glucose (mg/dL) × fasting insulin (μIU/mL)/405.
Histological analysis of liver tissue
The paraffin-embedded sections were stained with hematoxylin and eosin (H&E) or Masson’s trichrome. A single pathologist, who was blinded to the experimental details, using light microscopy (400 × magnification), performed liver histological analysis. The NAFLD activity score (NAS) was calculated using the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) scoring system61. This scoring system analyzes four components to evaluate histological changes of NAFLD, and each component is scored, based on the degree of morphological change, as follows: steatosis grade (0–3), lobular inflammation (0–3), hepatocellular ballooning (0–2), and fibrosis score (0, 1a, 1b, 1c, 2, 3, 4). The NAS was calculated as the sum of the scores for steatosis, lobular inflammation, and hepatocellular ballooning.
Quantitative real-time PCR
Total hepatic RNA was extracted using TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed using AMV reverse transcriptase (TaKaRa Bio., Kyoto, Japan). Real-time polymerase chain reactions (PCR) were conducted using the Power SYBR Green PCR master mix (Applied Biosystems Inc., Foster City, CA, USA) in the StepOne Plus real-time PCR system (Applied Biosystems). Bioneer (Daejeon, Republic of Korea) designed the PCR primers. Primer sequences and PCR conditions are shown in Table 3. Each mRNA level was normalized to the β-actin mRNA level.
Western blot analysis
Liver tissues were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (iNtRON Biotechnology, Seongnam-si, Gyeonggi-do, Republic of Korea). Samples were centrifuged at 13,000 rpm × 20 min at 4 ºC. Protein concentrations were quantified by the Bradford method. Proteins were loaded onto a 10% acrylamide gel, separated using SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were incubated overnight at 4 °C in Tris-buffered saline-Tween 20 (TBST) with primary antibodies (1:1000 dilution) against the following: immunoglobulin heavy chain-binding protein (BiP, Cell Signaling Biotechnology, Beverly, MA, USA), phosphorylated inositol-requiring enzyme 1 (p-IRE1, Abcam), total IRE1 (t-IRE1, Abcam), x-box binding protein 1 (XBP-1, Abcam), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated goat secondary antibodies (anti-rabbit or mouse antibodies, 1:5000 dilution, Thermo Scientific, Rockford, IL, USA) at room temperature for 1 h. Immunoreactive bands were enhanced and detected by chemiluminescence (ECL, Millipore), and band densities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). To simultaneously investigate the expression of several proteins of different sizes on one membrane, the membrane was cut into sections and identified by attaching different antibodies. Therefore, we provided a cropped image rather than a full-length western blot image.
Statistical analysis
Data were expressed as either mean ± SE or median with interquartile range. Each experiment was repeated in triplicate. Statistical analysis was performed using SPSS 18.0 software (Chicago, IL, USA). One-way analysis of variance was used followed by a post-hoc test with the Bonferroni procedure when data were normally distributed. Data not normally distributed were evaluated using the Kruskal–Wallis test; significant differences for multiple comparisons between groups were determined using the Mann–Whitney U-test with adjustment of the significance level for the decision criteria using a Bonferroni type adjustment. Statistical significance was defined as P < 0.05.
References
Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231 (2002).
Marchesini, G. et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37, 917–923 (2003).
Lazo, M. & Clark, J. M. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin. Liver Dis. 28, 339–350 (2008).
Lee, Y. H. et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab. J. 43, 31–45 (2019).
Cave, M. et al. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J. Nutr. Biochem. 18, 184–195 (2007).
Elliott, S. S., Keim, N. L., Stern, J. S., Teff, K. & Havel, P. J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 76, 911–922 (2002).
Lim, J. S., Mietus-Snyder, M., Valente, A., Schwarz, J. M. & Lustig, R. H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 7, 251–264 (2010).
Lanaspa, M. A. et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287, 40732–40744 (2012).
Basaranoglu, M., Basaranoglu, G., Sabuncu, T. & Senturk, H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 19, 1166–1172 (2013).
Cox, C. L. et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr. Metab. (Lond.) 9, 68 (2012).
Lee, K. Relationship between uric acid and hepatic steatosis among Koreans. Diabetes Metab. 35, 447–451 (2009).
Li, Y., Xu, C., Yu, C., Xu, L. & Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J. Hepatol. 50, 1029–1034 (2009).
Lee, B. W. et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research group of the Korean diabetes association. Diabetes Metab. J. 44, 382–401 (2020).
Lindor, K. D. et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 39, 770–778 (2004).
Adams, L. A., Zein, C. O., Angulo, P. & Lindor, K. D. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am. J. Gastroenterol. 99, 2365–2368 (2004).
Nelson, A., Torres, D. M., Morgan, A. E., Fincke, C. & Harrison, S. A. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J. Clin. Gastroenterol. 43, 990–994 (2009).
Belfort, R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355, 2297–2307 (2006).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Jeong, S. W. Nonalcoholic fatty liver disease: a drug revolution is coming. Diabetes Metab. J. 44, 640–657 (2020).
Kawano, K. et al. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41, 1422–1428 (1992).
Iba, A. et al. Insulin resistance increases the risk of urinary stone formation in a rat model of metabolic syndrome. BJU Int. 106, 1550–1554 (2010).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
El-Bassossy, H. M. & Watson, M. L. Xanthine oxidase inhibition alleviates the cardiac complications of insulin resistance: effect on low grade inflammation and the angiotensin system. J. Transl. Med. 13, 82 (2015).
Nakagawa, T. et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal. Physiol. 290, F625-631 (2006).
Najjar, S. M. & Perdomo, G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda) 34, 198–215 (2019).
Andrikopoulos, S., Blair, A. R., Deluca, N., Fam, B. C. & Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab 295, E1323–E1332 (2008).
Choi, Y. J. et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Invest. 94, 1114–1125 (2014).
Song, Y. S. et al. Time course of the development of nonalcoholic fatty liver disease in the Otsuka long-evans Tokushima fatty rat. Gastroenterol. Res. Pract. 2013, 342648 (2013).
McGarry, J. D. Malonyl-CoA and carnitine palmitoyltransferase I: an expanding partnership. Biochem. Soc. Trans. 23, 481–485 (1995).
Schoonjans, K., Staels, B. & Auwerx, J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 37, 907–925 (1996).
Nagai, Y. et al. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am. J. Physiol. Endocrinol. Metab. 282, E1180-1190 (2002).
Park, H. S. et al. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab. J. 40, 376–385 (2016).
Fortelny, N., Overall, C. M., Pavlidis, P. & Freue, G. V. C. Can we predict protein from mRNA levels?. Nature 26, E19–E20 (2017).
Povero, D. & Feldstein, A. E. Novel molecular mechanisms in the development of non-alcoholic steatohepatitis. Diabetes Metab. J. 40, 1–11 (2016).
Kanuri, G., Spruss, A., Wagnerberger, S., Bischoff, S. C. & Bergheim, I. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 22, 527–534 (2011).
Li, Z. et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37, 343–350 (2003).
Bergheim, I. et al. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology 130, 2099–2112 (2006).
Alessi, M. C. et al. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler. Thromb. Vasc. Biol. 23, 1262–1268 (2003).
Kanuri, G., Spruss, A., Wagnerberger, S., Bischoff, S. C. & Bergheim, I. Fructose-induced steatosis in mice: role of plasminogen activator inhibitor-1, microsomal triglyceride transfer protein and NKT cells. Lab. Invest. 91, 885–995 (2011).
Peglow, S., Toledo, A. H., Anaya-Prado, R., Lopez-Neblina, F. & Toledo-Pereyra, L. H. Allopurinol and xanthine oxidase inhibition in liver ischemia reperfusion. J. Hepatobiliary Pancreat. Sci. 18, 137–146 (2011).
Demirel, U. et al. Allopurinol ameliorates thioacetamide-induced acute liver failure by regulating cellular redox-sensitive transcription factors in rats. Inflammation 35, 1549–1557 (2012).
Zhou, H. & Liu, R. ER stress and hepatic lipid metabolism. Front Genet. 5, 112 (2014).
Schroder, M. Endoplasmic reticulum stress responses. Cell Mol. Life Sci. 65, 862–894 (2008).
Zheng, J. et al. Docosahexaenoic acid ameliorates fructose-induced hepatic steatosis involving ER stress response in primary mouse hepatocytes. Nutrients 20, 8 (2016).
Li, X. et al. Emodin ameliorates hepatic steatosis through endoplasmic reticulum-stress sterol regulatory element-binding protein 1c pathway in liquid fructose-feeding rats. Hepatol. Res. 46, E105-117 (2016).
Li, S., Zhao, F., Cheng, S., Wang, X. & Hao, Y. Uric acid-induced endoplasmic reticulum stress triggers phenotypic change in rat glomerular mesangial cells. Nephrology (Carlton) 18, 682–689 (2013).
Li, P., Zhang, L., Zhang, M., Zhou, C. & Lin, N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: a mechanism for uric acid-induced endothelial dysfunction. Int. J. Mol. Med. 37, 989–997 (2016).
Lee, A. H., Scapa, E. F., Cohen, D. E. & Glimcher, L. H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496 (2008).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Wang, S. et al. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 16, 473–486 (2012).
Zhang, K. et al. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 30, 1357–1375 (2011).
Wan, X. et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 64, 925–932 (2016).
Roncal, C. A. et al. Combination of captopril and allopurinol retards fructose-induced metabolic syndrome. Am. J. Nephrol. 30, 399–404 (2009).
Hu, Q. H., Zhang, X., Pan, Y., Li, Y. C. & Kong, L. D. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem. Pharmacol. 84, 113–125 (2012).
Suzuki, Y., Sudo, J. & Tanabe, T. Allopurinol toxicity: its toxic organ-specificity between the liver and the kidney in the rat. J. Toxicol. Sci. 9, 343–351 (1984).
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Pharmacology and toxicology, guidance for industry. Estimating the maximum safe dose in initial clinical trials for therapeutics in adult healthy volunteers, p. 7. http://www.fda.gov/cder/guidance/index.htm (2005).
Harb, A. A., Bustanji, Y. K., Almasri, I. M. & Abdalla, S. S. Eugenol reduces LDL cholesterol and hepatic steatosis in hypercholesterolemic rats by modulating TRPV1 receptor. Sci. Rep. 9, 14003–14012 (2019).
Hwang, Y. C. et al. Compound K attenuates glucose intolerance and hepatic steatosis through AMPK-dependent pathways in type 2 diabetic OLETF rats. Korean J. Intern. Med. 33, 347–355 (2018).
Phillips, D. I., Clark, P. M., Hales, C. N. & Osmond, C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet. Med. 11, 286–292 (1994).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported by the National Research Foundation of Korea (NRF) [Grant Number NRF-2011–0013729] funded by the Korean government (MEST); the Korean Diabetes Association [Grant Number I.J.C., 2016F-1], and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea [Grant Number HI17C2060].
Author information
Authors and Affiliations
Contributions
I.-J.C.: Conceptualization, Investigation, Data Curation, Writing-Original Draft; D.-H.O.: Investigation; J.Y.: Investigation; Y.-C.H.: Data Curation; K.J.A.: Data Curation; H.-Y.C.: Data Curation; S.W.J.: Investigation, Interpretation of data; J.-Y.M.: Investigation, Data Curation; S.-H.L.: Data Curation; Sung-Jig Lim: Investigation, Data Curation, Visualization; I.-K.J.: Conceptualization; Supervision; Funding acquisition; Writing-Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, IJ., Oh, DH., Yoo, J. et al. Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway. Sci Rep 11, 9894 (2021). https://doi.org/10.1038/s41598-021-88872-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88872-7
- Springer Nature Limited
This article is cited by
-
Sestrin2: multifaceted functions, molecular basis, and its implications in liver diseases
Cell Death & Disease (2023)
-
Liver-derived metabolites as signaling molecules in fatty liver disease
Cellular and Molecular Life Sciences (2023)
-
Convergence of Fructose-Induced NLRP3 Activation with Oxidative Stress and ER Stress Leading to Hepatic Steatosis
Inflammation (2023)