Abstract
Ultraconserved regions (UCRs) are 481 genomic sequences with 100% identity across humans, rats, and mice. Increasing evidence suggests that non-coding RNAs transcribed from UCRs are involved in various diseases, especially cancers. The human transformer 2β gene (TRA2B) encodes a UCR (uc.138) that spans exon 2 and its neighboring introns. TRA2B4 RNA is the only transcript that contains the whole exon 2 among five spliced TRA2B RNA variants (TRA2B1-5). TRA2B4 is upregulated in colon cancer cell lines, although it is not translated to Tra2β protein because of its nuclear retention. Nevertheless, the clinical significance and biological functions of uc.138 in colon cancer cells remain unclear. In this study, RNA in situ hybridization showed that TRA2B4 was predominantly overexpressed in the nucleus of colon adenocarcinoma and adenoma. Overexpression of TRA2B4 in colon cancer HCT116 cells promoted cell proliferation by changing the expression of G2/M-related cell cycle regulators. Moreover, TRA2B4 increased migration and cell viability in a uc.138 sequence-dependent manner. TRA2B4 significantly enhanced tumorigenesis in vivo. Taken together, uc.138 encoded in TRA2B4 plays an oncogenic role in tumor progression and may become a potential biomarker and therapeutic target in colon cancer.
Similar content being viewed by others
Introduction
Ultraconserved regions (UCRs) are 481 segments that are absolutely conserved longer than 200-bp in length between orthologous regions in human, rat, and mouse genomes1. Transcripts that are transcribed from the genomic loci containing UCRs are categorized as a novel group of functional RNAs, transcribed-UCRs (T-UCRs)2. Recent genome-wide expression profiling studies have reported that T-UCRs are dysregulated in several types of human diseases such as chronic lymphatic leukemia, cervical, colorectal, lung, and breast cancers3,4,5,6,7,8.
Transformer 2β (Tra2β) contains two serine/arginine domains and an RNA recognition motif, and it functions as a sequence-specific pre-mRNA splicing enhancer9, 10. Overexpression of the Tra2β protein is associated with the development of several cancers11,12,13,14. We previously reported that the protein and mRNA levels of Tra2β were upregulated in colon cancer cells, and silencing of Tra2β decreased colon cancer cell proliferation15. Tra2β interacts with the 3′-UTR of the antiapoptotic BCL2 mRNA and increases resistance to apoptosis16. Thus, Tra2β is supposed to act as an oncogenic protein to accelerate tumor progression15,17. The human TRA2B gene contains 10 exons and 9 introns and generates five mRNA isoforms (TRA2B1 to 5) through alternative splicing18. Among them, TRA2B1 mRNA lacking exon 2, which contains multiple premature termination codons (PTCs), is translated into the Tra2β protein18. Intriguingly, the TRA2B gene encodes a 419-bp UCR (uc.138) with perfect human-to-rodent sequence identity1. The UCR in the TRA2B gene spans exon 2 and its neighboring introns. Among the five spliced transcripts, only TRA2B4 contains a complete exon 2. In general, premature termination codon-containing RNAs such as TRA2B4 are considered byproducts of aberrant splicing and are degraded by RNA surveillance mechanisms such as nonsense-mediated mRNA decay (NMD). However, we have previously reported that the RNA-binding protein Hu antigen R (HuR) increased TRA2B4 production by upregulating the inclusion of exon 2 in response to oxidative stress19. In addition, TRA2B4 was preferentially retained in the nucleus of colon cancer cells by association with a nuclear protein nucleolin, which helped to escape degradation through nonsense-mediated mRNA decay20,21. Using cDNA libraries prepared from 24 patients with colon cancer, we determined that colon cancer tissues expressed significantly higher levels of TRA2B4 compared with surrounding normal tissues by qPCR20.
We also found that TRA2B4 inhibited p21-mediated cellular senescence by interrupting the binding between Sp1 and the CDKN1A promoter in colon cancer cells20. Although several T-UCRs exhibit disease-specific expression and affect pathological conditions, the underlying biological functions of T-UCRs are largely unknown.
In the present study, we demonstrated RNA in situ hybridization and found that uc.138-containing TRA2B4 was predominantly expressed in the nucleus of colon adenocarcinoma and adenoma cells. Then, we investigated the gene expression profiles in stable TRA2B4-overexpressed colon cancer cells and revealed that G2/M-related cell cycle regulators were significantly upregulated. Overexpression of the transcribed uc.138, as one of the spliced isoforms of TRA2B, promoted migration and cell proliferation of colon cancer cells. These results indicate that the ultraconserved uc.138 has oncogenic roles in the progression of colon cancer.
Results
Expression of TRA2B4 in human colon adenocarcinoma
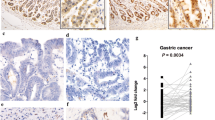
The TRA2B gene (185,914,558–185,938,103 located on Chromosome 3) contains an UCR (uc.138: length 419 bp, 185,931,503–185,931,921) that spans exon 2 (ENSE00003574041: length 276 bp, 185,931,852–185,931,577) and neighboring introns1,18. Among the TRA2B mRNA isoforms, only TRA2B4 includes exon 2 (Ensembl transcript ID: ENST00000456380.5.). First, we investigated whether the UCR-containing TRA2B4 is expressed in human colon carcinoma tissues. TRA2B4 expression was examined by RNA in situ hybridization targeting exon 2 using colon cancer tissue microarray (TMA) (Provitro, colon carcinoma tissues, no. 401 2211). TMA included 10 normal colon tissues, 10 adenomas, and 40 colon adenocarcinomas (International Union Against Cancer (UICC) stage I–IV). TRA2B4 was weakly expressed in normal colonic epithelial cells (Fig. 1A). However, robust expression of TRA2B4 was observed mainly in the nucleus of colorectal cancer cells (Fig. 1B–F). TRA2B4 expression was detected at different clinical stages of colon adenocarcinoma tissues using in situ hybridization (Fig. 1C–F). Proliferative nuclear marker Ki-67 was stained to confirm proliferating colon cancer cells (Fig. 1G–L). TRA2B4 was detected in Ki-67-positive colon cancer samples. Tissue arrays were stained with hematoxylin and eosin (H&E) to define the tumor region (Fig. 1M–R). A number of positive cells per area in each adenocarcinoma (ADC) tumor stages (UICC stages I–IV), adenoma (A), and normal colon (N) was measured. Although the levels of TRA2B4 were not correlated with the severity of UICC stage, its expression was significantly higher in tumors than that in the normal colon tissue (Fig. 1S).
Expression of TRA2B4 in normal colonic epithelium and colon tumor. (A–F) RNA in situ hybridization for the expression of TRA2B4 was performed using normal colon (A), adenoma (B), and colon adenocarcinomas (ADC) (C–F) tissue microarray (TMA). Magnified views of these areas are shown with lowercase letters. DIG-labeled antisense probe targeting for exon 2 in TRA2B4 was hybridized to normal colonic epithelium or primary tumor and then hematoxylin was used as the counterstain. (G–L) Immunohistochemical expression of Ki-67 in normal colon and colorectal cancer tissues. (M–R) Hematoxylin and eosin (H&E) stain in normal colon and colorectal cancer tissues. Scale bars: 50 μm. (S) A number of positive cells per area in each tumor stages (UICC stages I–IV), adenoma (A), and normal colon (N) were counted (n = 10 samples per group, with 10 fields per samples). *Significantly different by the unpaired Student’s t test compared with normal colon (p < 0.05).
Identification of uc.138-related gene expression profiles in colon cancer cells
Uc.138-containing TRA2B4 transcript encodes multiple PTCs in exon 2 (Fig. 2A). RNA variants with PTCs are preferentially subjected to NMD as aberrant RNAs and are not translated into proteins. To test whether TRA2B4 has protein-coding potential, we performed polysome profiling using sucrose gradient-based fractionation. Glucose gradient centrifugation fractionated the nonpolysome-associated (fractions 1–2), small (40S) and large (60S) ribosomal subunits, monosomes (80S) (fractions 3–8), and progressively larger polysomes (fractions 9–14) (Fig. 2B). The relative distribution of TRA2B4, TRA2B1 (Tra2β protein-coding transcript), ACTB, and non-coding RNA MALAT1 on polysome gradients was measured by qPCR. TRA2B1 and ACTB were enriched in the high molecular weight polysome fractions, whereas TRA2B4 and MALAT1 were not abundant in the polysome fractions, indicating that TRA2B4 did not interact with the translational machinery (Fig. 2C). To investigate the molecular mechanism underlying the influence of uc.138 expression, we first established stable cell lines that overexpressed full-length TRA2B4 (ex 1–ex 10) or TRA2B exon 2 encoding uc.138 (Fig. 2A). We introduced pEB-Multi constructs to human colorectal carcinoma (HCT116) cells and selected cells that stably expressed high levels of TRA2B4 or exon2 (TRA2B4 #1, TRA2B4 #2, exon2 #1, and exon2 #2) (Fig. 2D). The expression levels of TRA2B4 (primer S1-AS1), exon 2 (S1-AS2), and TRA2B1 (S2-AS1) were confirmed using real-time quantitative (q) PCR with specific primers, as shown in Fig. 2A. The expression levels of TRA2B4 and exon 2 were significantly increased in the stable lines, whereas the expression of another spliced isoform, TRA2B1, was not affected (Fig. 2D). We previously showed that expression levels of TRA2B1 did not affect TRA2B4 expression in HCT116 cells21. The introduction of TRA2B4 and exon 2 significantly increased cell growth (Fig. 2E). As shown in Fig. 2F, microarray-based global expression analysis showed that TRA2B4 #1 and TRA2B4 #2 stable cells differentially expressed 2469 and 2292 genes in total, compared with those in the mock-treated control cells, respectively (≥ 1.5-fold) (Fig. 2D) (NCBI Gene Expression Omnibus #GSE161169). We found that expression levels of 1354 genes, including 693 upregulated genes and 681 downregulated genes, were commonly altered in both TRA2B4 #1 and #2 overexpressing cells, compared with mock cells (≥ 1.5-fold). The cells specifically overexpressing exon 2 #1 and exon 2 #2, commonly altered the expression of 1880 genes, including 1178 upregulated genes and 702 downregulated genes (≥ 1.5-fold). Finally, we identified 1067 commonly altered genes among genetically diverse cells, TRA2B4 #1, TRA2B4 #2, exon2 #1 and exon2 #2, which were compared with mock cells (≥ 1.5-fold) (Fig. 2F, Supplementary Table 2). The differentially expressed genes were analyzed using ingenuity pathway analysis (IPA, Qiagen Bioinformatics) software to identify biological functions relevant to these genes. The enriched biofunctions of the genes regulated in uc.138-overexpressing cells were (1) cellular development (p = 9.05E−18), (2) cell growth and proliferation (p = 9.05E−18), (3) cell cycle (p = 6.55E−15), (4) cell death and survival (p = 8.19E−15), and (5) cancer (p = 2.04E−12) (Fig. 2G). These results suggest that uc.138 in exon 2 may facilitate proliferation of colon cancer cells.
Identification of uc.138-related gene expression profiles in colon cancer cells. (A) Schematic diagram of the human TRA2B gene. Exons are indicated by open boxes and Arabic numbers. Filled boxes denote the ultraconserved uc.138. Two major splice variants TRA2B1 and TRA2B4 are generated from the TRA2B gene and the use of each exon is shown. Black arrows show the specific primers used to detect each of the transcripts. Arrows in exon 1 indicate a translation start site. PTCs; premature termination codons. (B) The absorbance at 254 nm was measured during the fractionation of polysomes. (C) The abundance of TRA2B4, TRA2B1, ACTB, and MALAT1 in each fraction from the polysome profiling was determined by qPCR. (D) Relative expression levels of TRA2B1, TRA2B4, and exon 2 in human colorectal carcinoma (HCT116) cells that were stably transfected with pEB-multi vector encoding TRA2B4 (exon 1–exon 10) or exon 2 were measured by RT-qPCR. GAPDH was used as an endogenous control. Data are expressed as the mean fold changes ± standard deviation (SD; n = 6), compared with those in the mock-treated cells. (E) Effects of stable overexpression of TRA2B4 or exon 2 on cell proliferation were monitored by counting the number of cells at the indicated times. Values are mean ± SD from five independent experiments. *Significantly different by the unpaired Student’s t test compared with mock #1-treated cells (p < 0.05). (F) The number of differentially expressed genes in TRA2B4 or exon 2-overexpressed cells (≥ 1.5-fold), compared with mock #1-treated cells. (G) Commonly regulated 1067 genes were subjected to Ingenuity Pathway Analysis (QIAGEN Bioinformatics) to identify biologically relevant functions.
Uc.138 increases cell cycle progression in colon cancer cells
We analyzed whether dysregulation of the cell cycle caused abnormal proliferation of uc.138-overexpressing cells. The canonical pathway of ‘Cyclin and Cell cycle regulation’ was represented by differentially expressed 1067 genes identified by microarray analysis (Fig. 3A). The molecules encoded by up- or down-regulated genes are shown in red and green, respectively, in S/G2/M phases (Fig. 3A). Overexpression of uc.138 increased G2 and S phases-related gene expression, including CDK1, cyclin A, and cyclin B. Real-time PCR analysis showed that CCNA1 and CCNB2 mRNA levels were significantly increased in both TRA2B4 and exon 2-introducing cells (Fig. 3B,C). In contrast, the expression levels of CDKN1A encoding p21, an inhibitor of cyclin-dependent kinases, were reduced (Fig. 3D). Protein levels of cyclin A and cyclin B were increased, whereas p21 expression was down-regulated in these cells (Fig. 3E). We next confirmed that phosphorylation of mitotic protein kinase CDK1 was increased in these cells (Fig. 3E). The phosphorylation of cell cycle regulator Rb was elevated in TRA2B4 and exon 2-overexpressing cells. To investigate the possibility that uc.138 contributes to elevated cell proliferation, we investigated whether TRA2B4 or exon 2 expression in colon cancers induced cell cycle progression (Fig. 3F). Using cytofluorimetry, we found that overexpression of TRA2B4 or exon 2 was associated with an increase in the percentage of G2/M cells (Fig. 3G). These results suggested that overexpression of uc.138 significantly increased cell proliferation via upregulation of cell cycle-regulatory proteins.
Effects of uc.138 on cell cycle progression in colon cancer cells. (A) Canonical pathways for cell cycle regulation represented by the differentially expressed genes between uc.138 overexpression and mock-transfected cells were analyzed using Ingenuity Pathway Analysis (QIAGEN Bioinformatics). The genes up- and down-regulated in uc.138 overexpressed cells are shown in red and green, respectively. (B–D) RNA levels of CCNA1, CCNB, and CDKN1A, respectively, were determined using RT-qPCR in TRA2B4 or exon 2-overexpressed cells using GAPDH mRNA as an endogenous quantity control. Values are mean ± SD (n = 6). *Significantly different by the unpaired Student’s t test (p < 0.05). (E) Amounts of cell cycle related proteins and phosphorylation of Cdk1 and Rb were determined using western blotting with Gapdh as a loading control. (F) Effects of TRA2B4 or exon 2-overexpression on cell cycle distribution were measured by flow cytometry. (G) The quantitative statistics of cell number in each phase. Values are means ± SD (n = 6). *Significantly different by the unpaired Student’s t test (p < 0.05).
Uc.138 overexpression affects resistance to apoptosis
According to gene expression data from microarray analysis, one of the biofunctions enriched by differentially expressed genes was ‘cell death and survival’ (p = 8.19E−15) (Fig. 2A). Ingenuity pathway analysis showed that cell death-related genes were significantly down-regulated in uc.138-introduced cells (Fig. 4A). To further verify the cellular function of uc.138, we investigated whether the expression levels of uc.138 influenced the susceptibility to apoptotic cell death. As shown in Fig. 4B,D, overexpression of TRA2B4 and exon 2 significantly inhibited 5-fluorouracil (5-FU)- or adriamycin-induced cell death after 24 h treatment. Compared with mock-treated cells, the cell viability of TRA2B4 or exon 2-introduced cells were significantly increased at different time points (Fig. 4C,E). These results indicated that expression of uc.138 affected tumorigenic properties such as resistance to anticancer drugs in colon cancer cells.
Effect of uc.138 on susceptibility to an anticancer drug. (A) Canonical pathways for “apoptosis signaling” represented by the differentially expressed genes between uc.138 overexpression and mock-transfected cells were analyzed using Ingenuity Pathway Analysis (QIAGEN Bioinformatics). The genes up- and down-regulated in uc.138 overexpressed cells are shown in red and green, respectively. (B,D) Human colorectal carcinoma (HCT116) cells with stable overexpression of TRA2B4 or exon 2 were exposed to 5-fluorouracil (5-FU) or adriamycin for 24 h at indicated concentrations. Subsequently, growing cells were harvested and counted. (C,E) The number of cells with stable overexpression of TRA2B4 or exon 2 was measured after exposure to 10 μM 5-FU or 330 nM adriamycin at different time-points (0, 48 and 72 h). nt: non-treatment. Values are mean ± SD from four independent experiments. *Significantly different by the unpaired Student’s t test compared with mock-treated cells (p < 0.05).
Mutation of uc.138 impaired cell proliferation
In a previous study, we identified that uc.138 contains a stem-loop structure (449–488 nt) using the programs CentroidFold (http://rtools.cbrc.jp/centroidfold/) and M-FOLD (http://www.unafold.org/)20. Transient expression of TRA2B4 significantly decreased p21 levels and accelerated cell growth; however, the introduction of mutations in the stem-loop motif canceled these effects20. In this study, we established stable cells that overexpressed TRA2B4 with/without mutations and investigated the effect of uc.138 on the cell cycle. The Mfold program predicts a ΔG value of − 13.80 kcal/mol for wild-type stem-loop structure. As shown in Fig. 5A, two-point mutations were introduced to disrupt stem-loop formation (mut#1: 485-GGGG-488 to 485-AAGG-488, ΔG value of − 5.90 kcal/mol). Cells overexpressing TRA2B4 with mutations that did not influence the stem-loop structure (mut#2: 564-GGGG-567 to 564-AAGG-567, ΔG value of − 12.90 kcal/mol) were also generated. Stable overexpression of TRA2B4 or TRA2B4 mut#2 accelerated cell proliferation, whereas TRA2B4 mut#1 did not affect the proliferation rate (Fig. 5B). To measure the population of cells in the S phase, BrdU incorporation in vivo was analyzed by flow cytometry (Fig. 5C). The percentage of BrdU-positive cells was significantly increased by stable overexpression of TRA2B4 or TRA2B4 mut#2 (Fig. 5D); however, the expression of TRA2B4 mut#1 did not change BrdU incorporation. These results suggested that uc.138 was involved in the progression of the cell cycle in a sequence-dependent manner. We analyzed the subcellular localization of TRA2B4 wild-type and mutations. Corresponding with the cellular function, TRA2B4 wild-type and mut#2 transcripts were predominantly located in the nuclear of HCT116 cells (Supplementary Fig. S1). In contrast, mut#1 was more abundant in the cytoplasmic fraction (Supplementary Fig. S1). Using biotinylated RNA pull-down assays followed by LS/MS analysis, we reported that TRA2B4 interacted with several nuclear proteins such as Sp1, nucleolin, hnRNPA, and hnRNPU via exon 221. Thus, decreased nuclear expression of mut#1 may cause loss of cellular functions thorough defects of association with nuclear factors.
Effect of mutations in uc.138 on cell proliferation. (A) Nucleotide sequence of uc.138-encoding TRA2B4 exon 2. Predicted stem-loop structure (449–488 nt), the mutations of 485-GG-486 to 4865-AA-486 (mut#1) and 515-GG-516 to 515-AA-516 (mut#2) are indicated in the schema. (B) Effects of stable overexpression of TRA2B4 or TRA2B4 mutations on growth of human colorectal carcinoma (HCT116) cells were monitored by counting the number of cells at the indicated times. Values are mean ± SD from five independent experiments. *Significantly different by the unpaired Student’s t test compared with mock #1-treated cells (p < 0.05). (C) BrdU incorporation was analyzed using flow cytometry. Scatter plots of fluorescence intensities of BrdU incorporation (y-axis) against DNA content (x-axis) are shown. The numbers depict percentages of cells in S phase. 7-AAD; 7-amino-actinomycin D. (D) The quantitative statistics of percentage of the S phase. Values are mean ± SD (n = 3). *Significantly different by the unpaired Student’s t test compared with mock (p < 0.05).
Uc.138 promotes colon cancer cell migration and tumor growth
IPA of the 1067 differentially expressed genes showed Cellular Movement (P = 1.93E−03–4.86E−09) and Cell Morphology (P = 1.70E−03–6.66E−09) as significantly affected molecular and cellular functions. We tested cell migration as one of uc.138-related malignant phenotypes of cancer cells. To investigate whether uc.138 influences cancer cell migration, we performed transwell assays. Elevated expression of TRA2B4, exon 2, and TRA2B4 mut#2 caused an increase in cell migration (Fig. 6A,B). The overexpression of TRA2B4 mut#1 did not change migration activity.
Effect of uc.138 on cell migration and tumor progression. (A) Transwell migration assays were performed using human colorectal carcinoma (HCT116) cells with stable overexpressed TRA2B4, exon 2, TRA2B4 mut#1, or TRA2B4 mut#2. Migrated cells were stained by a Diff-Quick staining kit. (B) Quantification of cell migration was expressed by cell counting. Values represent mean ± SD (n = 8). *Significant difference versus TRA2B4 #1 (unpaired Student’s t test, p < 0.05). (C) Images of the tumors from the mice injected with cells which were stably overexpressed TRA2B4, TRA2B4 mut#1, or mock. (D) Tumor size in nude mice injected with HCT116 cells was measured every 7 days. Values represent mean ± SD (n = 6). *Significant difference versus mock (unpaired Student’s t test, p < 0.05).
To confirm the effect of uc.138 on tumor progression, we constructed a xenograft tumor model by injection of mock, TRA2B4- or TRA2B4 mut#1-overexpressed cells. When compared with mock injection, the TRA2B4 overexpressed cells exhibited a rapid rate of tumor growth as measured by tumor volumes (Fig. 6C,D). In contrast, injection of TRA2B4 mut#1-overexpressed cells did not accelerate tumor progression. We found that overexpression of TRA2B4 wild-type and mut#2 suppressed apoptosis induced after treatment of anticancer agents. Introduction of mut#2 did not affect resistance to apoptosis (Supplementary Fig. S2). Taken together, these results suggest that uc.138 enhances cell migration and tumor growth in colon cancer.
Discussion
There are 481 completely conserved sequences (referred to as UCRs) between the ortholog regions of human, mouse, and rat genomes1. Transcripts generated from UCRs (T-UCRs) are defined as a novel category of functional RNAs. Increasing evidence has indicated that aberrant expression of T-UCRs is shown in various types of cancers, and several T-UCRs act as oncogenes or tumor suppressors6. In this study, we show that uc.138, which is encoded in the TRA2B gene, is upregulated in human colon cancer tissues using RNA in situ hybridization. We assessed the effects of increased uc.138 expression on biological pathways by measuring changes in gene expression in stable uc.138-overexpressing cells. Stable overexpression of uc.138 in colon cancer cells significantly increased cell proliferation. Pathway analysis suggested that cancer-related pathways, including cellular development, cell proliferation, cell cycle, and cell death, were influenced by differentially expressed genes in uc.138-overexpressed cells. Stable overexpression of uc.138 increased cell migration in vitro and tumor formation in an in vivo murine xenograft model. Interestingly, the host gene of uc.138, TRA2B, transcribes five spliced variants (TRA2B1-TRA2B5)9. Among them, TRA2B4, which includes uc.138-containing exon 2, is retained in the nucleus and is not translated into the Tra2β protein21. TRA2B1 mRNA, which skips exon 2, can translocate to the cytoplasm and generate functional Tra2β protein. We previously reported that TRA2B1 mRNA and Tra2β were upregulated in colon cancer tissues15. Overexpression of Tra2β reduced colon cancer cell death by stabilizing the anti-apoptotic factor BCL216. These results suggest that different RNA variants, such as TRA2B1 and the functional RNA TRA2B4, can regulate cellular function through multiple pathways. Therefore, dysregulation of the TRA2B gene may be associated with promotion of tumorigenesis by not only Tra2β protein but also T-UCR uc.138.
Increasing evidence indicates that T-UCRs control physiological status and homeostasis, such as epithelial differentiation and renewal of the intestinal mucosa. For instance, uc.291 interacts with the chromatin remodeling protein ACTL6A and promotes epithelial differentiation22. Uc.173 is directly associated with and destabilizes pri-miR-195, resulting in the stimulation of intestinal epithelial renewal23. Uc.173 has an important role in intestinal epithelial barrier function by inhibiting the miR-29b/tight junction protein claudin-1 axis24. According to the recent studies about functions mediated by RNA-RNA interactions, we predicted microRNAs which were specifically associated with uc.138. We found that two putative miRNA target sites (miR-3925 and miR-6881, the minimum free energy of binding was − 16.4 and − 18.3 kcal/mol, respectively) in uc.138 using miRBase (http://www.mirbase.org/) and RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid). Although the functions of these miRNAs have not been reported yet, uc.138 sequences may contain the potential to post-transcriptionally interact with miRNAs.
Thus, dysregulation of T-UCRs in the intestinal epithelium may be associated with the development of diseases. In fact, the expression levels of uc.261 were elevated in intestinal mucosa in patients with Crohn’s disease and positively correlated with disease activity index and histological index7. Zhang et al. reported that uc.338 was upregulated in human colorectal cancer and increased cell proliferation by activation of the PI3K/AKT pathway25. The methylation status and expression levels of uc.160, uc.283, and uc.346 were dysregulated in neoplastic tissues from colorectal cancer, and uc.160 and uc.346 enhanced colorectal cancer cell progression26.
Although aberrant expression of T-UCRs occurs in many types of diseases, how T-UCRs contribute to cellular functions in general is still unclear. To date, several studies have shown that T-UCRs can regulate gene expression by binding to target proteins or mRNAs in a sequence-dependent manner. We previously reported that silencing of TRA2B4 inhibited cell growth through induction of p21/CDKN1A by accelerating interaction between Sp1 and the CDKN1A promoter20. Human fibroblast cell line (TIG-3) exhibited an age-associated reduction of TRA2B4 and expression levels of TRA2B4 and CDKN1A were negatively correlated20. Here, cell proliferation assay using stable cell lines suggested that p21 was one of the key regulators of uc.138-repated cell cycle progression. In non-small lung cancer cells, uc.339 functioned as a decay for microRNAs that targeted Cyclin E2 and promoted cell growth and migration27. Overexpression of uc.338 in cervical cancer cells promoted lymph node metastasis by binding to the 3′-UTR of tissue inhibitor of metalloproteinase 128. Uc.323 is associated with a histone methyltransferase EZH2 and decreased transcription of cardiac hypertrophy-related CPT1b, which ameliorated cardiac hypertrophy29.
In this study, we demonstrated that uc.138 was significantly upregulated in colon cancer tissues. Overexpression of uc.138 was associated with an increase in cell proliferation, migration, and tumor progression. Further studies are needed to reveal the pathological signatures that influence the aberrant expression of uc.138. Our previous study showed that the transcription of the TRA2B gene encoding uc.138 is upregulated by activation of HSF1 in response to oxidative stress in human colon cancer cells15. Since TRA2B4 contains PTCs, TRA2B4 is supposed to be degraded by NMD as an aberrant RNA. The expression levels of TRA2B4 were significantly lower than that of TRA2B1 in normal colon epithelial cells20. Oxidative stress-induced phosphorylation of HuR leads to aberrant alternative splicing of TRA2B pre-mRNAs and increases TRA2B4 generation in colon cancer cells19. In addition, TRA2B4 was protected in the nuclear by association with Nucleolin in colon cancer21. These posttranscriptional regulation of TRA2B4 might be the reason why TRA2B4 showed more significant increase in cancer cells as compared with TRA2B1. Altered expression of T-UCRs caused by cellular stimuli can disrupt homeostasis within tissue microenvironments. Taken together, these results suggest that tumorigenic T-UCR uc.138 may become a potential biomarker and novel therapeutic target in patients with colon cancer.
Materials and methods
Cell culture and transfection
Human colon cancer cell lines HCT116 (CCL-247) were obtained from American Type Culture Collection (ATCC) (Manassas, VA) and cultured in McCoy's 5A medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 5% (v/v) heat-inactivated fetal bovine serum and antibiotics (penicillin and streptomycin) at 37 °C in 5% CO2. The human full-length TRA2B4 (exon 1 to exon 10) and exon 2 were amplified using PCR with the primer set listed in Supplementary Table 1. The amplified products were separated with a gel extraction kit (Qiagen, Hilden, Germany) and cloned into the mammalian expression vector pEB-Multi-Bsd (Wako, Osaka, Japan) using XhoI and EcoRV sites. Point mutations were introduced into pEB-Multi TRA2B4 vector using a site-directed mutagenesis kit (KOD-Plus-Mutagenesis Kit; Toyobo, Osaka, Japan). The construct sequence was confirmed by DNA sequencing. HCT116 cells were then transfected with either empty, TRA2B4, or an exon 2-expression vector using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. The stably transfected cells were selected using medium containing 5 µg/mL of Blasticidin S. (InvivoGen, San Diego, CA) for ~ 7 days.
Western blotting
Whole cell lysates were prepared using RIPA buffer (Thermo Fisher Scientific) with a complete protease inhibitor cocktail (Roche, Mannheim, Germany) as previously described15. Ten micrograms of the extracted proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA, USA). After blocking with 5% nonfat dry milk (Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature, the membrane was incubated with anti-p21 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-cyclin A, anti-cyclin B, anti-Rb, anti-phospho-Rb, anti-phospho-Cdk1, and anti-Cdk1 (Cell Signaling Technology); or anti-glyceraldehyde 3-phosphate dehydrogenase (Gapdh) (Santa Cruz) antibody overnight at 4 °C. Following incubation with an appropriate secondary antibody for 1 h at room temperature, the bound antibodies were detected with Pierce Western Blotting Substrate (Thermo Fisher Scientific). The intensities of the bound antibodies were quantified using ImageJ (National Institutes of Health, USA).
qPCR
Total RNA was extracted from cells using RNAiso Plus (Takara, Tokyo, Japan) according to the manufacturer’s protocol. Isolated RNAs were reverse transcribed using ReverTra Ace qPCR RT Master Mix (Toyobo). The expression levels of TRA2B1, TRA2B4, CCNA1, CCNB2, CDKN1A, ACTB, and GAPDH mRNA were measured using specific primer sets and SYBR Green Master Mix (Thermo Fisher Scientific) (Supplementary Table S1).
RNA in situ hybridization
The colon cancer TMA was provided by Provitro (Berlin, Germany; colon carcinoma tissues, no. 401 2211). Digoxigenin-labeled antisense RNA complementary to exon 2 (313–588 nt) of TRA2B4 was generated using a DIG RNA labeling kit (Roche). The TMA sections were deparaffinized in xylene and then hydrated in graded concentrations of ethanol for 5 min each. These sections were incubated with 3% hydrogen peroxide and then hybridized at 55 °C with the RNA probes at 200 ng/mL overnight. After hybridization, tissue sections were washed in 5 × saline-sodium citrate (SSC), 50% formamide, and again with 0.5 × saline-sodium citrate. Then, the sections were incubated with 1:500 of an anti-digoxigenin antibody (Roche) overnight at 4 °C. After washing with TBS-T, sections were incubated with avidin–biotin complex solution (horseradish peroxidase-streptavidin–biotin complex, Vectastain ABC kit; Vector Laboratories) according to the manufacturer’s protocol. The final signal was developed with diaminobenzidine solution, and the tissues were counterstained with hematoxylin for 15 s. The signals were classified as negative (−) or positive (+) and counted.
Polysome profiling
For the isolation of polysome fractions and polysome profiling, 10 × 106 HCT116 cells were pretreated with cycloheximide at a final concentration of 100 µg/mL for 10 min. Cytoplasmic lysates were prepared using lysis buffer (20 mM Tris–HCl, pH 7.5, 100 mM KCl, 5 mM MgCl2, 0.3% IGEPAL, and protease inhibitors). Cytoplasmic extracts were fractionated by ultracentrifugation through 10–50% linear sucrose gradients. For measurements of RNA distributions, 14 fractions were collected, and RNA was extracted with RNAiso Plus (TaKaRa).
Cell migration assay
Cell migration was examined using transwell chambers (Becton-Dickinson Biosciences, Franklin Lakes, NJ, USA) as previously described30. In brief, after serum starvation for 48 h, cells were seeded in serum-free media on the upper side of a transwell chamber. The cells were allowed to migrate towards media containing 10% fetal bovine serum in the lower chamber. After incubation for 24 h, migrating cells were fixed and then stained with Diff-Quick stain (Sysmex, Kobe, Japan). The numbers of migrated cells were estimated as the average number of 5 random fields at 20 × magnification.
Cell cycle analysis
The cells were harvested and fixed in 70% ethanol. After washing with phosphate-buffered solution, the cells were treated with 5 μg/mL of RNAse A and then stained in a solution containing 10 μg/mL of propidium iodide (Sigma-Aldrich). After 30 min staining, the samples were analyzed by flow cytometry using a BD FACSVerse (BD Biosciences). BrdU incorporation in vivo was measured by flow cytometry using the APC BrdU Flow Kit (BD Biosciences) in accordance with the manufacturer’s instructions.
Microarray analysis
Total RNA was extracted from cells using a RNeasy kit (Qiagen) according to the manufacturer’s protocol. Purified RNA quality was assessed using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano Labchip kit (Agilent Technologies, Santa Clara, CA, USA). RNA samples with > 9.0 RNA integrity number were used for further experiments. Expression profiles were measured using a whole human genome microarray (SurePrint G3 Human; Agilent). The expression data were analyzed using GeneSpring 14.9 (Agilent). Differentially expressed genes were subjected to Ingenuity Pathway Analysis (Qaigen Bioinformatics) to identify biologically relevant functions.
Mouse xenograft experiments
Seven-week-old male athymic nude mice (Nippon SLC, Japan) were acclimated for 1 week. Mock, TRA2B4, or TRA2B4#1-overexpressed HCT116 cells (2 × 106) were suspended in 100 μL phosphate-buffered solution and injected subcutaneously into the flanks of nude mice. Tumor volumes were examined every 7 days after transplantation. Four weeks after the injection, the mice were sacrificed, and the tumor volumes (length × width2 × 0.5) were measured.
All animal procedures conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The experiments were carried out in accordance with the Guidelines for Animal Experiments of Tokushima University approved by Institutional Animal Care and Use Committee of Tokushima University (Approval number: T2820). All animal experiments were performed in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (https://arriveguidelines.org/arrive-guidelines).
Statistical analysis
All statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Results are expressed as mean ± SD. Significant differences between two groups were estimated by two-tailed Student’s t test. Non-parametric data were analyzed using the Wilcoxon–Mann–Whitney U test when comparing two groups. *p < 0.05 was considered statistically significant.
Abbreviations
- TRA2B :
-
Transformer 2-beta
- UCR:
-
Ultraconserved region
- T-UCR:
-
Transcribed-ultraconserved region
- qPCR:
-
Real-time quantitative polymerase chain reaction
References
Bejerano, G. et al. Ultraconserved elements in the human genome. Science 304, 1321–1325 (2004).
Scaruffi, P. The transcribed-ultraconserved regions: A novel class of long noncoding RNAs involved in cancer susceptibility. Sci. World J. 11, 340–352 (2011).
Calin, G. A. et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell 12, 215–229 (2007).
Braconi, C. et al. Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma. Proc. Natl. Acad. Sci. U.S.A. 108, 786–791 (2011).
Peng, J. C., Shen, J. & Ran, Z. H. Transcribed ultraconserved region in human cancers. RNA Biol. 10, 1771–1777 (2013).
Terracciano, D. et al. The role of a new class of long noncoding RNAs transcribed from ultraconserved regions in cancer. Biochim. Biophys. Acta Rev. Cancer 1868, 449–455 (2017).
Qian, X. et al. Transcribed ultraconserved region (T-UCR) uc.261 expression is closely correlated with disease activity and intestinal permeability in Crohn’s disease. Ther. Adv. Gastroenterol. 12, 1756284819880733 (2019).
das Chagas, P. F. et al. Ultraconserved long non-coding RNA uc.112 is highly expressed in childhood T versus B-cell acute lymphoblastic leukemia. Hematol. Transfus. Cell Ther. 20, 30005 (2020).
Dauwalder, B., Amaya-Manzanares, F. & Mattox, W. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc. Natl. Acad. Sci. 93, 9004–9009 (1996).
Tsuda, K. et al. Structural basis for the dual RNA-recognition modes of human Tra2-β RRM. Nucleic Acids Res. 39, 1538–1553 (2011).
Watermann, D. O. et al. Splicing factor Tra2-β1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer Res. 66, 4774–4780 (2006).
Best, A. et al. Expression of Tra2 ?? in cancer cells as a potential contributory factor to neoplasia and metastasis. Int. J. Cell Biol. 2013, 843781 (2013).
Li, F. et al. Identification of TRA2B-DNAH5 fusion as a novel oncogenic driver in human lung squamous cell carcinoma. Cell Res. 26, 1149–1164 (2016).
Paudel, D. et al. Expression of TRA2B in endometrial carcinoma and its regulatory roles in endometrial carcinoma cells. Oncol. Lett. 18, 2455–2463 (2019).
Kajita, K. et al. Ets1 and heat shock factor 1 regulate transcription of the Transformer 2β gene in human colon cancer cells. J. Gastroenterol. 48, 1222–1233 (2013).
Kuwano, Y. et al. Transformer 2β and miR-204 regulate apoptosis through competitive binding to 3′ UTR of BCL2 mRNA. Cell Death Differ. 22, 815–825 (2015).
Liu, J. et al. miR-335 inhibited cell proliferation of lung cancer cells by target Tra2β. Cancer Sci. 109, 289–296 (2018).
Nayler, O., Cap, C. & Stamm, S. Human transformer-2-beta gene (SFRS10): Complete nucleotide sequence, chromosomal localization, and generation of a tissue-specific isoform. Genomics 53, 191–202 (1998).
Akaike, Y. et al. HuR regulates alternative splicing of the TRA2 gene in human colon cancer cells under oxidative stress. Mol. Cell. Biol. 34, 2857–2873 (2014).
Kajita, K. et al. Ultraconserved region-containing Transformer 2β4 controls senescence of colon cancer cells. Oncogenesis 5, e213 (2016).
Satake, Y. et al. Nucleolin facilitates nuclear retention of an ultraconserved region containing TRA2β4 and accelerates colon cancer cell growth. Oncotarget 9, 26817–26833 (2018).
Panatta, E. et al. Long non-coding RNA uc.291 controls epithelial differentiation by interfering with the ACTL6A/BAF complex. EMBO Rep. 21, 1–14 (2020).
Xiao, L. et al. Long noncoding RNA uc.173 promotes renewal of the intestinal mucosa by inducing degradation of microRNA 195. Gastroenterology 154, 599–611 (2019).
Wang, J. et al. Regulation of intestinal epithelial barrier function by long noncoding RNA uc.173 through interaction with microRNA 29b. Mol. Cell. Biol. 38, e00010-18 (2018).
Zhang, Y. et al. Uc.338 targets p21 and cyclin D1 via PI3K/AKT pathway activation to promote cell proliferation in colorectal cancer. Oncol. Rep. 40, 1119–1128 (2018).
Kottorou, A. E. et al. Differentially methylated ultra-conserved regions Uc160 and Uc283 in adenomas and adenocarcinomas are associated with overall survival of colorectal cancer patients. Cancers 12, 895 (2020).
Vannini, I. et al. Transcribed ultraconserved region 339 promotes carcinogenesis by modulating tumor suppressor microRNAs. Nat. Commun. 8, 1–19 (2017).
Li, Q., Shen, F. & Wang, C. TUC338 promotes cell migration and invasion by targeting TIMP1 in cervical cancer. Oncol. Lett. 13, 4526–4532 (2017).
Sun, Y. et al. Transcribed ultraconserved regions, Uc.323, ameliorates cardiac hypertrophy by regulating the transcription of CPT1b (carnitine palmitoyl transferase 1b). Hypertension 75, 79–90 (2020).
Nishida, K., Kuwano, Y. & Rokutan, K. The microRNA-23b/27b/24 cluster facilitates colon cancer cell migration by targeting FOXP2. Cancers 12, 174 (2020).
Acknowledgements
This research was supported by research grants (#18K07941) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Y.K) and Takeda Science Foundation. This study was supported by Support Center for Advanced Medical Sciences, Institute of Biomedical Sciences, Tokushima University Graduate School.
Author information
Authors and Affiliations
Contributions
This study was conceived and supervised by Y.K. and K.R.; Y.K. and K.N. performed most of the experiments. K.N. contributed with biochemical and microscope analysis. Y.K. and K.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuwano, Y., Nishida, K. & Rokutan, K. Overexpression of the transcribed ultraconserved region Uc.138 accelerates colon cancer progression. Sci Rep 11, 8667 (2021). https://doi.org/10.1038/s41598-021-88123-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88123-9
- Springer Nature Limited
This article is cited by
-
Ultra-conserved RNA: a novel biological tool with diagnostic and therapeutic potential
Discover Oncology (2023)