Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is one of the most important differential diagnosis in patients with thunderclap headache (TCH). We aimed to develop a new scoring system for RCVS in patients with TCH. We retrospectively analyzed 72 patients enrolled in the prospective study of TCH conducted in 2015–2016 (derivation set). We identified possible predictors for the diagnosis of RCVS and constructed a prediction model (RCVS–TCH score) using the multivariable logistic regression model. Diagnostic performance was validated to an independent validation set from our headache registry. The derivation set comprised 41 patients with RCVS and 31 with non-RCVS, and the validation set included 253 patients with TCH (165 with RCVS and 88 with non-RCVS). The RCVS–TCH score (range: 0–12) contained four predictors: recurrent TCHs, female sex, triggering factor for TCH (single or multi) and blood pressure surge. The C-index of RCVS–TCH score was 0.929 (95% CI = 0.874–0.984). The RCVS–TCH score ≥ 7 had a sensitivity of 80% and a specificity of 97% in discriminating RCVS from non-RCVS. In the validation set, RCVS–TCH score showed a C-index of 0.861 (95% CI = 0.815–0.908). In our study, the RCVS–TCH showed good performance, which may aid the diagnosis of RCVS among patients with TCH.
Similar content being viewed by others
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) is a clinical and radiological syndrome characterized by recurrent thunderclap headaches (TCHs) and reversible cerebral vasoconstriction of the cerebral arteries1. It is one of the most important differential diagnosis in patients with TCH because a substantial proportion of patients with RCVS can have neurological complications such as ischemic stroke, cortical subarachnoid hemorrhage (SAH), intracerebral hemorrhage, and posterior reversible encephalopathy syndrome (PRES)2,3,4,5,6. However, the diagnosis of RCVS can be challenging because of overlapping clinical features with other disorders presenting with TCH and lower sensitivity of angiography during the earlier phases of disease7.
Recently, the RCVS2 score was proposed as a diagnostic tool to distinguish RCVS in patients with intracranial vasculopathies8. The score includes clinical and imaging features such as recurrent/single thunderclap headache, vasoconstrictive trigger, sex, SAH, and carotid artery involvement. This score showed excellent performance in distinguishing between RCVS and intracranial vasculopathies. However, the presence of thunderclap headache is the major component of the RCVS2 score, and this alone can lead to the diagnosis of RCVS. Therefore, the RCVS2 score would not be useful for the differential diagnosis of TCH, and any patients with TCH can be falsely classified as having RCVS using this score.
Therefore, in this study, we aimed to develop a new prediction model for the diagnosis of RCVS in patients with TCH. We validated the performance of our prediction model and compared it with the RCVS2 score in unselected patients with TCH.
Methods
Study setting
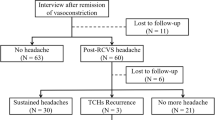
For the derivation set, we retrospectively included 72 patients with TCH who participated in a prospective imaging study conducted from April 2015 to July 2016 at the Samsung Medical Center, Seoul, Korea9. Patients who (1) clearly remembered the mode of onset, (2) reported the time from headache onset to its peak to be < 60 s, and (3) visited within 1 month after the first attack were included, whereas those with (1) aneurysmal SAH, (2) contraindications to magnetic resonance imaging (MRI) or gadolinium enhancement, and (3) clinical manifestations suggestive of infectious meningitis were excluded. Diagnoses were based on neuroimaging findings and the International Classification of Headache Disorders (ICHD)–3 beta version10. Definite RCVS were diagnosed when the multifocal vasoconstrictions were not explained by SAH and normalized within 3–6 months without immunotherapy. The diagnoses of probable RCVS and primary TCH were based on ICHD–3 beta version criteria10. Forty-one patients had RCVS, and 31 had primary TCH or other secondary causes. The Institutional Review Board of Samsung Medical Center approved this study. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained for all patients at the inclusion visit.
Clinical and imaging evaluation
Our protocol for evaluating TCH was described previously9. To summarize, the protocol was depended on the site of recruitment: emergency room (ER), outpatient headache clinic, or inpatient consultation. All patients presenting to ER underwent emergent non-contrast brain computed tomography (CT) and post-contrast CT angiography (CTA) to exclude aneurysmal SAH. In equivocal cases, lumbar puncture was done for detecting xanthochromia. In case of suspected SAH, transfemoral cerebral angiography was performed to confirm the presence of a ruptured aneurysm. The same protocol was applied in case of inpatient consultation. In the outpatient headache clinic, patients were primarily evaluated using brain MRI and MRA, whereas patients with persistent headaches were referred to the ER and the emergency protocol was then applied. For patients who were suspected of having RCVS, neuroimaging was followed-up for 3–6 months to confirm reversibility of vasoconstrictions.
We extracted clinical data of patients from a structured questionnaire, which included headache characteristics specifically designed for the evaluation of TCHs11. We collected information on the recurrence pattern (single or recurrent TCHs), triggering factors (situations or activities known to trigger TCH in RCVS, such as sexual activity, exertion, Valsalva maneuvers, emotion, bending, bathing and/or showering), the presence of a premorbid migraine, and blood pressure (BP) surge defined as systolic BP of > 160 mmHg during headache attacks or > 30 mmHg from baseline3. In the case of RCVS, the cause was classified into idiopathic or secondary (having medical conditions known to cause RCVS, such as postpartum period or vasoactive drugs). We investigated neurological manifestations such as transient focal neurological symptoms and seizure reported by patients or reliable informants. We assessed brain MRI and MRA to analyze neurological complications such as ischemic stroke, hemorrhage (parenchymal or subarachnoid) and PRES, and the distribution of vasoconstriction. We also analyzed other structural or vascular lesions that could be the secondary cause of TCH occurrence.
Development of a new prediction model (the RCVS–TCH score)
To develop a prediction model for RCVS using the derivation set, we performed the univariable logistic regression analysis of clinical and imaging factors associated with RCVS. Then, we selected a list of candidate predictors of RCVS that were significant in univariable logistic regression with a p value of < 0.05. Selected variables were entered into the multivariable logistic regression model to assign a score value to each variable. The integer value closest to the beta coefficient of each variable was assigned as the weighted score. The sum of the weighted score was named as the “RCVS–TCH score.” For the diagnostic performance of the RCVS–TCH score, discriminative power was assessed using the concordance index (C-index), and calibration power was assessed by using the Hosmer and Lemeshow goodness-of-fit test12. The specificity, sensitivity, and positive and negative predictive values were calculated by analyzing the receiver operating characteristic curve (ROC). The Youden index was used to determine the optimal cut-off score. The accuracy and likelihood ratio were calculated for a cut‐off score based on the sensitivity and specificity13.
Validation of the RCVS–TCH score and comparison with the RCVS2 score
For validation, we screened patients from the prospective headache registry of the Samsung Medical Center and extracted 253 patients with TCH who completed neuroimaging work-up within 1 month after onset. Of them, 165 had RCVS, 72 had primary TCH, and 16 had other secondary causes of TCH including intracranial artery dissection (n = 9), sentinel headache (n = 5), cardiac cephalalgia (n = 1) and airplane headache with sinus barotrauma (n = 1). These patients served as an independent validation set. Using this validation set, the diagnostic performance of the RCVS–TCH score was validated and compared with that of the RCVS2 score8.
As mentioned earlier, the RCVS2 score comprises five predictors. The RCVS2 score is the sum of the component scores (range − 2 to + 10), and a higher RCVS2 score indicates a higher possibility of RCVS. A total RCVS2 score of ≥ 5 was suggested as a cut-off score for diagnosing RCVS. Because “vasoconstrictive trigger,” one of the RCVS2 score components, might be confused with “triggering factor” for TCH, we used “etiology of RCVS” instead of vasoconstrictive trigger.
Statistical analysis
Data are presented as number (percentage) or median (interquartile range, IQR), unless otherwise specified. Categorical variables were compared using the Chi-square test or Fisher’s exact test, and continuous variables were analyzed using the Student’s t-test or Mann–Whitney U test. Missing data were not imputed and were excluded from the associated analysis. Statistical analyses were performed using IBM SPSS 22.0 (IBM, Inc.) and R 3.6.0 (Vienna, Austria; http://www.R-project.org/). A two-tailed p value of < 0.05 was considered significant.
Results
Patients
Demographics and clinical characteristics of patients in the derivation and validation sets are shown in Table 1. When the RCVS group was compared with the non-RCVS group, female predominance was observed. Recurrent TCHs were reported in 181 (87.9%) patients. Triggering factors for TCH were reported in 163 (79.1%) patients. Among them, 82 (38.9%) patients reported two or more triggering factors. BP surge was observed in 82 (39.8%) patients. When compared with the non-RCVS, female sex, recurrent TCHs, multi-triggers for TCH, and BP surge were more frequently seen in the RCVS group in both datasets. Nine (4.4%) patients with RCVS had secondary causes of RCVS.
Development of a prediction model: the RCVS–TCH score
In the univariable logistic regression analysis of the derivation set, recurrent TCHs (Beta = 2.31, OR = 10.08, 95% CI = 3.37–30.13, p < 0.001), female sex (Beta = 1.65, OR = 5.18, 95% CI = 1.27–21.19, p = 0.022), the presence of triggering factors for TCH (single, Beta = 2.37, OR = 10.68, 95% CI = 2.62–43.48, p = 0.001; multiple, Beta = 2.85, OR = 17.25, 95% CI = 4.41–67.44, p = 0.001), and BP surge (Beta = 3.25, OR = 25.91, 95% CI = 3.22–208.38, p = 0.002) were associated with RCVS. Age, etiology of RCVS, carotid artery involvement, and neurological complications were not significant.
Table 2 shows results of multivariable regression analysis and score of the developed prediction model for the diagnosis of RCVS: RCVS–TCH score (range: 0–12). The ROC curve of RCVS–TCH score in the derivation data set was shown in Fig. 1. The C-index was 0.929 (95% CI = 0.874–0.984), and the Hosmer and Lemeshow goodness of fit was not significant (χ2 = 3.651, p = 0.887). RCVS–TCH score ≥ 7 was selected as the cut-off score for the diagnosis of RCVS. RCVS–TCH score ≥ 7 yielded an accuracy of 87%, a positive likelihood ratio of 26.7, a negative likelihood ratio of 0.2, a sensitivity of 80%, a specificity of 97%, and a positive predictive value of 97% and negative predictive value of 79% (Table 3).
Receiver operating characteristic curve analysis for the diagnosis of reversible cerebral vasoconstriction syndrome (RCVS). Data were analyzed using the derivation data set. Solid red curve represents the RCVS–TCH score with Area Under the Curve (AUC) of 0.929 (95% CI = 0.874–0.984). Diagonal dashed line is a reference line (AUC = 0.5).
Diagnostic performance of the RCVS–TCH and RCVS2 scores
The RCVS–TCH score was validated using the validation data set. The C-index was 0.861 (95% CI = 0.815–0.908), and the Hosmer and Lemeshow goodness of fit was not significant (χ2 = 7.038, p = 0.533). RCVS–TCH score ≥ 7 yielded an accuracy of 77%, a positive likelihood ratio of 3.5, a negative likelihood ratio of 0.3, a sensitivity of 77%, a specificity of 78%, and a positive predictive value of 88% and negative predictive value of 61%. When the RCVS2 score was validated using the same dataset, the C-index was 0.601 (95% CI = 0.526–0.677). RCVS2 score ≥ 5 yielded an accuracy of 65%, a positive likelihood ratio of 1.0, a negative likelihood ratio of 0.5, a sensitivity of 96%, a specificity of 8%, and a positive predictive value of 66% and negative predictive value of 50%. The ROC curves between RCVS–TCH score and RCVS2 score were compared in Fig. 2. Figure 3 shows the distribution of scores in the RCVS and non-RCVS groups. In the derivation set, 33 patients with RCVS and only one non-RCVS patient (score 7, the patient with primary TCH) (Fig. 3A) scored ≥ 7. In the validation data set, 119 patients with RCVS, 12 with primary TCH, and 5 with other secondary causes of TCH (sentinel headache, n = 2 and intracranial arterial dissection without RCVS, n = 3) scored ≥ 7 (Fig. 3B).
Receiver operating characteristic curve (ROC) analysis for the diagnosis of reversible cerebral vasoconstriction syndrome (RCVS). Data were analyzed using the validation data set. Solid red line represents the ROC of RCVS–TCH score with Area Under the Curve (AUC) of 0.861 (95% CI = 0.815–0.908). Solid blue line represents the ROC of RCVS2 score with AUC of 0.601 (95% CI = 0.526–0.677). Diagonal dashed line us a reference line (AUC = 0.5).
Distribution of the RCVS–TCH scores in the derivation and validation datasets. Histograms show the distribution of the RCVS–TCH score between the RCVS and non-RCVS groups in each derivation (A) and validation (B) set. Black bars indicate the number of patients with RCVS, and grey bars indicate the number of patients with non-RCVS.
Discussions
We developed a new prediction model, the RCVS–TCH score, to aid the diagnosis of RCVS in patients with TCH. Our model reliably predicted the diagnosis of RCVS based on clinical features. The RCVS–TCH score showed a superior performance to the RCVS2 score.
In this study, the RCVS–TCH score showed a good performance, with a high discriminative power and good calibration power. Our score also showed good accuracy and strong likelihood ratio with high sensitivity and specificity to predict RCVS among patients with TCH. In contrast, the RCVS2 score showed poor performance with poor accuracy in this patient population. In a previous study, the RCVS2 score showed an excellent performance to predict RCVS in patients with arteriopathies8. However, the score of TCH was highest, which led that the presence of TCH alone would be enough to classify RCVS under the RCVS2 score system. However, several disorders other than RCVS can cause TCH, but the RCVS2 score would not be useful in the differential diagnosis of TCH. Furthermore, aneurysmal SAH is most important differential diagnosis because it requires an emergent therapeutic approach, otherwise it would cause significant morbidity or even death14,15. This should be considered first in patients with TCH. Brain non-contrast CT, CTA, and lumbar puncture have high specificities and sensitivities to exclude aneurysmal SAH, so they should be conducted before MRI and MRA when aneurysmal SAH is suspected16. This cannot be replaced by clinical scoring such as the RCVS2 and RCVS–TCH scores. We recruited patients with TCH after carefully excluding aneurysmal SAH as a validation set. Among them, 33% had disorders other than RCVS. This finding again highlights that not all TCHs are RCVS. The involvement of the internal carotid artery was not included in our score model. This might be because our study participants were all Asians in whom asymptomatic intracranial atherosclerosis is common17. Nevertheless, our finding is in line with that of Rocha et al.’s study8 because the internal carotid artery was rarely involved in RCVS, even though it was similarly rare in patients with non-RCVS in our study.
In this study, female sex, recurrent TCHs, and triggering factors for TCH were used for the development of the RCVS–TCH score. This is in line with previous studies reporting that 81–90% of patients with RCVS were women, 78–100% had recurrent TCHs, and 75–80% had one or more triggering factors for TCH2,6,18. Among these clinical factors, recurrent TCHs and the presence of triggers are included in the ICHD-3 criteria for acute headache attributed to RCVS (6.7.3.1) or probable RCVS (6.7.3.2)10,19. While our study results support the feasibility of the ICHD-3 criteria, the addition of female sex could be considered in the next version of ICHD10,19. Although we reported the importance of female sex for the diagnosis of RCVS, it should not be overlooked that despite a small proportion, male sex could be diagnosed with RCVS. We also found that multiple triggering factors were more associated with RCVS compared to single triggering factor. This finding suggests that the multiplicity of triggering factors might be worthy to be considered when revising the diagnostic criteria of RCVS.
In our study, BP surge was observed in 47% of patients with RCVS, which is similar to the findings in previous studies2,6. BP surge was the most powerful predictor of RCVS in the RCVS–TCH score. The pathophysiology of BP surge in RCVS has not been fully elucidated, but it is thought that BP surge might result from either sympathetic overactivity or a stress response to excruciating headaches20. In our study, only a small proportion of non-RCVS patients with TCH had BP surge, suggesting that BP surge may be caused not by a response to severe headache but by the unique pathophysiology of RCVS.
Timely and accurate diagnosis of RCVS is necessary to ensure appropriate patient care and avoid unnecessary diagnostic tests. Early recognition of RCVS is important because RCVS can cause neurological complications such as intracranial hemorrhage and PRES during the early stage6,21. The diagnosis of RCVS is based on typical clinical features and reversibility of multiple vasoconstrictions. However, the diagnosis is often challenging because RCVS can have diverse clinical manifestations and angiographic findings can be negative during the early phase of disease22,23. A previous study showed that the first angiogram within 1 week was normal in up to 30% of patients who were suspicious of RCVS6. Furthermore, the diagnosis of RCVS can be challenging because its clinical features overlap with other thunderclap headache disorders24,25,26,27. Even though clinical and angiographic features are compatible with RCVS, the definitive diagnosis of RCVS can be delayed until reversibility of vasoconstriction is confirmed. It can be difficult to differentiate vasoconstrictions of RCVS with those of other various diseases such as intracranial atherosclerosis, intracranial dissection, primary angiitis of the central nervous system, and moyamoya disease.
In our study, the RCVS–TCH score showed high specificity and sensitivity for discriminating RCVS in patients with TCH. We suggest using the RCVS–TCH score for the differential diagnosis of TCH after aneurysmal SAH has been excluded using appropriate diagnostic methods. The RCVS–TCH score can be useful particularly when the angiographic finding is negative or equivocal. The RCVS2 score was not discriminative of RCVS in patients with TCH. On the other hand, RCVS2 score would be helpful in the differential diagnosis of intracranial angiopathies8. These scores may help physicians to determine the necessity of further work-ups such as repeated noninvasive angiography, transfemoral conventional angiography, and brain biopsy and initial tentative treatment (calcium-channel blockers for RCVS vs. steroid for angiitis).
This is the first study to develop a prediction model for the diagnosis of RCVS in patients with TCH. To develop this model, we used high-quality data collected in a previous prospective study. Validation was performed using a large group of unselected patients with TCHs. Nevertheless, there are several limitations to this study. First, the sample size was relatively small in the derivation set. In addition, the data were obtained from a single center and a single ethnicity (i.e. Asians). These factors may limit the generalizability of study findings. However, we validated using large, unselected and prospectively recruited patients and the prevalence of key factors used in the RCVS–TCH score was similar between our cohort and others. Nevertheless, future studies using this scoring system on a more heterogeneous group of patients are needed. Second, patients were included after excluding aneurysmal subarachnoid hemorrhages. So, our study findings are not applicable to all patients with TCH presenting to the emergency department. Careful exclusion of aneurysmal SAH should be performed first. Third, non-RCVS patients mainly comprised those with primary TCH. Considering the low prevalence of other secondary causes among patients with TCH, it might be that there were few patients with secondary causes. External validation is required to confirm that this model will be useful to distinguish RCVS from other secondary causes. Fourth, it was challenging to distinguish RCVS from intracranial artery dissection because 25% of patients with intracranial artery dissection were more than cut-off score. This may be because of similar clinical features between intracranial artery dissection and RCVS. In addition to our results, future studies should investigate the high-resolution vessel wall imaging to discriminate between them more accurately. Lastly, in terms of patient population, our study and the study of Rocca et al. differ that our patient population was those with thunderclap headache whereas RCVS2 score was proposed as a diagnostic tool to distinguish RCVS in patients with intracranial vasculopathies (regardless of the presence of headache). These important differences motivated the design and conceptualization of our study. We recommend to choose the RCVS–TCH score or RCVS2 score according to the clinical setting. The RCVS–TCH score should be considered first in the differential diagnosis of thunderclap headache, whereas RCVS2 score would be helpful in the differential diagnosis of intracranial angiopathies.
Conclusions
The RCVS–TCH score, a new prediction model for RCVS among patients with TCH, showed good performance in distinguishing RCVS from primary TCH or other secondary causes of TCH. Our findings would aid the diagnosis of RCVS among patients with TCH when the aneurysmal subarachnoid hemorrhages were excluded.
Data availability
Any data not published within this article will be shared, in an anonymized data, will be shared by request from any qualified investigator.
References
Calabrese, L. H., Dodick, D. W., Schwedt, T. J. & Singhal, A. B. Narrative review: reversible cerebral vasoconstriction syndromes. Ann. Intern. Med. 146, 34–44 (2007).
Chen, S. P. et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann. Neurol. 67, 648–656 (2010).
Chen, S. P., Fuh, J. L., Lirng, J. F., Chang, F. C. & Wang, S. J. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder?. Neurology 67, 2164–2169. https://doi.org/10.1212/01.wnl.0000249115.63436.6d (2006).
Dodick, D. W., Brown, R. Jr., Britton, J. & Huston, J. III. Nonaneurysmal thunderclap headache with diffuse, multifocal, segmental, and reversible vasospasm. Cephalalgia 19, 118–123 (1999).
Ducros, A. et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 130, 3091–3101 (2007).
Ducros, A. et al. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke 41, 2505–2511 (2010).
Ducros, A. & Bousser, M. G. Thunderclap headache. BMJ (Clin. Res. Ed.) 346, e8557. https://doi.org/10.1136/bmj.e8557 (2013).
Rocha, E. A., Topcuoglu, M. A., Silva, G. S. & Singhal, A. B. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology 92, e639–e647 (2019).
Lee, M. J. et al. Blood–brain barrier breakdown in reversible cerebral vasoconstriction syndrome: Implications for pathophysiology and diagnosis. Ann. Neurol. 81, 454–466 (2017).
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33, 629–808. https://doi.org/10.1177/0333102413485658 (2013).
Lee, M. J., Choi, H. A., Choi, H. & Chung, C.-S. Serial testing of the ICHD-3 beta diagnostic criteria for probable reversible cerebral vasoconstriction syndrome: a prospective validation study. Cephalalgia 38, 1665–1671 (2018).
Steyerberg, E. W. et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21, 128–138. https://doi.org/10.1097/EDE.0b013e3181c30fb2 (2010).
Deeks, J. J. & Altman, D. G. Diagnostic tests 4: likelihood ratios. BMJ (Clin. Res. Ed.) 329, 168–169 (2004).
Schievink, W. I., Wijdicks, E. F., Parisi, J. E., Piepgras, D. G. & Whisnant, J. P. Sudden death from aneurysmal subarachnoid hemorrhage. Neurology 45, 871–874. https://doi.org/10.1212/wnl.45.5.871 (1995).
van Gijn, J., Kerr, R. S. & Rinkel, G. J. Subarachnoid haemorrhage. Lancet 369, 306–318. https://doi.org/10.1016/s0140-6736(07)60153-6 (2007).
Schwedt, T. J., Matharu, M. S. & Dodick, D. W. Thunderclap headache. Lancet Neurol. 5, 621–631 (2006).
Bang, O. Y. Intracranial atherosclerosis: current understanding and perspectives. J. Stroke 16, 27 (2014).
Singhal, A. B. et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch. Neurol. 68, 1005–1012. https://doi.org/10.1001/archneurol.2011.68 (2011).
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38, 1–211. https://doi.org/10.1177/0333102417738202 (2018).
Chen, S. P., Fuh, J. L. & Wang, S. J. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother 11, 1265–1276. https://doi.org/10.1586/ern.11.112 (2011).
Topcuoglu, M. A. & Singhal, A. B. Hemorrhagic reversible cerebral vasoconstriction syndrome: features and mechanisms. Stroke 47, 1742–1747 (2016).
Marder, C. P., Donohue, M. M., Weinstein, J. R. & Fink, K. R. Multimodal imaging of reversible cerebral vasoconstriction syndrome: a series of 6 cases. Am. J. Neuroradiol. 33, 1403–1410. https://doi.org/10.3174/ajnr.A2964 (2012).
Fugate, J. E. et al. Variable presentations of postpartum angiopathy. Stroke 43, 670–676. https://doi.org/10.1161/strokeaha.111.639575 (2012).
de Bruijn, S. F., Stam, J. & Kappelle, L. J. Thunderclap headache as first symptom of cerebral venous sinus thrombosis. CVST Study Group. Lancet 348, 1623–1625. https://doi.org/10.1016/s0140-6736(96)07294-7 (1996).
Arnold, M. et al. Pain as the only symptom of cervical artery dissection. J. Neurol. Neurosurg. Psychiatry 77, 1021–1024. https://doi.org/10.1136/jnnp.2006.094359 (2006).
Day, J. W. & Raskin, N. H. Thunderclap headache: symptom of unruptured cerebral aneurysm. Lancet 2, 1247–1248 (1986).
Wang, S. J. & Fuh, J. L. The, “other” headaches: primary cough, exertion, sex, and primary stabbing headaches. Curr. Pain Headache Rep. 14, 41–46. https://doi.org/10.1007/s11916-009-0083-0 (2010).
Acknowledgements
The authors thank M. Jung for data management.
Funding
This study was supported by the National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIP) (Nos. 2020R1A2B5B01001826 and 2017R1A2B4007254). The Yuhan Company and Samjin pharm supported the data management. The funding bodies had no role in study design, data collection, analysis, interpretation, or writing of this report.
Author information
Authors and Affiliations
Contributions
C.S.C. conceived the study and designed the trial. C.S.C. and M.J.L. supervised the conduction of the trial conduct and were also involved in data collection. S.C. and M.J.L. analyzed all images and data. S.C. and M.J.L. drafted the manuscript, and all authors substantially contributed to its revision. Y.E.G. supported statistical analysis. C.S.C. take responsibility for the content of the paper. S.C. and M.J.L. contributed equally to this work as co-first authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, S., Lee, M.J., Gil, Y.E. et al. RCVS–TCH score can predict reversible cerebral vasoconstriction syndrome in patients with thunderclap headache. Sci Rep 11, 7750 (2021). https://doi.org/10.1038/s41598-021-87412-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87412-7
- Springer Nature Limited
This article is cited by
-
Reversible cerebral vasoconstriction syndrome: review of neuroimaging findings
La radiologia medica (2022)