Abstract
The detection of underlying atrial fibrillation (AF) has become increasingly possible by insertable cardiac monitoring (ICM). During hospitalization for cryptogenic stroke, factors related to the early and late development of AF have not been studied. CHALLENGE ESUS/CS is a multicenter registry of cryptogenic stroke patients undergoing transesophageal echocardiography. Twelve-lead electrocardiogram, continuous cardiac monitoring, and 24-h Holter electrocardiogram were all used for the detection of AF. Early and late detection of AF was determined with an allocation ratio of 1:1 among patients with AF. A total of 677 patients (68.7 ± 12.8 years; 455 men) were enrolled, and 64 patients developed AF during hospitalization. Four days after admission was identified as the approximate median day to classify early and late phases to detect AF: ≤ 4 days, 37 patients; > 4 days, 27 patients. Multiple logistic regression analysis showed that spontaneous echo contrast (SEC) (OR 5.91; 95% CI 2.19–15.97; p < 0.001) was associated with AF ≤ 4 days, whereas a large infarction > 3 cm in diameter (OR 3.28; 95% CI 1.35–7.97; p = 0.009) was associated with AF > 4 days. SEC and large infarctions were important predictors of in-hospital AF detection, particularly in the early and late stages, respectively; thus, they could serve as indications for recommending ICM.
Similar content being viewed by others
Introduction
Cryptogenic stroke, known as ischemic stroke with an undermined etiology, is not uncommon and comprises about a fifth to a quarter of all ischemic strokes1. The majority of cryptogenic strokes are caused by embolic mechanisms; thus, an embolic stroke of undetermined source (ESUS) was advocated in 20141,2. Underlying atrial fibrillation (AF) is a primary embolic source in cryptogenic stroke and ESUS among various potential embolic diseases, including arteriogenic embolisms and paradoxical embolisms3,4. Short-term monitoring, such as continuous inpatient telemetry and Holter monitoring, has been shown to detect approximately 8% of covert AF cases in cryptogenic stroke patients; meanwhile, detection of AF increased to approximately 30% with extended cardiac monitoring, such as insertable cardiac monitoring (ICM), during 3 years of follow-up5,6,7. In recent clinical trials, direct oral anticoagulants did not reduce the risk of stroke in comparison to aspirin among individuals with ESUS8,9. Thus, determining the optimal anticoagulant therapy is essential to elucidate the clinical manifestations of AF among embolic diversities in cryptogenic stroke and ESUS.
Recent studies have focused on the clinical significance of the AF development stage10,11,12. Late-onset of AF during an acute myocardial infarction increased the risk of stroke11. After catheter ablation, early- and late-phase AF recurrence displayed different clinical characteristics10,12. Furthermore, although considerable evidence has found that extended cardiac monitoring, such as ICM, substantially increased AF identification, the related clinical characteristics are yet to be elucidated. Despite these interests, there is no available evidence regarding the impact of early- and late-phase AF detection, and for clarifying the clinical manifestations of patients with late-phase AF detection to enable stratifying patients for whom prolonged cardiac monitoring, such as ICM, is indicated in cryptogenic stroke.
Transesophageal echocardiography (TEE) is critical to assess cardiac parameters related to AF, screen for diverse potential embolic sources in cryptogenic stroke and ESUS, and guide optimal therapeutic management3,13. The presence of a thrombus, spontaneous echo contrast (SEC), and decreased left atrial appendage (LAA) emptying velocity are reported markers of thromboembolic risk in AF14,15,16. We have recently initiated the Mechanisms of Embolic Stroke Clarified by Transesophageal Echocardiography for ESUS/Cryptogenic Stroke (CHALLENGE ESUS/CS) registry, enrolling cryptogenic stroke patients undergoing TEE as well as maintaining a comprehensive database among multiple centers in Japan. Using data from CHALLENGE ESUS/CS registry with comprehensive data including detection of AF, we explored the clinical characteristics of patients with early and late development of AF in the hospital after cryptogenic stroke.
Methods
The CHALLENGE ESUS/CS registry is a multicenter, retrospective registry that enrolled consecutive patients with cryptogenic stroke who underwent TEE within eight participating hospitals in Japan between April 2014 and December 2016, and was registered at http://www.umin.ac.jp/ctr/ (UMIN000032957). Details of the rationale, study design, factors to evaluate, TEE protocol, and characteristics of the participants in the CHALLENGE ESUS/CS registry have been published elsewhere17,18. We used clinical information obtained from medical records, and therefore, the need to obtain written informed consent from each patient was waived for this retrospective study. The Independent Ethics Committee of Juntendo University Hospital, the Independent Ethics Committee of Iwate Prefectural Central Hospital, the Research Ethics Committee of the National Cerebral and Cardiovascular Center, the Ethics Committee of St. Marianna University Hospital, the Nagasaki University Hospital Clinical Research Ethics Committee, the Ethics Committee of Showa University Koto Toyosu Hospital, the Independent Ethics Committee of Dokkyo Medical University Hospital, and the Independent Ethics Committee of Juntendo University Urayasu Hospital all approved the protocol. This study was conducted following the Declaration of Helsinki.
Inclusion and exclusion criteria for the CHALLENGE ESUS/CS registry
Inclusion criteria for the current study were as follows: (1) ischemic stroke within seven days of onset; (2) non-lacunar infarction on neuroradiological imaging; (3) absence of an arterial stenosis > 50% or occlusive lesions in a corresponding cervical or large cerebral artery; (4) absence of major cardiac embolic sources; and (5) lack of other determined stroke etiologies. In the diagnostic criteria of ESUS, cardiac monitoring for > 24 h is recommended; thus, AF that was detected < 24 h after admission was not suitable for the diagnosis of ESUS and was not registered in the CHALLENGE ESUS/CS registry.
Atherosclerotic risk factors
Hypertension was defined as a history of using antihypertensive agents, systolic blood pressure > 140 mmHg, or diastolic blood pressure > 90 mmHg at 14 days after stroke. Diabetes mellitus was defined as the use of oral hypoglycemic agents or insulin, fasting blood glucose level > 126 mg/dL, or glycosylated hemoglobin ≥ 6.5%. Dyslipidemia was defined as use of antihyperlipidemic agents, serum low-density lipoprotein cholesterol ≥ 140 mg/dL, high-density lipoprotein cholesterol < 40 mg/dL, or triglycerides ≥ 150 mg/dL. Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2.
TEE
TEE was performed as explained in our previous work4,13. Subjects were awake and had fasted for at least 4 h before the examination. To examine the heart and aortic arch, a multiplane probe was manipulated to provide appropriate views, including axial and sagittal images. The presence of a right-to-left shunt was assessed by injecting agitated saline and having patients perform the Valsalva maneuver, and then numbers of microbubbles with and without contrast agents were compared. Atrial septal aneurysm was defined as a ≥ 10 mm excursion into either the left or right atrium, or a sum of total excursion into the left or right atrium of ≥ 15 mm. Aortic arch plaques with thickness ≥ 4 mm, mobile plaques seen swinging on their peduncles, or ulcerative plaques with width and maximum depth of at least 2 mm were defined as aortic complicated lesions. Examinations were performed by two or three experienced sonographers in each institution.
In-hospital detection of AF and temporal classification
After admission, all enrolled patients underwent a 12-lead electrocardiogram, continuous cardiac monitoring, and a 24-h Holter electrocardiogram after admission. To date, evidence regarding classification into the early to late stages of in-hospital AF detection has been unavailable. The cutoff period to classify early and late detection of AF was determined by a ratio approximating 1:1 allocation.
Data collection and analyses
Collection of baseline clinical information including cardiovascular risk factors, laboratory and radiological data on admission, and echocardiographic findings was conducted with permission by the steering committee of the CHALLENGE ESUS/CS. These variables were compared according to the absence of AF, early detection of AF, and late detection of AF. Independent factors associated were assessed in those with early and late AF detections.
Statistical analysis
Numerical values are reported as the mean ± standard deviation. Data were analyzed using the chi-squared test for categorical variables and the Kruskal–Wallis test for nonparametric analyses among patients with early detection of AF after admission, late detection of AF after admission, and no AF. All variables with values of p < 0.05 on univariate analyses were entered into a multinomial regression analysis to identify independent variables for patients with the development of AF in the early and late phases. Predictors for in-hospital AF detection were analyzed using the Kaplan–Meier analysis. A value of p < 0.05 was considered statistically significant. All data analyses were conducted using SPSS for Mac version 26.0 (SPSS Inc., Chicago, IL, USA).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Results
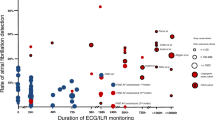
A total of 677 patients with cryptogenic strokes (mean age, 68.7 ± 12.8 years; 455 males) were enrolled in the present study. The median baseline National Institutes of Health Stroke Scale (NIHSS) score was 2, and AF was detected in 64 patients (9.5%). The median length of hospital stay was 17 days. The temporal profile of AF detection during hospitalization is shown in Fig. 1 among patients with detection. Four days after admission was identified as the approximate median day for detection of AF and was used to classify early and late AF detection. Based on this, AF was found in ≤ 4 days in 37 patients and in > 4 days in 27 patients.
Clinical characterizations according to early, late, and no detection of AF in cryptogenic stroke
Baseline characteristics of the entire study population, cryptogenic stroke patients with in-hospital early and late AF development, and absence of AF detection are summarized in Table 1. The mean age of patients was in the following order: early AF development, late AF development, and absence of AF (77.6 ± 7.4, 75.6 ± 10.6, 67.9 ± 12.8 years, respectively, p < 0.001). The female sex and CKD were more predominant in patients with early AF detection than in other groups (49%, p = 0.034; 57%, p = 0.039), whereas the frequency of cigarette smoking was lower in patients with early AF development (3%, p < 0.001). The median NIHSS score was 5 in patients with early AF development, which was higher than in other groups (p < 0.001). Functional outcomes, based on the modified Rankin Scale, at discharge were not different among the groups. Findings from magnetic resonance imaging showed that a large infarction (> 3 cm in diameter) was significantly more common in patients with late AF development than in patients with early AF development and without AF (59%, p = 0.001). In echocardiographic parameters, left atrial (LA) diameter was larger in patients with early and late AF development (38.9 ± 5.9, 38.6 ± 5.0 mm, respectively, p < 0.001) than in those without AF. In comparison, LAA flow was lower in patients with early and late AF development than in patients without AF (48.1 ± 16.0, 47.5 ± 14.7 cm/s, respectively, p < 0.001). The SEC frequency observed in the patients was in the following order: early AF development, late AF development, and without AF (32%, 19%, and 3%, respectively, p < 0.001). Aortic arch plaques on TEE were more frequent in patients without AF detection (39%, p = 0.018). In laboratory data, brain natriuretic peptide (BNP) levels of patients were in the following order: early AF development, late AF development, and without AF (175.5 ± 131.3, 134.1 ± 116.5, 94.3 ± 166.9 pg/mL, respectively, p < 0.001).
Independent variables associated with early and late detection of paroxysmal AF (PAF)
Age, female sex, cigarette smoking, CKD, NIHSS score, large infarct > 3 cm, LA diameter, LAA flow, SEC, aortic arch plaques, and BNP were entered into multinomial logistic regression analyses. Age (odds ratio [OR] 1.06; 95% confidence interval [CI] 1.01–1.11; p = 0.019), LA diameter (OR 1.07; 95% CI 1.01–1.14; p = 0.036), and SEC (OR 5.91; 95% CI 2.19–15.97; p < 0.001) were associated with early detection of AF, whereas cigarette smoking (OR 0.12; 95% CI 0.02–0.92; p = 0.042) and aortic arch plaques (OR 0.21; 95% CI 0.08–0.61; p = 0.004) were inversely related (Table 2). Age (OR 1.07; 95% CI 1.01–1.12; p = 0.012) and a large infarction > 3 cm in diameter (OR 3.28; 95% CI 1.35–7.97; p = 0.009) were associated with late detection of AF (Table 2).
Discussion
The current study elucidated the factors related to in-hospital detection of AF in cryptogenic stroke, depending on the early and late phases of hospitalization using data from the CHALLENGE ESUS/CS registry. The principal findings are that AF was detected during hospitalization in 9.5% of cryptogenic stroke patients, and using 4 days as the approximate cutoff after admission, SEC and large infarct size > 3 cm in diameter were found to be significantly related to AF development in the early and late phases, respectively.
Detection of covert AF is critical in cryptogenic stroke and ESUS patients. Although our data did not show a significant difference in functional outcomes at discharge in patients with early and late AF development, and absence of AF detection, covert AF has been shown to increase the risk of a recurrent stroke long-term among patients with embolic diseases19. Many previous studies have focused on the factors that predicted covert AF in cryptogenic stroke and ESUS. Electrophysiological substrates, such as P wave alterations and supraventricular extrasystole20,21, and blood biomarkers, such as BNP and NT-proBNP22,23, could predict occult AF in cryptogenic stroke and ESUS. Importantly, LA enlargement, SEC, and LAA dysfunction were related to not only covert AF in cryptogenic stroke and ESUS24,25, but also to embolic events in patients with AF14,15,16. In the sub-analysis of NAVIGATE ESUS, patients with an LA diameter > 46 mm displayed lower stroke recurrence after rivaroxaban treatment26. Thus, echocardiographic parameters are important to predict AF in cryptogenic stroke and ESUS. On the other hand, some studies have explored the clinical significance of early and late development of AF. After catheter ablation, early and late recurrences of AF showed different clinical characteristics, and an early recurrence of AF ≤ 7 days predicted a late recurrence of AF10,12. In acute myocardial infarctions, comorbidity with AF occurring in the late phase of > 24 h, but not AF resolved within ≤ 24 h, displayed a higher incidence of stroke rate compared to no AF11. In our data, increasing age was related to both early and late AF detection, which was consistent with previous studies2,27. Conversely, current smoking habits were shown to be negatively associated with early detection of PAF. In a previous study, current smoking status was more closely related to stroke patients aged < 65 years than to those aged ≥ 75 years28. Thus, it is suggested that older patients did not have smoking habits, reflecting the current results of the association between aging and AF detection. More importantly, among various important characterizations related to detection in early and late stages with a cutoff of 4 days, SEC and large infarction were closely linked with the early and late occurrence of AF, respectively.
In recent years, there has been growing interest in the detection of AF using prolonged cardiac monitoring using ICM. A recent meta-analysis identified that the detection rates of AF with ICM were 5%, 21%, 26%, and 34% in < 6 months, 6–12 months, 12–24 months, and ≥ 24 months, respectively; increasing age was correlated with AF detection, whereas sex, atherosclerotic risk factors, CHADS2 score, and CHA2DS2-VASc score were not29. Meanwhile, AF was detected in a small number of patients with ESUS in large-scale clinical trials where systematic assessment for AF by ICM was not carried out8,9. Therefore, there is a possibility that AF detection by ICM may ensure the efficacy of direct oral anticoagulants in patients with cryptogenic stroke or ESUS. In our data, SEC was closely related to the early detection of AF and was also relatively linked with the late development of AF, whereas a large infarction was an independent factor for the late development of AF in cryptogenic stroke. So far, clinical characteristics related to long-term AF detection are not well understood. Furthermore, it was shown that SEC was related to AF30, while the association of radiological parameters such as large infarctions, shown in the current study, with long-term AF detection on ICM are yet to be elucidated. Thus, not only SEC, but also large infarctions could be important characteristics for an ICM recommendation, even though AF was not detected during hospitalization in those patients; further studies are warranted.
This study has some limitations when interpreting the present results. First, this was a retrospective study with a small sample size of early and late detection of AF. Furthermore, the period of continuous cardiac monitoring and the timing of a 24-h Holter electrocardiogram differed among individuals and were determined at the discretion of the treating physicians; this raised a potential bias for AF detection. In addition, unknown stroke patients with detection of AF < 24 h were diagnosed with cardioembolic stroke and not registered in the CHALLENGE ESUS/CS database. Second, selection bias may have been involved in the performance of TEE in patients with cryptogenic stroke at participating hospitals. Third, radiological, echocardiographic, and laboratory data were lacking in a fraction of cases.
Conclusion
The CHALLENGE ESUS/CS registry enrolled a large number of cryptogenic stroke patients with comprehensive data. Our results showed that SEC and large infarct size were important predictors of AF detection, particularly in the early and late phases, respectively. Clinical characteristics including radiological parameters related to long-term AF detection are not well understood. The presence of SEC and a large infarction could be indications for recommending ICM.
References
Hart, R. G. et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 13, 429–438. https://doi.org/10.1016/S1474-4422(13)70310-7 (2014).
Hart, R. G., Catanese, L., Perera, K. S., Ntaios, G. & Connolly, S. J. Embolic stroke of undetermined source: A systematic review and clinical update. Stroke 48, 867–872. https://doi.org/10.1161/STROKEAHA.116.016414 (2017).
Katsanos, A. H. et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology 87, 988–995. https://doi.org/10.1212/WNL.0000000000003063 (2016).
Ueno, Y. et al. Emerging risk factors for recurrent vascular events in patients with embolic stroke of undetermined source. Stroke 47, 2714–2721. https://doi.org/10.1161/STROKEAHA.116.013878 (2016).
Rizos, T. et al. Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 43, 2689–2694. https://doi.org/10.1161/STROKEAHA.112.654954 (2012).
Sanna, T. et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 370, 2478–2486. https://doi.org/10.1056/NEJMoa1313600 (2014).
Gladstone, D. J. et al. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 370, 2467–2477. https://doi.org/10.1056/NEJMoa1311376 (2014).
Hart, R. G. et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N. Engl. J. Med. 378, 2191–2201. https://doi.org/10.1056/NEJMoa1802686 (2018).
Diener, H. C. et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N. Engl. J. Med. 380, 1906–1917. https://doi.org/10.1056/NEJMoa1813959 (2019).
Lee, S. H. et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J. Interv. Card. Electrophysiol. 10, 221–226. https://doi.org/10.1023/B:JICE.0000026915.02503.92 (2004).
Axelrod, M., Gilutz, H., Plakht, Y., Greenberg, D. & Novack, L. Early atrial fibrillation during acute myocardial infarction may not be an indication for long-term anticoagulation. Angiology 71, 559–566. https://doi.org/10.1177/0003319720908760 (2020).
Yang, D. Y. et al. Noninvasive electrocardiography monitoring for very early recurrence predicts long-term outcome in patients after atrial fibrillation ablation. Ann. Noninvasive Electrocardiol. https://doi.org/10.1111/anec.12785 (2020).
Ueno, Y. et al. Rosuvastatin may stabilize atherosclerotic aortic plaque: Transesophageal echocardiographic study in the EPISTEME trial. Atherosclerosis 239, 476–482. https://doi.org/10.1016/j.atherosclerosis.2015.02.021 (2015).
SPAF-Investigators. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann. Intern. Med. 128, 639–647. https://doi.org/10.7326/0003-4819-128-8-199804150-00005 (1998).
Fatkin, D., Kelly, R. P. & Feneley, M. P. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J. Am. Coll. Cardiol. 23, 961–969. https://doi.org/10.1016/0735-1097(94)90644-0 (1994).
Zabalgoitia, M. et al. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J. Am. Coll. Cardiol. 31, 1622–1626. https://doi.org/10.1016/s0735-1097(98)00146-6 (1998).
Ueno, Y. et al. Large aortic arch plaques correlate with CHADS2 and CHA2DS2-VASc scores in cryptogenic stroke. Atherosclerosis 284, 181–186. https://doi.org/10.1016/j.atherosclerosis.2019.03.009 (2019).
Kikuno, M. et al. Underlying embolic and pathologic differentiation by cerebral microbleeds in cryptogenic stroke. J. Neurol. 267, 1482–1490. https://doi.org/10.1007/s00415-020-09732-4 (2020).
Ntaios, G. et al. Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. J. Am. Heart Assoc. 8, e012858. https://doi.org/10.1161/JAHA.119.012858 (2019).
Ntaios, G. et al. Supraventricular extrasystoles on standard 12-lead electrocardiogram predict new incident atrial fibrillation after embolic stroke of undetermined source: The AF-ESUS study. J. Stroke Cerebrovasc. Dis. 29, 104626. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104626 (2020).
Goda, T. et al. P-wave terminal force in lead v1 predicts paroxysmal atrial fibrillation in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 26, 1912–1915. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.06.031 (2017).
Rodriguez-Yanez, M. et al. High pro-BNP levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology 81, 444–447. https://doi.org/10.1212/WNL.0b013e31829d8773 (2013).
Todo, K. et al. Frequent premature atrial contractions in cryptogenic stroke predict atrial fibrillation detection with insertable cardiac monitoring. Cerebrovasc. Dis. 49, 144–150. https://doi.org/10.1159/000505958 (2020).
Shimizu, T. et al. Association between paroxysmal atrial fibrillation and the left atrial appendage ejection fraction during sinus rhythm in the acute stage of stroke: A transesophageal echocardiographic study. J. Stroke Cerebrovasc. Dis. 22, 1370–1376. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.03.020 (2013).
Ohya, Y. et al. Usefulness of transesophageal echocardiography for predicting covert paroxysmal atrial fibrillation in patients with embolic stroke of undetermined source. Cerebrovasc. Dis. Extra 9, 98–106. https://doi.org/10.1159/000502713 (2019).
Healey, J. S. et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: Secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. 76, 764–773. https://doi.org/10.1001/jamaneurol.2019.0617 (2019).
Pagola, J. et al. Large vessel occlusion is independently associated with atrial fibrillation detection. Eur. J. Neurol. https://doi.org/10.1111/ene.14281 (2020).
Ueno, Y. et al. Age stratification and impact of eicosapentaenoic acid and docosahexaenoic acid to arachidonic acid ratios in ischemic stroke patients. J. Atheroscler. Thromb. 25, 593–605. https://doi.org/10.5551/jat.40691 (2018).
Tsivgoulis, G. et al. Duration of implantable cardiac monitoring and detection of atrial fibrillation in ischemic stroke patients: A systematic review and meta-analysis. J. Stroke 21, 302–311. https://doi.org/10.5853/jos.2019.01067 (2019).
Zhang, E., Liu, T., Li, Z., Zhao, J. & Li, G. High CHA2DS2-VASc score predicts left atrial thrombus or spontaneous echo contrast detected by transesophageal echocardiography. Int. J. Cardiol. 184, 540–542. https://doi.org/10.1016/j.ijcard.2015.02.109 (2015).
Acknowledgements
The authors especially thank the CHALLENGE ESUS/C.S. participants for their enthusiastic collaboration.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study concept and design: R.D., Y.U., T.S., Y.T., A.K., and H.T. Acquisition of data: R.D., Y.U., M.K., T.S., Y.T., A.K., H.T., Y.S., K.K., Y.K., E.Y., M.K., M.I., A.T., K.H., Y.H., T.K., and N.H. Analysis and interpretation of data: R.D., Y.U., M.K., T.S., Y.T., A.K., H.T., Y.S., K.K., Y.K., E.Y., M.K., M.I., A.T., K.H., Y.H., T.K., and N.H. Drafting of the manuscript: R.D. and Y.U. Critical revision of the manuscript for important intellectual content: Y.U. and T.U. Study supervision: Y.U.
Corresponding author
Ethics declarations
Competing interests
Y.U received lecture fees from OHARA Pharmaceutical Co., Ltd. and research funds from Bristol-Myers Squibb. H.T received lecture fees from Pfizer Japan Inc. and Daiichi Sankyo Co., Ltd. M.K received lecture fees from Bayer Pharmaceutical Co., M.I received a research grant from Shimadzu Corporation, Otsuka Pharmaceutical, and Panasonic Corporation, and lecture fees from Daiichi Sankyo Co., Ltd, Eisai Co. Ltd. Bayer Pharmaceutical Co. K.H received lecture fees from MSD Co., Ltd., Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., Novartis Pharma K.K., and AbbVie GK, and research funds from Eisai Co., Ltd., Pfizer Japan Inc., Novartis Pharma K.K., Takeda Pharmaceutical Co., Ltd., TAIYO Co., Ltd., Kyowa Minami Hospital, Shirasawa Hospital, Shiobara Onsen Hospital, Utsunomiya Chuo Hospital, Nishikata Hospital, and Moka Hospital. Y.H received lecture fees from Bayer Pharmaceutical Co., N.H was an advisory member of Dai-Nippon Sumitomo Pharma Co., Ltd., Hisamitsu Pharmaceutical Co., Inc, Biogen Idec Japan Ltd., received lecture fees from Dai-Nippon Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical, Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kyowa Hakko-Kirin Co., Ltd., FP Pharmaceutical Corporation, Eisai Co., Ltd., Novartis Pharma K.K., and AbbVie, and received departmental endowments by commercial entities from Kyowa Hakko-Kirin Co., Ltd., Nippon Boehringer Ingelheim, Co., Ltd., AbbVie GK, FP Pharmaceutical Corporation, Otsuka Pharmaceutical, Co., Ltd., Dai-Nippon Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., Nihon Medi-physics Co., Ltd., Asahi Kasei Medical Co., Ltd., Ono Pharmaceutical Co., Ltd., MiZ Co., Ltd., AbbVie GK, OHARA Pharmaceutical Co., Ltd., Nihon Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Boston Scientific Corporation, and Medtronic Inc. T.U received lecture fees from Daiichi Sankyo Co., Ltd., Boehringer Ingelheim, Otsuka Pharmaceutical Co., Ltd., Bayer Pharmaceutical Co., AstraZeneca K.K., and AbbVie GK., and research funds from Otsuka Pharmaceutical Co., Ltd., Astellas Pharma Inc., Bayer Pharmaceutical Co. Ltd., AbbVie GK, and Eisai Co., Ltd. Other authors have no conflicts of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doijiri, R., Ueno, Y., Kikuno, M. et al. Different aspects of early and late development of atrial fibrillation during hospitalization in cryptogenic stroke. Sci Rep 11, 7127 (2021). https://doi.org/10.1038/s41598-021-86620-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86620-5
- Springer Nature Limited