Abstract
The formula of a standardized extract of Centella asiatica (ECa 233) was modified to improve its dissolution, with implications for pharmacokinetics and metabolomic profile. This study aimed to understand the resultant changes in disposition kinetics of ECa 233 and alterations to human metabolome after oral administration. This study was a two-sequence of dosages (250 and 500 mg), with an open-label phase I clinical trial. The modified formula was administered in single and multiple doses to twelve healthy Thai volunteers. The major parent compounds, madecassoside and asiaticoside, were rarely absorbed, instead undergoing biotransformation into active metabolites, madecassic acid and asiatic acid with possibility to be eliminated via fecal route. Increasing the dose of ECa 233 resulted in significantly greater plasma levels of those active metabolites, with accumulation of asiatic acid after multiple oral administration for seven days. Examining the impacts of accumulation behavior on metabolomics, the study traced changes in levels pre- and post-dose of five relevant human metabolites. Administration of ECa 233 was found to be significantly associated with an increase of choline, an endogenous metabolite with documented benefits for learning and memory. Therefore, ECa 233 may be useful in mitigating cognitive impairment, through its role in modulating human metabolites.
Similar content being viewed by others
Introduction
Centella asiatica (Linn.), commonly known as Asiatic pennywort or Gotu kola, belonging to the family Apiaceae, has been widely used in traditional medicine and possesses well-documented neuroprotective effects1. A standardized extract of C. asiatica was fully researched and developed as ECa 233 and was then characterized to contain more than 81% of triterpenoid glycosides2. Recent reports have shown the tolerability of ECa 233 in preclinical toxicology studies with NOAEL ≥ 1000 mg/kg/day in rats3. Pretreatment with 10 mg/kg of ECa 233 in mice was effective in mitigating the Alzheimer-like cognitive deficits induced by β-amyloid peptide4,5. Hence, ECa 233 has a potential to become a phytomedicine candidate for human use in terms of improving patients’ cognitive function.
Pharmacokinetics profiles of ECa 233 were first determined in a rat model6. Later, 250- and 500-mg capsules of ECa 233 were reported to be well tolerated in a phase 1 study in healthy subjects7. The parent compounds of ECa 233, which are MDS and ASS, were extensively biotransformed into active metabolites, MDA and ASA, respectively7. Most of the pharmacokinetic parameters of ECa 233’s parent compounds and active metabolites were illustrated; however, data about excretion and metabolomics are still limited7. To date, information in regard to the metabolome may directly reflect changes in the physiological state after oral administration of a specific drug and has been applied in the process of identifying biomarkers in metabolic disorder-involved diseases8. Understanding the associations between changes in the levels of candidate endogenous metabolites and the metabolite-related biomarkers of neurodegenerative disease9 is the way to support the next step in the use of ECa 233 in clinical practice.
A previous study found that ECa 233 had limited dissolution of formulation, leading to a restriction of its oral bioavailability7. Hence, the modified formula to improve oral bioavailability of ECa 233 was developed and was used in this clinical study. This research aimed to investigate the clinical pharmacokinetics profile and excretion pathway including metabolomic profiles of the modified-formula ECa 233 capsules in healthy Thai volunteers.
Materials and methods
Chemicals
The standardized C. asiatica extract is a white to off-white powder that contains the triterpenoid glycosides MDS (53.1%) and ASS (32.3%) as determined by the LC–MS/MS method. For this clinical study the extract was provided by Siam Herbal Innovation Co., Ltd (Thailand) (Lot number MRA 0816001). The modified formula of ECa 233 capsules (Lot number 18EC003005) was manufactured by Pharma Nueva Co., Ltd. (Thailand) under Good Manufacturing Practice quality standards. The appropriate dosage of C. asiatica extract used in this clinical study was determined by calculating the first dose in humans (FIH dose)10. The estimated maximum recommended starting dose (MRSD) was converted from the human equivalent dose (HED)7 based on the results of safety and pharmacological studies of ECa 233.
For LC–MS/MS analysis, methanol (HPLC grade) was obtained from Merck (Germany). The internal standards of glycyrrhizin (purity > 90%) and glycyrrhetinic acid (purity > 98%), and the reference standard of ASS (purity > 98.5%) were purchased from Sigma-Aldrich (Corp. USA). MDS (purity > 96.7%) was purchased from Chromadex, (Inc. USA). MDA and ASA, determined to be > 97.5% and 97.0% purity, respectively, were purchased from Wako Pure Chemical Industries, (Ltd. Japan).
For NMR analysis, deuterium oxide (D2O) was purchased from Cambridge Isotope Laboratories, (Inc. USA). Sodium hydrogen phosphate (Na2HPO4) for phosphate buffer preparation, sodium azide (NaN3) and trimethylsilyl propionic acid sodium salt (TSP) were purchased from Sigma-Aldrich, (Corp. USA).
Ethical statement and informed consent
This study protocol was approved by the Institutional Review Board of Chula Clinical Research Center, Chulalongkorn University (IRB number: 479/61, COA number 850/2018, date of approval: 06/09/2018) and was registered in the Thai Clinical Trials Registry, a trial registration data set required by World Health Organization, (TCTR identification number: TCTR20180922001, date of first registration: 20/09/2018). The clinical study was conducted at Chula Pharmacokinetic Center, Maha Chakri Sirindhorn, Clinical Research Center, Faculty of Medicine, Chulalongkorn University, under the International Conference on Harmonization—Good Clinical Practice, and in accordance with the Declaration of Helsinki. All participants provided written informed consent for their participation prior to the start of the study.
Eligibility criteria for participants
Twelve Thai healthy volunteers aged between 18 and 50 years were enrolled and recruited from March to April 2018. Inclusion criteria were BMI between 18.0 and 25.0 kg/m2, with normal medical histories, physical examinations, vital signs, and clinical laboratory tests. Participants were excluded if they smoked more than ten cigarettes/day, had a history of alcoholism or of moderate drinking (more than three drinks/day), were pregnant or breastfeeding, or planned to become pregnant during the study period. The number of subjects was defined according to the general guidelines for clinical pharmacokinetic studies, a concise guide used in clinical research11. Sample size determination was calculated for a 95% level of confidence according to the standard normal distribution (Z1-α/2 = 1.96), and a tolerated margin of error at 15% of the mean (γ = 0.15)12.
Study design and sample collection
This was a single-center, with an open-label, two-sequence of dosages (250 and 500 mg), two-periods of study (single and multiple dose) conducted in fasting condition (Fig. 1). In study period 1, all participants fasted overnight for at least 10 h before drug administration. The 250 mg of ECa 233 with 240 mL of drinking water were administered orally. Blood samples (3 mL) were collected from each individual at pre-dose (0 h) and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, and 72 h post dose. All blood samples were centrifuged at 4 °C, 3200 × g for 10 min. Urine samples were collected at 0 h (the day prior to dosing) and 0–4, 4–8, 8–12, and 12–24 h after dosing. Feces were also collected from one of the volunteers for detecting the possible presence of any eliminated compounds. Feces samples were collected each time that a volunteer defecated on day 1–3, 4–6 and 7–9 during the study period. These samples were immediately fixed with methanol and prepared by our previously described method of protein precipitation. After a 3-week washout period, all participants continued period 2 of the study, where they received a 500-mg dose of ECa 233, and sample collection procedures remained consistent with period 1 (Fig. 2). Plasma, urine and fecal samples were then stored at − 80 °C until analysis.
For multiple oral dosing, participants continued to take 250 mg or 500 mg of ECa 233 every morning for 7 consecutive days. Then they had another set of blood and urine samples collected similar to the single dose study. The primary outcome was determination of pharmacokinetic parameters, including the profiles of the metabolites, and the secondary outcome was consideration of the safety profiles of ECa 233 after period 2 (end of the study).
Sample preparation and pharmacokinetic analysis by LC–MS/MS
Sample preparation was performed by protein precipitation with two internal standards (glycerrhizin and glycerrhetinic acid) to correct the quantitative analysis of parent compounds and of their metabolites. Briefly, 200 µL of methanol was added followed by vortex mixing with 50 µL of each sample for 10 min and then centrifuged at 12,000 × g, 4 °C, 10 min. Quantitative determination of the parent compounds and their active metabolites in plasma and urine were carried out by using liquid chromatography tandem mass spectrometry (LC–MS/MS): LCMS-8060 (Shimadzu Corp. Japan) according to a previously validated and published bioanalytical method7. Chromatographic separation of the extracted sample was then performed on a Phenomenex Synergi 4u Fusion-RP (80A, 4 microns, size 50 × 2.0 mm) C18 column (column temperature = 40 °C) and eluted by two mobile phases: 0.2% formic acid in water (solvent A) and 100% methanol (solvent B), following gradients 0–0.5 min 10% B, 0.5–3 min 90% B, and 3–5 min 10% B, at a constant flow rate of 0.5 mL/min with 10 µL of injection volume. The MS/MS system was operated using an ESI source in negative mode with MRM. For m/z transition; MDS, ASS, MDA, ASA, glycyrrhizin, and glycyrrhetinic acid showed a precursor ion and product ion at 973.50/503.30, 957.55/469.00, 503.20/437.20, 487.60/409.25, 821.25/350.95, and 469.45/409.40, respectively (Supplementary Figs. 1S, 2S and 3S).
Sample preparation and metabolomics analysis by 1H-NMR spectroscopy
Plasma samples at predose, 0.5, 1, and 2 h were used in the metabolomics part of the study. In brief, 400 µL of plasma were mixed with 400 µL of 0.142 M phosphate buffer in D2O solution (pH 7.4) containing 3 mM sodium azide (NaN3) used as a bacteriostatic reagent, and 0.08% trimethylsilylpropanoic acid (TSP) used as a chemical shift reference. The mixer was vortexed, sonicated at 25 °C for 15 min and then centrifuged at 13,000 × g, 4 °C for 10 min. Lastly, 600 µL of supernatant was transferred into a 5-mm diameter NMR tube for analysis.
All 1H NMR spectra of plasma samples were acquired at 600.13 MHz on a 600 MHz NMR spectroscopy (Bruker Avance III, Bruker Biospin, Germany) and were determined using a modified version of a previously published method13. The samples were measured at a temperature of 300 K using the presaturation sequence (64 scans). 1H NMR spectra were recorded by a standard one-dimensional (1-D) with the suppression of water signal, CPMG pulse. The spectra region was set to a range from − 1 to 10 ppm and the parameters were as follows: 64 K data points; relaxation delay of 2 s and mixing time (tm) of 100 ms.
Data processing and statistical analysis of pharmacokinetics
Non-compartmental analysis was applied to determine pharmacokinetic parameters and performed by PK solutions software (version 2.0). AUC(0-t) was calculated using the linear trapezoidal rule. t½ was calculated by t(1/2) = ιn2/kel . The apparent clearance (Cl/F) after oral administration was calculated as Cl/F = Dose/AUC, where F is the absolute oral bioavailability. The apparent volume of distribution (Vd/F) was estimated using Vd/F = Dose/(AUC × kel). The Cmax and Tmax were determined directly from the observed concentration–time curve.
For statistical analysis in the pharmacokinetics study, all data were expressed as mean ± SD except for Tmax, which was expressed as median (IQR). The descriptive data were used to describe demographic characteristics and to summarize the continuous variables. Data processing was performed using STATA software (version 10.0; StataCorp, USA) and displayed in the graphical charts by using GraphPad Prism 8. A paired t-test or Wilcoxson Signed Rank Test was used to compare pharmacokinetics parameters between single and multiple doses. To determine statistically significant differences of the pharmacokinetics parameters of two dosages between 250- and 500-mg, Student’s t-test or Mann–Whitney U test was used where appropriate. A p-value of less than 0.05 was considered statistically significant.
Data pre-processing, multivariate statistical analysis, and metabolite identification for metabolomics
All plasma spectra were pre-processed using TopSpin 3.1 software (Bruker, Germany) including baseline correction and TSP calibration at 0.00 ppm. The NMR spectra were subsequently transferred into MATLAB software (MathWorks, R2014a, USA). The spectral regions containing TSP peak (δ1H − 1 to 0.66 ppm) and water peak (δ1H 4.599 to 5.053 ppm) were omitted. The spectral alignment was then applied to adjust the position for all remaining peaks, and those aligned NMR spectra were imported into SIMCA 14.0 (Umetrics Umea, Sweden). In order to provide a general overview on clustering, data were initially analyzed by an unsupervised principal component analysis (PCA) with unit variance (UV) scaling. In considering the possible influences of ECa 233, a clear separation of suspicious metabolome changes between pre- (T0) and post-dose (T0.5, 1, 2 h, on day 7) was further analyzed by OPLS-DA in MATLAB (R2014a) using the in-house developed scripts at Imperial College London. The OPLS-DA model was evaluated according to two parameters: R2X explained the fitness and Q2Y represented the predictive capability of the model. The permutation test was assessed by using 1000 times for the validation of all models involved in this study. The coefficient loadings plot of OPLS-DA models was used to indicate the significantly different metabolites as compared between pre-dose and post-dose. The permutation p-values were used to illustrate the level of differences that were considered as statistically significant at p-values less than 0.05.
For metabolite identification, plasma endogenous metabolites were determined using Chenomx NMR Suite 8.2 (Chenomx Inc., Canada), and were further justified in statistical total correction spectroscopy (STOCSY) using MATLAB software. In addition, the human metabolome database (HMDB) as well as the previously published assignments and in-house chemical shift databases were also used to support the metabolite identification in this study.
Results
Participants baseline clinical characteristics
All subject demographics and characteristics are summarized in Table 1. The age limit for study volunteers was 18–50 years; people in this age range were eligible to participate. The age of enrolled participants, however, was within a smaller range, from 21 to 38 years. One subject withdrew consent during the second period of the study due to personal reasons, which were neither ECa 233-related problems nor intolerable side effects. Therefore, there were eleven subjects that completed the study and remained for the per-protocol analysis.
Pharmacokinetics
Pharmacokinetic profiles of the modified formula ECa 233
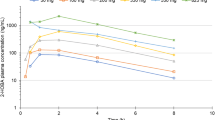
Pharmacokinetic profiles after oral administration of modified formula ECa 233 capsules are represented in plasma concentration–time curves of the parent compounds (MDS or ASS) and metabolites (MDA or ASA) (Fig. 3a–d). Cmax of MDS or ASS was observed within 1 h after dosing, followed by the appearance of MDA or ASA that reached their Cmax within 1–2 h. A notably higher Cmax with a greater AUC was observed in both parent compounds and metabolite profiles of the modified formula of ECa 233 when compared to its previous formulation.
In considering a dose comparison between the 250 mg and 500 mg, the Cmax of MDA and ASA was found to increase significantly; however, no significant changes were observed in Tmax and t1/2 (Table 2). Likewise, the apparent volume of distribution (Vd/F) and the apparent clearance (Cl/F) for MDA and ASA did not show significant differences when compared between the two dosage regimens.
Multiple oral dosing of the modified formula ECa 233 capsule resulted in greater plasma concentrations of ASA as compared to the single oral dosing. The AUC and Cmax of ASA on day 7 were significantly higher than those on day 1 (p = 0.001 and 0.007, respectively), indicating that there was an accumulation of active metabolites after the repeated doses. The accumulation ratio of ASA was approximately 1.891.
The unchanged forms of MDS and ASS in plasma were primarily eliminated into the urine from the first 4 h until 24 h after dosing (Fig. 4). The highest cumulative contents of MDS and ASS were within 8 h, where only a small amount of the unchanged compounds was eliminated through the kidneys during 12–24 h. The active metabolites (MDA and ASA), however, did not follow the same pattern observed for their respective parent compounds, as evidenced by the absence of the metabolites in the urine. Fecal samples collected from one of the volunteers also corroborated this assumption, as MDA and ASA were both found in the feces.
Metabolomics
Human metabolomic profiling
Following oral administration of a once-daily dose of 250 mg or 500 mg of modified-formula ECa 233 for 7 consecutive days, metabolomic profiling detected a total of 31 endogenous metabolites from 1D 1H-NMR spectra. Twenty-nine metabolites in plasma were identified according to the Chenomx NMR and human metabolome database (HMDB; Supplementary Fig. 4S). Clustering between pre- and post-dose receiving the repeated 500 mg of modified-formula was clearly separated based on the OPLS-DA score plot, indicating the discrimination of metabolites observed among those two clusters (Fig. 5).
The results on OPLS-DA score plot suggested that a two-dose treatment (250 and 500 mg in multiple dose) had the same patterns of metabolome changes. The permutation p-value of the model validity was only significant for the group of 500 mg. Therefore, these findings may indicate a dose-dependent relationship in metabolomics. The OPLS-DA coefficient loadings plot was further used to investigate in the group of 500 mg repeated doses. The correlation of variables with classification of OPLS-DA models is shown in Fig. 6. Based on the results in the loadings plot, five potential metabolites (Table 3) were indicated as possible biomarkers for the metabolomics alterations related effects when taking repeated doses of 500 mg modified-formula ECa 233.
OPLS-DA coefficient loadings plot of different metabolites that were altered in the pairwise comparison (predose, T0 vs postdose, T1h). Resonances pointing upwards reflect metabolites that were greater in the pre-dose group relative to the post-dose group, whereas resonances pointing downwards indicate lower concentrations of metabolites in the pre-dose group relative to the post-dose group.
The findings demonstrated the significant differences in levels of metabolites that varied in the pairwise comparison (predose T0 vs postdose T1h), (Fig. 6). In contrast, focusing on the metabolites at post-dose (T1h, day 7), these results showed that relative plasma concentrations of L-homoserine significantly decreased while levels of citrulline, O-succinyl-L-homoserine, homocarnosine, and choline were significantly increased after receiving modified formula ECa 233 in comparison to pre-dose levels at T0.
Metabolomics shifts
A shift in human endogenous metabolite profiles as a result of administered modified formula ECa 233 was observed between a single low dose (250 mg dose taken once) and multiple high dose (500 mg dose taken once daily for 7 days), (Fig. 7). The PCA trajectory scores plot between single low dose and multiple high dose at different time points (0, 0.5, 1, 2 and 4 h) revealed remarkable metabolic alterations. At pre-dose, the first time point demonstrates similar metabolic baselines of both groups suggesting that human endogenous metabolites at the beginning of the study are similar in both groups (Fig. 7). After receiving the modified formula ECa 233, distinct metabolic shifts were observed during 4 h at different magnitudes (Fig. 7).
Safety and tolerability
The modified formula ECa 233 was well-tolerated throughout the study (Table 4). No serious adverse events were reported. The most commonly reported AEs were associated with the gastrointestinal system; mild abdominal discomfort was occasionally (n = 2/12) found during the 250-mg multiple oral dosing, and moderate flatulence (n = 1/11) with constipation (n = 1/11) were rarely reported in the group that received 500 mg doses. One participant reported serious drowsiness after receiving the multiple high doses of ECa 233; this AE was also included in the previous report after oral administration with high doses of C. asiatica14.
Discussion
This study found that daily doses of the modified formula ECa 233 was safe and appropriate for clinical uses. This newly developed ECa 233 capsule causes changes not only in disposition kinetics but also in endogenous human metabolomics profiling.
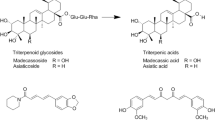
In comparison with the previous formulation7, AUC and Cmax of MDS and ASS exhibited the improved absorption kinetics of this modified-formula, which indicates an increased solubility of the parent compounds. This can be explained by the formula’s enhanced dissolution from the addition of a small amount of solubilizer, an excipient that increases the bioavailability of pharmaceutical ingredients. The higher dissolution of this formula could lead to the increased MDS and ASS absorption and result in the improved ECa 233 oral bioavailability. Interestingly, a significantly greater systemic exposure was clearly seen in their respective metabolites (MDA and ASA). These results reflect a complete biotransformation of the parent compounds, the unabsorbed triterpenoid glycosides that can be completely hydrolyzed to become their respective metabolites (triterpenic acids). This process is carried out by an enzyme produced by intestinal bacteria that has the role of hydrolyzing glycosides15. In addition, the previous study reported the possible hydrolysis mechanism of ASS. This mechanism involves the hydrolysis of the sugar moiety of the glycoside (ASS) under acidic conditions, converting ASS into aglycone (ASA)16, which becomes the active component of C. asiatica extract that has pharmacological effects17. This modified formula ECa 233 had greater accumulation of active metabolites as seen in the increased AUC and Cmax of ASA (p < 0.05), corresponding to the increased amount of ECa 233 in the administered dose. This accumulation supports the role of ASA in inhibition of P-glycoprotein (P-gp)18, an efflux membrane transporter that limits cellular uptake of xenobiotics. As a consequence of inhibiting P-gp expression, ASA (a substrate of P-gp) increases, then accumulates in the systemic circulation19. Other studies have also found an accumulation of ASA after oral administration of C. asiatica extract20.
In regard to the excretion behaviors, the study findings fill a gap in the knowledge around ECa 233. MDS and ASS are rarely absorbed but rather undergo extensive biotransformation with minimal renal excretion. Their active metabolites, MDA and ASA, which are highly lipophilic molecules, were likely eliminated through the hepatobiliary system and then mainly excreted by the fecal route. The results suggest that both major parent compounds were excreted mainly as metabolites via the feces. This is supported by the findings of other research in elimination pathways of C. asiatica extracts, which indicates that the excretion (0–72 h) of MDS was 0.25% in urine and 24.68% in feces21. Another study showed that the extract was excreted in feces over 24–76 h, while only a small amount of extract was eliminated via the kidneys20.
The accumulation behavior of the modified formula ECa 233 affected human metabolomic profiles, partly through an alteration of endogenous metabolites detected in plasma between pre- and post-dose. The changes in 5 relevant human metabolites were thoroughly considered to identify the candidate biomarkers; including L-homoserine, citrulline, O-succinyl L-homoserine, homocarnosine, and choline. In particular, increasing doses of ECa 233 to a 500-mg multiple oral dosing had a significant effect on increasing levels of choline, an endogenous metabolite reported to have benefits for learning and memory. It has been reported that higher concentrations of choline are believed to slow the progression of cognitive impairment9. The previous studies have suggested that a possible mechanism by which C. asiatica enhances cognitive function is through inhibition of acetylcholinesterase activity (AChEI). The studies also demonstrated this extract’s protective effect against ß-amyloid formation. The results indicated that ECa 233, an extract from of C. asiatica, might be considered as an AChEI alternative and responsible for increasing level of choline. Choline is a precursor of acetylcholine, a neurotransmitter that serves the neural activities in the learning and memory system. Furthermore, the reduction of AChE activity in cholinergic neurons is also involved with cognitive impairment as detected in Alzheimer’s disease22,23. This finding in healthy volunteers suggests that the recommended dose of ECa 233 should not be less than 500 mg/day in future studies investigating metabolite changes or exploring metabolic pathways related to cognitive deficits in patients.
Metabolite shifts were observed over 4 h after administration of multiple doses of ECa 233 (Fig. 7). The results indicated that changes of metabolomic profiling could be obviously detected over 1–2 h post-dose. This interval time is also considered as Tmax in the pharmacokinetic results (Tmax = 1–1.5 h, Table 2). These findings could imply that the relationship between drug disposition kinetics and the changes in human endogenous metabolites are affected by time after administration of high oral doses of ECa 233. Lastly, at 4 h later, the metabolic features had returned to nearly the starting point or closely to the initial metabolites again (Fig. 7), presumably because of the decreased concentration of the parent compounds and their active metabolites in plasma (Fig. 3). This pattern at end points conformed with the termination phase of ECa 233 as reflected in its excretion.
Conclusion
This study presents the first reported evidence on the elimination kinetics of standardized extract of C. asiatica (ECa 233), indicating that the unchanged forms (MDS and ASS) are excreted partly through renal elimination. Fecal excretion is assumed to be the major pathway for their active metabolites (MDA and ASA). The enhanced-formula ECa 233 indicated higher absorption, which significantly increased its bioactive metabolites in healthy volunteers. This study reveals that administering a daily dose of at least 500 mg of ECa 233 can alter human metabolic profiling. Five human endogenous metabolites (L-homoserine, citrulline, O-succinyl-L-homoserine, homocarnosine, and choline) should be further investigated as candidate biomarkers in a phase II clinical trial.
Change history
09 June 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-92113-2
Abbreviations
- AChE:
-
Acetylcholinesterase
- AChEI:
-
Acetylcholinesterase inhibitor or cholinesterase inhibitor
- AEs:
-
Adverse events
- ASA:
-
Asiatic acid
- ASS:
-
Asiaticoside
- AUC:
-
Area under plasma concentration–time curve
- BMI:
-
Body mass index
- Cl/F:
-
Apparent total clearance of the test substance removed from plasma after oral administration
- Cmax :
-
Maximum plasma concentration
- CPMG:
-
Carr-Purcell-Meiboom-Gill pulse
- ECa 233:
-
Standardized extract of Centella asiatica
- ESI:
-
Electrospray ionization
- F:
-
Absolute oral bioavailability
- HMDB:
-
Human metabolome database
- HPLC:
-
High performance liquid chromatography
- IQR:
-
Interquartile range
- IS:
-
Internal standard
- LC–MS/MS:
-
Liquid chromatography tandem mass spectrometry
- LLOQ:
-
Lower limit of quantification
- MDA:
-
Madecassic acid
- MDS:
-
Madecassoside
- MRM:
-
Multiple reaction monitoring
- NMR:
-
Nuclear magnetic resonance spectroscopy
- NOAEL:
-
No observed adverse effect level
- OPLS-DA:
-
Orthogonal-signal correction-projection to latent structure-discriminant analysis
- PCA:
-
Principal component analysis
- P-gp:
-
P-glycoprotein
- p.o. (Per os):
-
Oral administration
- SD:
-
Standard deviation
- STOCSY:
-
Statistical total correction spectroscopy
- Tmax :
-
Time to reach maximum plasma concentration
- TSP:
-
Trimethylsilylpropanoic acid
- t1 / 2 :
-
Elimination half-life
- Vd/F:
-
Apparent volume of distribution after non-intravenous administration
References
Gray, N. E. et al. Centella asiatica—Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 17, 161–194 (2018).
Saifah, E. et al. Preparation methods of colorless mixture between madecassoside and asiaticoside from Centella asiatica. In, Department of Intellectual Property MoC ed C07H; 2009.
Chivapat, S., Chavalittumrong, P. & Tantisira, M. H. Acute and sub-chronic toxicity studies of a standardized extract of Centella asiatica ECa 233. Thai. J. Pharm. Sci. 35, 55–64 (2011).
Tantisira, M. H. et al. Effect of a standardized extract of Centella asiatica ECa 233 on learning and memory impairment induced by transient bilateral common carotid artery occlusion in mice. Thai. J. Pharmacol. 32, 22–33 (2010).
Kam-eg, A., Tantisira, B. & Tantisira, M. H. Preliminary study on effects of standardized extract of Centella asiatica, ECa 233 on deficit of learning and memory induced by an intracerebroventricular injection of β-amyloid peptide in mice. Thai. J. Pharmacol. 33, 79–82 (2009).
Anukunwithaya, T., Tantisira, M. H., Tantisira, B. & Khemawoot, P. Pharmacokinetics of a standardized extract of Centella asiatica ECa 233 in rats. Planta Med. 83, 710–717 (2017).
Songvut, P., Chariyavilaskul, P., Tantisira, M. H. & Khemawoot, P. Safety and pharmacokinetics of standardized extract of Centella asiatica (ECa 233) capsules in healthy Thai volunteers: a phase 1 clinical study. Planta Med. 85, 483–490 (2019).
Botas, A., Campbell, H. M., Han, X. & Maletic-Savatic, M. Metabolomics of neurodegenerative diseases. Int. Rev. Neurobiol. 122, 53–80 (2015).
Graham, S. F., Holscher, C., McClean, P., Elliott, C. T. & Green, B. D. 1H NMR metabolomics investigation of an Alzheimer’s disease (AD) mouse model pinpoints important biochemical disturbances in brain and plasma. Metabolomics 9, 974–983 (2013).
FDA. Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. In; 2005.
Hackshaw, A. A Concise Guide to Clinical Trials (John Wiley & Sons, 2011).
Sakpal, T. V. Sample size estimation in clinical trial. Perspect. Clin. Res. 1, 67–69 (2010).
Phetcharaburanin, J. et al. Systemic characterization of an obese phenotype in the zucker rat model defining metabolic axes of energy metabolism and host-microbial interactions. J. Proteome Res. 15, 1897–1906 (2016).
Gohil, K. J., Patel, J. A. & Gajjar, A. K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 72, 546–556 (2010).
Grimaldi, R. et al. Pharmacokinetics of the total triterpenic fraction of Centella asiatica after single and multiple administrations to healthy volunteers. A new assay for asiatic acid. J. Ethnopharmacol. 28, 235–241 (1990).
Borhan, M. Z., Ahmad, R., Rusop, M. & Abdullah, S. Green extraction: Enhanced extraction yield of asiatic acid from Centella asiatica (L.) nanopowders. J. Appl. Chem. 2, 2 (2013).
Nagoor Meeran, M. F. et al. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: A pentacyclic triterpenoid of therapeutic promise. Front. Pharmacol. 9, 892–892 (2018).
Cheng, Q., Liao, M., Hu, H., Li, H. & Wu, L. Asiatic acid (AA) sensitizes multidrug-resistant human lung adenocarcinoma A549/DDP cells to cisplatin (DDP) via down regulation of p-glycoprotein (MDR1) and its targets. Cell Physiol. Biochem. 47, 279–292 (2018).
Guo, L., Cui, Y. & Hao, K. Effects of glycyrrhizin on the pharmacokinetics of asiatic acid in rats and its potential mechanism. Pharm. Biol. 56, 119–123 (2018).
EMA. Centellae asiaticae extractum summary report. (1998).
Leng, D.-D., Han, W.-J., Rui, Y., Dai, Y. & Xia, Y.-F. In vivo disposition and metabolism of madecassoside, a major bioactive constituent in Centella asiatica (L.) Urb. J. Ethnopharmacol. 150, 601–608 (2013).
Mukherjee, P. K., Kumar, V. & Houghton, P. J. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother. Res. 21, 1142–1145 (2007).
Soumyanath, A. et al. Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: investigation of a possible mechanism of action. Int. J. Alzheimers Dis. https://doi.org/10.1155/2012/381974 (2012).
Acknowledgements
This study was supported by the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship and the 90th Anniversary Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund). The authors would like to thank Maha Chakri Sirindhorn, Clinical Research Center, Faculty of Medicine, Chulalongkorn University; and Khon Kaen, University International Phenome Laboratory (KKUIPL) for their generosity in supporting this research. We would like to express sincere gratitude to Dr.Jutarop Phetcharaburanin for sharing his immense expertise in metabolomics, NMR data analysis and interpretation. The authors would like to thank Professor Daren Cornforth for proofreading. Lastly, we are grateful to all participants who kindly participated in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization and grant finding were done by P.C. and P.K. Subject management and sample collection were done by P.C. and P.S. Test compound preparation and pharmacokinetic analysis were carried out by P.S. and P.K. P.S. and R.T. performed metabolomic analysis, data management, statistical analysis and wrote the main manuscript. R.T., P.C. and P.K. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article modifications have been made to Phisit Khemawoot and Rossarin Tansawat’s affiliations. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Songvut, P., Chariyavilaskul, P., Khemawoot, P. et al. Pharmacokinetics and metabolomics investigation of an orally modified formula of standardized Centella asiatica extract in healthy volunteers. Sci Rep 11, 6850 (2021). https://doi.org/10.1038/s41598-021-86267-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86267-2
- Springer Nature Limited
This article is cited by
-
Bioenhancing effects of piperine and curcumin on triterpenoid pharmacokinetics and neurodegenerative metabolomes from Centella asiatica extract in beagle dogs
Scientific Reports (2022)
-
Increase water solubility of Centella asiatica extract by indigenous bioenhancers could improve oral bioavailability and disposition kinetics of triterpenoid glycosides in beagle dogs
Scientific Reports (2022)