Abstract
Factors influencing the efficacy of insectivorous vertebrates in providing natural pest control services inside crops at increasing distances from the crop edge are poorly understood. We investigated the identity of vertebrate predators (birds and bats) and removal of sentinel prey (mealworms and beetles) from experimental feeding trays in cotton crops using prey removal trials, camera traps and observations. More prey was removed during the day than at night, but prey removal was variable at the crop edge and dependent on the month (reflecting crop growth and cover) and time of day. Overall, the predation of mealworms and beetles was 1-times and 13-times greater during the day than night, respectively, with predation on mealworms 3–5 times greater during the day than night at the crop edge compared to 95 m inside the crop. Camera traps identified many insectivorous birds and bats over crops near the feeding trays, but there was no evidence of bats or small passerines removing experimental prey. A predation gradient from the crop edge was evident, but only in some months. This corresponded to the foraging preferences of open-space generalist predators (magpies) in low crop cover versus the shrubby habitat preferred by small passerines, likely facilitating foraging away from the crop edge later in the season. Our results are in line with Optimal Foraging Theory and suggest that predators trade-off foraging behaviour with predation risk at different distances from the crop edge and levels of crop cover. Understanding the optimal farm configuration to support insectivorous bird and bat populations can assist farmers to make informed decisions regarding in-crop natural pest control and maximise the predation services provided by farm biodiversity.
Similar content being viewed by others
Introduction
Finding solutions to reduce pesticide use in crops and enhance the abundance of natural enemies of crop pests in agroecosystems is a pressing global issue. Worldwide, pests and diseases are responsible for an estimated 20–40% of crop yield losses1. Natural enemies can suppress insect pests below economic thresholds and therefore provide a viable alternative to pesticide use2,3. Natural enemies of pests, including vertebrate and invertebrate insectivores (birds, bats and arthropods) and insect parasitoids, provide a service worth billions of dollars to global agriculture4,5,6. As such, natural pest control is one of the most important and economically significant ecosystem services to agriculture7. Yet, many aspects of natural pest control in crops remain unclear. In particular, the identity of the vertebrate predators providing this service is generally unknown and the efficacy of predation inside crops at increasing distances from the crop edge is poorly understood. This information is important for farmers who implement habitat modifications under agri-environmental schemes for natural pest control to determine how far inside crops the benefits of non-crop habitat may extend.
Insectivorous birds and bats protect crops from pest arthropod damage and are economically important natural enemies in agricultural systems8,9,10,11,12,13,14. Maintaining non-crop habitat for these natural enemies of crop pests can therefore provide both ecological and economic benefits15. Birds and bats have high energetic demands due to flight, and consume large amounts of prey to fuel high rates of energy expenditure16,17. Globally, 400–500 million tonnes of arthropods are consumed by birds annually, with 50% of birds considered insectivorous and an additional 25% of birds consuming invertebrates occasionally18. The efficacy of insectivorous birds in suppressing arthropods in agricultural and forest systems is well documented19. Bird-mediated reductions in arthropod abundance in crops typically vary from 20 to 70%12,19,20,21. For example, insectivorous birds significantly reduced densities of large arthropods and damage due to foliage-chewing insects by 46% in cacao crops21, and reduced infestation of the coffee berry corer (Hypothenemus hampei) by 50%12. Similarly, predation of the corn earworm by Tadarida brasiliensis (Brazilian free-tailed bat) is estimated to increase corn crop yields by 1.4%, with bats tracking and exploiting moth irruptions22,23. In inland eastern Australia, agricultural pests comprised 65% of the identified diet of insectivorous bats in cotton crops and were the six most frequently consumed prey items, suggesting a significant pest regulation service24.
Much of the work on insect pest control by insectivorous birds and bats has occurred in tropical forest and agricultural production systems13,25,26,27, with few studies in subtropical or temperate agroecosystems, aside from cotton crops in Northern America28, macadamia orchards in South Africa and Australia29, wheat11,30 and brassicas31,32. Studies attempting to separate and quantify the insect pest control service provided by birds and bats use exclosures12,13,25,33, or plasticine model caterpillars as a proxy for lepidopteran control34. Although these studies generally show that birds suppress more arthropods than bats, few link this service to pest consumption rates and some, but not all, infer insect predation via reduced leaf herbivory and increased yields34,35,36,37. Furthermore, the contribution of small nocturnal mammals to insect pest control is often overlooked, yet a growing body of evidence suggests that they provide an important pest regulation service38,39.
Diurnal and nocturnal vertebrate predators suppress different groups of arthropods and thus impact trophic processes (such as leaf herbivory) differently12. Insectivorous bats mostly target soft-bodied Lepidoptera above crops or glean from leaf surfaces22,23, whilst small insectivorous passerines forage in the shrub layer from the ground up and consume mainly ants, wasps, flies and beetles with a mean prey size of around 4–8 mm40,41,42,43. Larger passerines consume a range of small and large invertebrates and provide effective pest control of fossorial scarab larvae44 and above-ground pests including crickets, grasshoppers and beetles. Yet, remarkably little information exists about the identity, predation rate or potential pest control service provided by the range of vertebrate fauna present in any agricultural system.
Crop edge habitats provide a different physical and microclimatic environment compared with crop interiors. The activity of vertebrate predators is often greater at crop edges than in crop interiors45,46, with a concomitant increase in predation of invertebrates close to the crop edge47,48. Crop edges provide flyways for foraging birds; less complex echolocation for bats to navigate, target and catch prey than adjacent non-crop woody vegetation; access to abundant crop insect prey resources, and reduced predation risk compared to crop interiors. Crop edges are thus optimal foraging habitat for insectivorous birds and bats in agricultural mosaic landscapes due to access to prey45,49. Under optimal foraging theory (OFT), foraging decisions made by an animal to maximise energy consumption in the least amount of time possible are constrained by the detection, handling and capture of their prey and the risk of predation of the foraging animal itself50. Thus, foraging over open crops at increasing distance from the crop edge—woody vegetation interface is a trade-off between flight capabilities, foraging behaviour and the risk of predation. In terms of bats, echolocation constraints imposed by habitat clutter determine whether they usually locate and capture prey in narrow or cluttered flyways, at habitat edges, or in open habitats51,52. Whilst edge-space bats typically forage along vegetation interfaces in open areas such as inside crops edges53,54, most bats show some plasticity in foraging strategy and echolocation behaviour54,55, suggesting the potential adaptive capability for bats to forage away from the crop edge in the crop interior.
Cotton (Gossypium spp.) is a fibre crop and the most valuable non-food agricultural commodity worldwide56. However, cotton is also the second-top global host for arthropod pests, with several cotton pests considered major pests worldwide57. Hence, the control of insect pests (particularly Helicoverpa spp., cotton bollworms) is a major issue. Integrated pest management (IPM) in Australian cotton crops typically includes encouraging a mix of beneficial arthropods (such as predatory beetles and parasitic wasps)6,58,59,60,61, transgenic crop biotechnology and minimising pesticide use. The effectiveness of IPM in Australian cotton crops would be enhanced with information about the diversity of vertebrate predators providing natural pest control. Cotton-growing regions in northern New South Wales (NSW) and southern Queensland host an abundance of naturally occurring vertebrate predators more than one-third of all Australian land birds62,63, over one-quarter of insectivorous bat species (H. Kolkert et al., unpublished data) and several threatened insectivorous marsupial dasyurids and other small native rodents—that likely play an integral role in suppressing insects in crops, but are underutilised in IPM programs. In this study, we identified vertebrate predators and assessed predation rates in cotton crops across the growing season. We used a combination of prey removal trials and camera traps in cotton crops at incremental distances from the crop edge as well as bird census, to investigate predation rates of diurnal and nocturnal vertebrate predators on two types of sentinel prey (live mealworms and dead beetles). Ants interacted with prey at feeding stations and were identified as possible important predators and were also included in our analysis. We asked four questions: (1) Which vertebrate taxa are present and remove insect prey in cotton crops? (2) Is predation greater by day or by night? (3) Does proximity to the crop edge influence prey removal? (4) How do prey removal rates change over the cotton growing season?
Results
Mealworm removal
Around 11% (1814) of mealworms were removed out of the 16,300 provided, ased on 3260 sampling observations across an Australian summer cotton growing season. Mealworms were 12.5% more likely to be taken by day than night (Odds Ratio [OR] 0.88, Supplementary Table S2 online). More mealworms were also removed later in the season (Fig. 1a). In February and March, diurnal mealworm predation doubled, whilst nocturnal mealworm predation increased five-fold compared to December and January.
Mean number of (a) mealworms and (b) beetles removed, averaged over feeding station and sampling session by month (with 95% confidence intervals) and (c) predicted removal of mealworms at increasing distances from the crop edge by time of day (day vs night) and month, showing the 3-way interaction. Shaded areas represent 95% confidence intervals.
Distance from the crop edge significantly affected mealworm predation but depended on month (Fig. 1a) and time of day (Table 1, Fig. 1c). Over the whole season, 3–5 times more mealworms were removed at the crop edge than at 95 m inside the crop during the night and day, respectively (Fig. 1c and Supplementary Table S3 online). Predation on mealworms did not vary with distance into the crop in December (Fig. 1c and Supplementary Table S3). In January, predation on mealworms significantly declined with increasing distance from the crop edge and similarly by night in February (Fig. 1c and Supplementary Table S1). However, at night in March, this trend reversed and more mealworms were removed in the crop interior than edge (Fig. 1c and Supplementary Table S3).
Beetle removal
Around 8% (1254) of beetles were removed of the 15,060 provided. Beetles were 13 times more likely to be removed during the day than at night, holding all other variables constant (OR 14.00, Supplementary Table S2 online). However, the interaction between time of day and month had a significant effect on beetle predation (Table 1). Predation on beetles was greatest by day in December, with 5-times the number of beetles removed than at night in December or in other months either by day or night (Fig. 1b). Significantly more beetles (around 2.5-times more) were removed by day than night in January and February, but there was no difference in March (Fig. 1b and Supplementary Table S1). The effect of distance to the crop edge on beetle predation was not significant (Table 1).
Compared to beetles, mealworms were 87% more at risk of predation (OR 0.13), holding all other variables constant (Supplementary Table S2 online). There was a high probability that a predator consumed zero beetles in the experiment (predicted probability 26%, zero inflation model).
Likely predators
Camera images
In total, 972 camera images with vertebrate species present were tagged (Table 2) from a total of 6449 day-camera hours and 5209 night-camera hours. Camera images revealed that prey was removed from the feeding stations by a variety of fauna (Table 3a and Supplementary Fig. S1 online). Five bird species common in agricultural landscapes were recorded, including two nocturnal and three diurnal species: eastern barn owl (Tyto alba), southern boobook (Ninox boobook), willie wagtail (Rhipidura leucophrys), Australian magpie (Gymnorhina tibicen) and pied butcherbird (Cracticus nigrogularis). Nocturnal mammals could not be confidently identified to species level and were grouped as either insectivorous bats or other small mammals (Table 3a).
Australian magpies were the main predators during the day (and were the main consumer of beetles, H. Kolkert, pers. obs.) whilst camera traps (CT) revealed that insectivorous bats were the main predator in crops during the night (Table 3a). However, bats were not captured on camera gleaning experimental prey or landing on the feeding trays. Rather they were captured foraging above the cotton close to the feeding trays. Insectivorous bats were present in 10-times more images than diurnal fauna (birds), with most visitations (tagged images) occurring in December and January. At night, January had the highest visitation rate to feeding trays (~ 24 CT/h; Table 2).
In the day, December had the highest visitation rate and length of visits (measured as an activity index, 0.93 CT/h and 4 m 31 s AI/h; Table 2) with magpies responsible for nearly all the prey items removed from feeding stations, as indicated by camera trap images. Visitation length at night (in March) was strongly skewed by two incidents where a small scansorial mammal sat on the feeding station for several hours. During the day, twice as many visits were recorded close to the cotton edge than inside the crop (Table 2). At night, seven times more visitations were recorded close to the cotton edge than inside the crop. However, visitation length was the inverse, skewed by the lengthy small mammal visits. Hence, visitation length was not a reliable predictor of prey removal in this experiment.
Birds, scats and ant counts
Eight species of bird were recorded in the sampling areas, totalling 170 bird observations (Table 3b). Mean monthly bird richness, bird abundance, ant abundance and scat abundance during morning and evening censuses were greatest late in the season (February and March) compared to earlier in the season (Fig. 2). This pattern reflects the monthly pattern of mealworm predation (Fig. 1a). Potential predators and climate variables were significantly correlated with patterns of mean total prey removal, mealworm removal and beetle removal rates, but the relationships were weak (r ≤ 0.16, Table S4). Additional species of small insectivorous bird were noted inside cotton crops, but these were recorded outside the sampling areas (H. Kolkert, pers. obs.).
Discussion
Understanding the optimal timing and location of beneficial insectivorous birds and bats can support decision-making to configure farm layout to maximise the insect pest predation services provided by farm biodiversity. In this study, we identified diurnal and nocturnal vertebrate insectivores that were present or removed experimental prey in a high-value commodity crop. A clear predation gradient from the crop edge was identified, but the gradient was only evident in some months. The variation in mealworm removal rates likely reflects predator foraging behaviours in response to crop cover versus open habitat, in-line with OFT64. For example, small insectivorous passerines (thornbills, cisticolas and fairywrens) are cover-dependent and were present in crops later in the growing season (Table 3b), when established cotton plants provided greater foliage cover, shelter, and protection from predators. Mature, shrubby cotton (mainly ~ 150 cm, but up to 200 cm high) facilitated small bird movement and access inside the crop, and best explained the lack of mealworm predation gradient from the edge into the crop during March. Increased foraging opportunities for cover-dependent species during February and March may also explain the higher mealworm removal rate (both by day and by night) than in other months. The lack of predation gradient in mealworm removal in December may be attributed to the absence of small birds traversing the sharp interface between crop edge habitat and the open field of young cotton. The low height and foliage cover of cotton plants (~ 30 cm high) in December also favoured larger birds of open habitat (Australian magpies) that are less sensitive to foraging away from the crop edge. These results support previous studies that identify shrubby habitat as a mediating factor for the movement of small birds across contrasting edge habitats in agricultural and forest systems45,65. It also highlights the importance of sufficient understorey cover in habitat immediately adjacent to the crop for edge-sensitive insectivorous birds and bats to facilitate movement and foraging in crops.
The removal of beetles primarily by the Australian magpie and peak beetle removal in December suggest that generalist predators suited to hunting in open habitat are less sensitive to crop edge proximity (moving throughout the crop), and explains the lack of an edge predation gradient early in the season. Increased foraging opportunities that benefit ground-feeding omnivores like Australian magpies occur early when ground cover is sparse. Therefore, our results suggest that predators favouring open foraging habitat benefit farmers during the initial stages of crop development (when bare soil is exposed). Small insectivorous birds did not consume beetles from feeding stations, to our knowledge. The size of the prey items offered may have excluded smaller predators from the experiment due to differences in prey size and thus availability to predators. This highlights the importance of maintaining predator functional diversity in farming landscapes in order to suppress different sized pest species for integrated pest control66,67.
Camera trap images also indicated that more visits occurred closer to the crop edge, by day and night (Table 2). However, small passerines were not captured on camera traps (Table 3b) and their contribution to prey removal was uncertain. Whilst more prey items were generally removed from feeding stations during the day, a greater number of camera images (mainly of insectivorous bats) were tagged at night. Maximum insectivorous bat activity was recorded in January (which corresponds to young bats becoming volant); yet experimental prey removed from the feeding stations was least in this month. Newly volant bats may not have removed prey from feeding stations because the predation risk was too high, due to reduced crop cover. A similar finding by Nelson and Gillam68 showed that during vulnerable life stages (pregnancy and lactation), bats prefer edge habitat due to the reduced predation risk. Furthermore, we hypothesise that, like birds69,70, newly volant juvenile bats are at greater risk of predation than adult bats, and that bold risk-taking behaviour in foraging bats increases with age. This may explain the high nocturnal mealworm removal in February and March, but not January.
Images of insectivorous bats did not necessarily reflect prey removal, although the presence of bats in the crop presumably inferred foraging behaviour and it is likely that bats were responsible for some mealworm removal with gleaning bat species present (Kolkert et al. unpublished data). This highlights the fact that artificial prey experiments may not accurately reflect the predation service to be estimated. Bats captured on camera were likely foraging for alternate food resources in the crop i.e. abundant crop insects. This suggested that bats were selecting a preferred food source inside the crop (i.e. moths), consistent with Optimal Foraging Theory (OFT), that predicts when prey is abundant (i.e. ~ 450 species of cotton insects) predators become selective with prey choice. Insectivorous bats in this area are known to consume agricultural arthropods, particularly moths, in cotton crops24. Therefore, bats captured on camera were likely hunting moths. This finding suggests the potential for camera traps to infer predation rates of crop pests by insectivorous bats in cropping agroecosystems. Further work is needed to calibrate the number of camera trap images of bats with pest predation and consumption volumes.
Artificial predation experiments are limited as they do not accurately reflect the visual, behavioural or physiological cues relied on to detect prey71,72,73. Although we attempted to account for predator cues by using live mealworms and regionally abundant dead beetles, beetles do not comprise a large component of insectivorous bat diets in crops in this region (H. Kolkert et al., unpublished data) and are less nutritious than mealworms74, which may explain why more mealworms were removed, and why the effects of proximity to crop edge were stronger with mealworms. For these reasons we suspect mealworms were the preferred food item for other small insectivorous birds, such as fairywrens, cisticolas and willie wagtails, with larger generalist predators, such as owls, tawny frogmouths, Australian magpies, also consuming beetles. Prey choice and participation in the predation experiment for a variety of predators was likely influenced by the abundant and diverse insect supply in the Bt-cotton61,72. It is possible that that attack rates on experimental prey may be under-inflated due to low predation pressure72. Nevertheless, we attempted to provide live stimuli and different sized prey to encourage participation in the experiment by a diversity of insectivore predators.
Future studies should aim to test other prey resources (potentially pest species) to further understand foraging mechanisms and prey selection by whole predator communities at the crop edge. Regardless, our camera trap and experimental prey removal results suggest that insectivorous predators forage in crops as predicted by OFT50, where the trade-off between resource maximisation and the risk of predation of the predators increased away from the crop edge. Whilst this was also true for bats, it is likely that echolocation constraints also contributed to foraging closer to the crop edge. Knowing that fear of predation, rather than food availability drives the spatial foraging patterns of insectivorous predators close to the crop interface (except for large generalist open space foragers like Australian magpies) can encourage land managers to provide suitable non-crop habitat adjacent to crop edges.
In conclusion, our results showed clear patterns between the removal of sentinel insect prey in crops, proximity to the cotton crop edge and the presence of potential predators. Information should be provided about how to encourage open space generalist predators early in the growing season and cover-dependent small passerines later in the season for pest control of different sized insects. Furthermore, information about the predation gradient from the crop edge and the rate of change in natural pest control with increasing distance into the crop over the growing season can be used to measure the outcome of biodiversity modifications on farms to benefit natural pest control. We conclude that optimal natural pest control in cotton at different phenological stages likely occurs via a diversity of predator functional groups. Maintaining a mix of structural vegetation components adjacent to the crop edge75 could buffer the variability in predator communities, particularly small passerines that avoid open habitat.
Methods
Ethics
Animal ethics approval was obtained from the University of New England Animal Ethics Committee (approval no. AEC13-060) in addition to a New South Wales (NSW) scientific licence (licence no. SL101296) issued by the NSW Office of Environment and Heritage. All methods were performed in accordance with the relevant guidelines and regulations.
Study sites
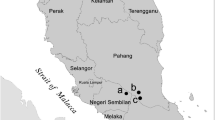
This research was conducted during the 2014–15 summer cotton growing season on three irrigated cotton farms near Boggabri (30°43′15.4"S 150°04′52.5"E) in northern NSW, Australia (Fig. 3). The combined farm area totalled 1654 ha, with 925 ha dedicated to irrigated cotton, 185 ha to dryland cotton, 335 ha to grazing and the remainder to houses, outbuildings, roads and irrigation channels. The research took place in three fields of irrigated cotton (one on each farm): 45 ha, 25 ha and 12 ha in size. The distance between each field was 4.0 km, 2.5 km and 6.0 km respectively. Cotton was planted in early October 2014 and no insecticide was applied during the growing season. At these farms (consistent with cotton grown in subtropical zones around Australia), the cotton flowers around December, cotton bolls develop in January and vegetative growth peaks around February. By March the majority of bolls open and crop harvesting occurs between March and April. Irrigated Bollgard II cotton was grown on these farms, planted in a configuration of one row of cotton per metre. Bollgard II cotton (Bt-cotton) contains two genes derived from the soil bacterium, Bacillus thuringiensis, producing toxins that are lethal to the larvae of Lepidoptera. About 10% of the cropping area at each farm was dedicated to unsprayed refuge crops (non-Bt, cotton and pigeon pea). Refuge crops delay the resistance of moths to the Bt toxin, by allowing Bt-resistant and Bt-susceptible moths to mate and disperse in crops.
These farms occur in the sub-tropical Brigalow Belt South Bioregion76 on black self-mulching clay alluvial plains adjacent to the Namoi River77. Remnant vegetation adjacent to each cotton field was predominantly forest, floodplain woodland and semi-arid grassland78 most commonly dominated by river red gum (Eucalyptus camaldulensis) open woodland associated with the Namoi River and adjacent floodplain. Native vegetation adjacent to each cotton field was unmanaged and extended along the entirety of the Namoi River in the region.
Trial design
At each farm, a 100 × 100 m (1 ha) sampling area of cotton crop was selected adjacent to remnant vegetation. Each 1 ha sampling area was divided into a 10 × 10 m grid with ten successive predation distances from the crop edge and ten successive bands of cotton, perpendicular to the crop edge. The first predation distance was 5 m from the edge of the crop (fifth row of cotton) with subsequent predation distances at 10-m intervals (15, 25 … 95 m; Fig. 3). At each predation distance, random number generation determined in which band a feeding station was placed. Each feeding station consisted of a plastic green tray (250 × 250 × 40 mm) nailed to a wooden stake, 1 m above the ground in the cotton row and left in situ for the duration of the experiment (total n = 60, 20 sampling points per site with two stations per distance). The possibility of predators learning the location of feeding stations and associating them with a food resource was accounted for by leaving the stations in-situ outside of sampling times.
Previously frozen and thawed Sericesthis geminata (Scarabaeoidea, Coleoptera, collected in Armidale and Boggabri, NSW) and live mealworms (the larvae of Tenebrio molitor; Pisces Enterprises) were placed on each feeding station to simulate a prey resource. Experimental trials indicated that live Helicoverpa armigera larvae (Lepidoptera: Noctuidae, a major pest of cotton) could not withstand the heat of the day (≥ 40˚C) on the feeding station. This led to the decision to use live mealworms with a hard exoskeleton and beetles to attract a diversity of predators. Sericesthis geminata are common in Boggabri during the cotton growing season and emerge en masse and swarm on warm nights. It is plausible that nocturnal fauna including insectivorous bats were attracted to this prey resource.
Each feeding station had 15 mealworms (15–20 mm long, 3 mm diameter) and 10–15 beetles (11–16 mm long). The number of beetles was always consistent within a sampling period, between farms. Prey removal was recorded, and prey replenished every morning and evening within an hour of sunrise and sunset. Prey removal was measured over the cotton growing season between December and March inclusive. Sampling occurred over 29 days (8 consecutive days in January; 7 consecutive days in other months) on three farms day and night. Sampling was missed on eleven occasions due to inclement weather. This resulted in 3260 sampling observations of feeding stations on the three farms across the season.
Identification of potential predators
Infrared motion-activated trail cameras (ScoutGuard SG550) were deployed at eight feeding stations per sampling area to record the animals visiting feeding stations. When motion was detected, one image per second for 3 secs was captured. Cameras were set to record for the duration of each sampling session and placed in the same position at each subsequent sampling session. Fauna images were identified to species where possible and categorised as day or night.
Bird species richness and abundance undertaken from the crop edge (point counts) over each sampling area for a period of 20 min each morning and evening (within an hour of sunrise and sunset) while replenishing feeding trays (n = 348 sampling events, 29 days by three farms day and night). The order of fields visited for bird observations was randomised. Scats deposited on trays and ant abundance were recorded at each feeding station and plates cleared of scats every morning and afternoon. These methods were used to evaluate the potential role of insectivorous birds and ants as predators of the experimental prey. Climate data from the Bureau of Meteorology (station no. 055202) was recorded each day.
Data analysis
Data analysis was performed in R. software (version 4.0.2)79. We used the glmmTMB package (v1.0.2.1, published 2020–07-01) to fit binomially distributed data using maximum likelihood estimation80. To determine the probability of a predation event, the number of prey removed (mealworms or beetles) of the pre-set number on offer was the response variable. Generalised linear mixed-effects models (GLMMs) were fitted with a beta-binomial distribution that captured overdispersion in the binomially distributed data and accounted for varying numbers of prey at feeding stations.
We tested whether the probability of a predation event was affected by the following fixed effects and interactions: time of day (day or night), month (December to March) and foraging distance (i.e. proximity to cotton edge; 5, 15, 25 … 95 m). Farm (three levels) and feeding stations were treated as random effects. A separate conditional model was created for each of the two prey resources (mealworms and beetles). A zero-inflation parameter was applied to the beetle model, where the probability of producing a structural zero was a function for all observations (ziformula ≃ 1). The zero-inflation parameter described the probability of observing an extra (i.e. structural) zero that was not generated by the conditional model. Predicted marginal effects (predicted probabilities for logistic models) of the mealworm removal rate from the crop edge were made using the ‘ggeffects’ package (v0.7.0, published 2018–11-17)81, taking random effects into account. Wald-type 95% confidence intervals for predicted average count were calculated. Residuals and diagnostics of models were checked using the DHARMa package (v0.2.0, published 2018–06-06), a simulation-based approach for checking model assumptions and diagnostics82. We identified the best fitting model based on Dharma diagnostics. The EMMEANS package (v1.5.1) was used to calculate estimated marginal means using the Tukey method to determine which pairwise differences were significant83.
To derive an estimate of the change in predation risk, we adopted an odds-based approach where odds-ratios (OR) were calculated by exponentiating the model coefficients (β). Odds-ratios were converted to a percent and calculated as (1 – exp(β) × 100) where OR ≤ 1, and (exp(β) – 1 × 100) where OR > 1. The resulting OR then describes the factor by which predation odds change for a 1-unit increase in the corresponding factor84.
Camera images with an animal present were tagged as a visitation event. Visitation events were quantified in two ways: (1) counts of visitations at each feeding station (or in the camera field of view for bats) were calculated as camera trap images per hour (CT/h), and (2) the length of time of visitations at each feeding station was calculated as an activity index (AI/h). Each method was adjusted for the sampling time at each location and standardised by the number of tagged images (1) or hours of recording (2). Visitation events were then allocated to a distance from the crop edge (close: 5–45 m; far: 55–95 m).
Bird abundance, bird richness, ant abundance and scat abundance data for every sampling observation and site were tabulated. Pearson correlation coefficients (r) were calculated between climate data (minimum and maximum temperature, daily rainfall, daily evaporation, maximum daily wind speed) and the predation rate of mealworms, beetles and total predation rate (combined mealworm and beetle predation).
Data availability
Should the manuscript be accepted, the data supporting the results will be archived in an appropriate public repository such as Dryad or Figshare and the data DOI will be included at the end of the article.
References
FAO. United Nations Food Agricultural Organisation. High Level Expert Forum (FAO, Rome, 2009).
Puig-Montserrat, X. et al. Pest control service provided by bats in Mediterranean rice paddies: Linking agroecosystems structure to ecological functions. Mamm. Biol. 80, 237–245. https://doi.org/10.1016/j.mambio.2015.03.008 (2015).
Cleveland, C. J. et al. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front. Ecol. Environ. 4, 238–243. https://doi.org/10.1890/1540-9295(2006)004[0238:Evotpc]2.0.Co;2 (2006).
Boyles, J. G., Cryan, P. M., McCracken, G. F. & Kunz, T. H. Conservation. Economic importance of bats in agriculture. Science 332, 41–42. https://doi.org/10.1126/science.1201366 (2011).
Naylor, R. L. & Ehrlich, P. R. In Nature’s Services: Societal Dependence on Natural Ecosystems (ed. Daily, G. C.) 151–174 (Island Press, New York, 1997).
Losey, J. E. & Vaughan, M. The economic value of ecological services provided by insects. Bioscience 56, 311–323. https://doi.org/10.1641/0006-3568(2006)56[311:Tevoes]2.0.Co;2 (2006).
Power, A. G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. Lond. B 365, 2959–2971. https://doi.org/10.1098/rstb.2010.0143 (2010).
Maine, J. J. & Boyles, J. G. Bats initiate vital agroecological interactions in corn. Proc. Natl. Acad. Sci. U.S.A. 112, 12438–12443. https://doi.org/10.1073/pnas.1505413112 (2015).
Tremblay, A., Mineau, P. & Stewart, R. K. Effects of bird predation on some pest insect populations in corn. Agric. Ecosyst. Environ. 83, 143–152. https://doi.org/10.1016/S0167-8809(00)00247-4 (2001).
Van Bael, S. A. et al. Birds as predators in tropical agroforestry systems. Ecology 89, 928–934 (2008).
Grass, I., Lehmann, K., Thies, C. & Tscharntke, T. Insectivorous birds disrupt biological control of cereal aphids. Ecology 98, 1583–1590. https://doi.org/10.1002/ecy.1814 (2017).
Karp, D. S. et al. Forest bolsters bird abundance, pest control and coffee yield. Ecol. Lett. 16, 1339–1347. https://doi.org/10.1111/ele.12173 (2013).
Maas, B. et al. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol. Rev. Camb. Philos. Soc. 91, 1081–1101. https://doi.org/10.1111/brv.12211 (2016).
Cohen, Y., Bar-David, S., Nielsen, M., Bohmann, K. & Korine, C. An appetite for pests: Synanthropic insectivorous bats exploit cotton pest irruptions and consume various deleterious arthropods. Mol. Ecol. 29, 1185–1198. https://doi.org/10.1111/mec.15393 (2020).
Chaplin-Kramer, R., de Valpine, P., Mills, N. J. & Kremen, C. Detecting pest control services across spatial and temporal scales. Agric. Ecosyst. Environ. 181, 206–212. https://doi.org/10.1016/j.agee.2013.10.007 (2013).
Speakman, J. R. & Thomas, D. W. In Bat ecology (eds Kunz, T. H. & Fenton, M. B.) 430–490 (University of Chicago Press, Chicago, 2003).
Norberg, U. M. Avian Energetics and Nutritional Ecology 199–249 (Springer, Berlin, 1996).
Nyffeler, M., Şekercioğlu, Ç. H. & Whelan, C. J. Insectivorous birds consume an estimated 400–500 million tons of prey annually. Sci. Nat. 105, 47. https://doi.org/10.1007/s00114-018-1571-z (2018).
Sekercioglu, C. H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471. https://doi.org/10.1016/j.tree.2006.05.007 (2006).
Mols, C. M. M. & Visser, M. E. Great tits can reduce caterpillar damage in apple orchards. J. Appl. Ecol. 39, 888–899. https://doi.org/10.1046/j.1365-2664.2002.00761.x (2002).
Van Bael, S. A., Bichier, P. & Greenberg, R. Bird predation on insects reduces damage to the foliage of cocoa trees (Theobroma cacao) in western Panama. J. Trop. Ecol. 23, 715–719. https://doi.org/10.1017/s0266467407004440 (2007).
Federico, P. et al. Brazilian free-tailed bats as insect pest regulators in transgenic and conventional cotton crops. Ecol. Appl. 18, 826–837. https://doi.org/10.1890/07-0556.1 (2008).
McCracken, G. F. et al. Bats track and exploit changes in insect pest populations. PLoS ONE 7, e43839. https://doi.org/10.1371/journal.pone.0043839 (2012).
Kolkert, H., Andrew, R., Smith, R., Rader, R. & Reid, N. Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol. Evol. 10, 371–388. https://doi.org/10.1002/ece3.5901 (2020).
Maas, B., Clough, Y. & Tscharntke, T. Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol. Lett. 16, 1480–1487. https://doi.org/10.1111/ele.12194 (2013).
Kalka, M. B., Smith, A. R. & Kalko, E. K. Bats limit arthropods and herbivory in a tropical forest. Science 320, 71. https://doi.org/10.1126/science.1153352 (2008).
Williams-Guillen, K., Perfecto, I. & Vandermeer, J. Bats limit insects in a neotropical agroforestry system. Science 320, 70. https://doi.org/10.1126/science.1152944 (2008).
Kunz, T. H., de Torrez, E. B., Bauer, D., Lobova, T. & Fleming, T. H. In Year in Ecology and Conservation Biology (eds Ostfeld, R. S. & Schlesinger, W. H.) 1–38 (New York Academy of Sciences, New York, 2011).
Taylor, P. J., Grass, I., Alberts, A. J., Joubert, E. & Tscharntke, T. Economic value of bat predation services: A review and new estimates from macadamia orchards. Ecosyst. Serv. 30, 372–381. https://doi.org/10.1016/j.ecoser.2017.11.015 (2018).
Redlich, S., Martin Emily, A. & Steffan-Dewenter, I. Landscape-level crop diversity benefits biological pest control. J. Appl. Ecol. https://doi.org/10.1111/1365-2664.13126 (2018).
Hooks, C., Pandey, R. R., & Johnson, M. W. Unlikely guardians of cropping systems: Can birds and spiders protect broccoli from caterpillar pests? Insect Pests (2007).
Martin, E. A., Reineking, B., Seo, B. & Steffan-Dewenter, I. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc. Natl. Acad. Sci. U.S.A. 110, 5534–5539. https://doi.org/10.1073/pnas.1215725110 (2013).
Karp, D. S. & Daily, G. C. Cascading effects of insectivorous birds and bats in tropical coffee plantations. Ecology 95, 1065–1074. https://doi.org/10.1890/13-1012.1 (2014).
Barbaro, L. et al. Avian pest control in vineyards is driven by interactions between bird functional diversity and landscape heterogeneity. J. Appl. Ecol. 54, 500–508. https://doi.org/10.1111/1365-2664.12740 (2017).
Rey Benayas, J. M., Meltzer, J., de las Heras-Bravo, D. & Cayuela, L. Potential of pest regulation by insectivorous birds in Mediterranean woody crops. PLoS ONE 12, 15. https://doi.org/10.1371/journal.pone.0180702 (2017).
Morrison, E. B. & Lindell, C. A. Birds and bats reduce insect biomass and leaf damage in tropical forest restoration sites. Ecol. Appl. 22, 1526–1534 (2012).
Ndanganga, P. K., Njoroge, J. B. M. & Vickery, J. Quantifying the contribution of birds to the control of arthropod pests on kale, Brassica oleracea acephala, a key crop in East African highland farmland. Int. J. Pest Manage. https://doi.org/10.1080/09670874.2013.820005 (2013).
Tschumi, M., Ekroos, J., Hjort, C., Smith, H. G. & Birkhofer, K. Rodents, not birds, dominate predation-related ecosystem services and disservices in vertebrate communities of agricultural landscapes. Oecologia 188, 863–873. https://doi.org/10.1007/s00442-018-4242-z (2018).
Elkinton, J. S., Liebhold, A. M. & Muzika, R.-M. Effects of alternative prey on predation by small mammals on gypsy moth pupae. Popul. Ecol. 46, 171–178. https://doi.org/10.1007/s10144-004-0175-y (2004).
Dyrcz, A. Breeding biology and behaviour of the willie wagtail (Rhipidura leucophrys) in the madang region Papua New Guinea. Emu 94, 17–26. https://doi.org/10.1071/MU9940017 (1994).
Adriano, S. & Calver, M. C. Diet of breeding willie wagtails (Rhipidura leucophrys) in suburban Western Australia. Emu 95, 138–141. https://doi.org/10.1071/MU9950138 (1995).
Razeng, E. & Watson, D. M. What do declining woodland birds eat? A synthesis of dietary records. Emu 112, 149–156. https://doi.org/10.1071/MU11099 (2012).
Brandl, R., Kristín, A. & Leisler, B. Dietary niche breadth in a local community of passerine birds: An analysis using phylogenetic contrasts. Oecologia 98, 109–116. https://doi.org/10.1007/bf00326096 (1994).
Kaplan, G. Australian Magpie: Biology and Behaviour of an Unusual Songbird (CSIRO Publishing, Clayton, 2019).
Puckett, H. L., Brandle, J. R. & Johnson, R. J. Avian foraging patterns in crop field edges adjacent to woody habitat. Agric. Ecosyst. Environ. 131, 9–15 (2009).
Best, L. B., Whitmore, R. C. & Booth, G. M. Use of cornfields by birds during the breeding season: the importance of edge habitat. Am. Midl. Nat. 123, 84–99. https://doi.org/10.2307/2425762 (1990).
Hansen, N. A., Sato, C. F., Michael, D. R., Lindenmayer, D. B. & Driscoll, D. A. Predation risk for reptiles is highest at remnant edges in agricultural landscapes. J. Appl. Ecol. 56, 31–43. https://doi.org/10.1111/1365-2664.13269 (2019).
Storch, I., Woitke, E. & Krieger, S. Landscape-scale edge effect in predation risk in forest-farmland mosaics of Central Europe. Landsc. Ecol. 20, 927–940. https://doi.org/10.1007/s10980-005-7005-2 (2005).
Douglas, D. J. T., Vickery, J. A. & Benton, T. G. Improving the value of field margins as foraging habitat for farmland birds. J. Appl. Ecol. 46, 353–362. https://doi.org/10.1111/j.1365-2664.2009.01613.x (2009).
Stephens, D. W. & Krebs, J. R. Foraging Theory (University Press, 1986).
Denzinger, A. & Schnitzler, H.-U. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 4, 164. https://doi.org/10.3389/fphys.2013.00164 (2013).
Schnitzler, H.-U. & Kalko, E. K. V. Echolocation by insect-eating bats. Vol. 51 (SPIE, 2001).
Neuweiler, G. Foraging, echolocation and audition in bats. Naturwissenschaften 71, 446–455. https://doi.org/10.1007/BF00455897 (1984).
Fenton, M. B. The foraging behaviour and ecology of animal-eating bats. Can. J. Zool. 68, 411–422. https://doi.org/10.1139/z90-061 (1990).
Jantzen, M. K. & Fenton, M. B. The depth of edge influence among insectivorous bats at forest–field interfaces. Can. J. Zool. 91, 287–292. https://doi.org/10.1139/cjz-2012-0282 (2013).
Estur, G. Cotton Exporter's Guide. (International Trade Centre UNCTAD/WTO, 2007).
RBG In State of the World’s Plants 2017 (ed. Willis, K.) 64–71 (Board of Trustees of the Royal Botanic Gardens, Kew, 2017).
Rencken, I. An Investigation of the Importance of Native and Non-Crop Vegetation to Beneficial Generalist Predators in Australian Cotton Agroecosystems PhD thesis, University of New England (2006).
Holloway, J. C., Furlong, M. J. & Bowden, P. I. Management of beneficial invertebrates and their potential role in integrated pest management for Australian grain systems. Aust. J. Exp. Agric. 48, 1531–1542. https://doi.org/10.1071/EA07424 (2008).
Schellhorn, N. A., Bianchi, F. J. & Hsu, C. L. Movement of entomophagous arthropods in agricultural landscapes: Links to pest suppression. Annu. Rev. Entomol. 59, 559–581. https://doi.org/10.1146/annurev-ento-011613-161952 (2014).
Whitehouse, M. E. A., Wilson, L. J. & Fitt, G. P. A comparison of arthropod communities in transgenic Bt and conventional cotton in Australia. Environ. Entomol. 34, 1224–1241 (2005).
Smith, R., Reid, J., Scott-Morales, L., Green, S. & Reid, N. A baseline survey of birds in native vegetation on cotton farms in inland eastern Australia. Wildl. Res. 46, 304–316. https://doi.org/10.1071/WR18038 (2019).
Ford, G. & Thomson, N. Birds on Cotton Farms: A Guide to Common Species and Habitat Management (Cotton Catchment Communities CRC, Boca Raton, 2006).
Whelan, C. J., Wenny, D. G. & Marquis, R. J. In Year in Ecology and Conservation Biology (eds Ostfeld, R. S. & Schlesinger, W. H.) 25–60 (Annals of the New York Academy of Sciences, New York, 2008).
Rodríguez, A., Andrén, H. & Jansson, G. Habitat-mediated predation risk and decision making of small birds at forest edges. Oikos 95, 383–396 (2001).
Sekercioglu, C. H. Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J. Ornithol. 153, 153–161. https://doi.org/10.1007/s10336-012-0869-4 (2012).
Greenop, A., Woodcock, B. A., Wilby, A., Cook, S. M. & Pywell, R. F. Functional diversity positively affects prey suppression by invertebrate predators: a meta-analysis. Ecology 99, 1771–1782. https://doi.org/10.1002/ecy.2378 (2018).
Nelson, J. J. & Gillam, E. H. Selection of foraging habitat by female little brown bats (Myotis lucifugus). J. Mammal. 98, 222–231. https://doi.org/10.1093/jmammal/gyw181 (2016).
Rohner, C. & Krebs, C. J. Owl predation on snowshoe hares: Consequences of antipredator behaviour. Oecologia 108, 303–310. https://doi.org/10.1007/bf00334655 (1996).
Rockwell, C., Gabriel, P. O. & Black, J. M. Bolder, older, and selective: Factors of individual-specific foraging behaviors in Steller’s jays. Behav. Ecol. 23, 676–683. https://doi.org/10.1093/beheco/ars015 (2012).
Krebs, J. R. In Perspectives in Ethology (eds Bateson, P. P. G. & Klopfer, P. H.) 73–111 (Springer US, Berlin, 1973).
Lövei, G. L. & Ferrante, M. A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Sci. 24, 528–542. https://doi.org/10.1111/1744-7917.12405 (2017).
Nagy, R. K., Schellhorn, N. A. & Zalucki, M. P. Fresh, frozen or fake: A comparison of predation rates measured by various types of sentinel prey. J. Appl. Entomol. 144, 407–416. https://doi.org/10.1111/jen.12745 (2020).
Ravzanaadii, N., Kim, S.-H., Choi, W. H., Seong-Jin, H. & Kim, N. J. Nutritional value of mealworm, tenebrio molitor as food source. Int. J. Ind. Ergon. 25, 93–98 (2012).
Barbaro, L., Giffard, B., Charbonnier, Y., van Halder, I. & Brockerhoff, E. G. Bird functional diversity enhances insectivory at forest edges: A transcontinental experiment. Divers. Distrib. 20, 149–159. https://doi.org/10.1111/ddi.12132 (2014).
Environment Australia. Revision of the Interim Biogeographic Regionalisation of Australia (IBRA) and Development of Version 5.0: Summary Report. (Department of Environment and Heritage, Canberra, 2000).
OEH. NSW (Mitchell) Landscapes. Vol. version 3.1 (State of New South Wales and Office of Environment and Heritage 2002).
Keith, D. A. Ocean Shores to Desert Dunes: The Native Vegetation of NEW South Wales and the ACT (Department of Environment and Conservation, 2004).
R Core Team, R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/, 2018).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 400. https://doi.org/10.3929/ethz-b-000240890 (2017).
Lüdecke, D. ggeffects: Create Tidy Data Frames of Marginal Effects for ‘ggplot’ from Model Outputs (v0.16.0). https://CRAN.R-project.org/package=ggeffects. (2017).
Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-level/mixed) Regression Models (v0.3.3). https://CRAN.R-project.org/package=DHARMa (2017).
Lenth, R., Singmann, H., Love, J., Buerkner, P. & Herve, M. Estimated Marginal Means, aka Least-Squares Means (v1.5.3). https://www.rdocumentation.org/packages/emmeans (2019).
Szumilas, M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry 19, 227–229 (2010).
Acknowledgements
We would like to thank the Watson family who allowed us to work on their property. Field assistance provided by Sarah Hartman and Dr Shannon Currie. Camera traps were kindly lent to us by Dr Guy Ballard. This project was funded by the Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation, the NSW North West Local Land Services (LLS) and the Brigalow-Nandewar Biolinks Project (Australia Government Biodiversity Fund – Projects LSP-991865-1429 and LSP-944752-1076) managed by the North West and Northern Tablelands LLS.
Author information
Authors and Affiliations
Contributions
All authors conceived the project and contributed to project design, statistical direction and manuscript revisions. H.K. collected the data, analysed the data and wrote the first draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kolkert, H.L., Smith, R., Rader, R. et al. Prey removal in cotton crops next to woodland reveals periodic diurnal and nocturnal invertebrate predation gradients from the crop edge by birds and bats. Sci Rep 11, 5256 (2021). https://doi.org/10.1038/s41598-021-84633-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84633-8
- Springer Nature Limited