Abstract
Two subspecies of rice, Oryza sativa ssp. indica and O. sativa ssp. japonica, with reproductive isolation and differences in morphology and phenotypic differences, were established during the process of rice domestication. To understand how domestication has changed the transcriptomes of the two rice subspecies and given rise to the phenotypic differences, we obtained approximately 700 Gb RNA-Seq data from 26 indica and 25 japonica accessions, and identified 97,005 transcribed fragments and 4579 novel transcriptionally active regions. The two rice subspecies had significantly different gene expression profiles, we identified 1,357 (3.3% in all genes) differentially expressed genes (DEGs) between indica and japonica rice. Combining existing gene function studies, it is found that some of these differential genes are related to the differentiation of the two subspecies, such as grain shape and cold tolerance, etc. Functional annotation of these DEGs indicates that they are involved in cell wall biosynthesis and reproductive processes. Furthermore, compared with the non-DEGs, the DEGs from both subspecies had more 5′flanking regions with low polymorphism to divergence ratios, indicating a stronger positive selection pressure on the regulation of the DEGs. This study improves our understanding of the rice genome by comparatively analyzing the transcriptomes of indica and japonica rice and identifies DEGs those may be responsible for the reproductive isolation and phenotypic differences between the two rice subspecies.
Similar content being viewed by others
Introduction
In addition to being a staple food that feeds over 50% of the world’s population, rice has also been used as a model plant for molecular, genetics and comparative genomic studies1,2. Rice varieties are traditionally classified into two major subspecies, Oryza sativa ssp. indica and O. sativa ssp. japonica, during the long process of rice domestication. These two rice subspecies are different in terms of yield, grain quality and stress resistance2,3,4,5. Indica rice is mainly cultivated in tropical and subtropical regions, whereas japonica rice is planted mainly in temperate regions or at higher altitudes in tropical and subtropical regions. During the process of domestication and breeding, these two subspecies have evolved characteristic morphological and agronomic traits those may contribute to intraspecific phenotypic adaptations6,7. The morphological features, including leaf color, seed size and apiculus hair length, cannot be used alone to definitively distinguish between the two subspecies due to the presence of morphological variants8,9,10. According to a previous report, multiple hybridization events between japonica and indica, both before and after the divergence of the indica cultivars11. However, the molecular mechanisms underlying the reproductive isolation and phenotypic differences remain largely unknown.
Thus far, there have been many studies on the molecular basis of the phenotypic differences between the two subspecies using different methods12,13. Among the methods used to search for candidate genes from hybrids of indica and japonica rice, the most important one is the quantitative trait locus (QTL)14,15. Using this method, several studies have managed to isolate and characterize candidate genes those are expressed in key tissues or at certain developmental stages from the two subspecies, indicating that gene expression variations contribute greatly to the phenotypic differences between the two subspecies16,17,18,19,20,21. Although there have been a number of transcriptomic studies and microarray analyses in several rice varieties, currently there are no transcriptomic studies carried out with multiple rice genotypes to establish a statistically significant genotype–phenotype correlation between two subspecies22. It has been suggested that nucleotide diversity in gene regulatory regions has a major impact on the expression of genes associated with the phenotypic differences between the two subspecies. In addition, it has been speculated that these gene regulatory regions may have evolved under positive selection pressure23,24,25,26. Although the gene-coding regions have been compared between indica and japonica rice, there is no method to effectively investigate the gene regulatory regions.
Studies on gene expression profiles and gene regulatory regions are limited by the high cost and inherent cloning bias of conventional high-throughput sequencing approaches and incomplete cDNA or expressed sequence tag (EST) libraries19. With the high sequencing efficiency and quality of paired-end-tag next generation sequencing, we are able to obtain large amounts of RNA-Seq data from a large number of samples and use these data to investigate gene expression profiles and study gene regulatory regions27,28,29,30,31,32. In this study, we analyzed the transcriptomes in the young panicle of 26 indica and 25 japonica accessions using RNA- Seq and identified 97,005 transcribed fragments and 4579 novel transcriptionally active regions. On this basis, we compared the transcriptomes of the two subspecies and identified 1357 differentially expressed genes (DEGs) between indica and japonica rice. Some of the identified DEGs were then confirmed by reverse transcription polymerase chain reactions (RT-PCR). This study has the following conclusions: (1) we report that the two rice subspecies have 1357 different gene expression profiles; (2) we propose the relationship between the DEGs and the phenotypic differences, including grain shape, cold tolerance and nitrate-absorption; and (3) we speculate that nucleotide diversity in gene regulatory regions may associate with the phenotypic differences and play an important role in crop domestication. Collectively, the results of this study improve our understanding of the rice genome.
Results
High-throughput transcriptome sequencing and read mapping in two rice subspecies
To characterize the gene expression profiles at the reproductive stage of the two rice subspecies, we extracted total RNA from 40-mm-long young panicles of 26 indica and 25 japonica accessions (Table S1). For each plant, equal amounts of total RNA isolated from three panicles was mixed to establish an RNA pool, which was then further processed using a previously described method33 with minor modifications, and the samples were subjected to shotgun sequencing by an Illumina GAII instrument. We obtained 2.45 billion reads for the 26 indica accessions and 2.41 billion reads for the 25 japonica accessions at a 125 base-pair resolution (Table S2). After filtering out low-quality reads and reads containing adaptor sequences (7% of all reads), we obtained approximately 4.52 billion clean reads (345.48 and 331.99 Gb for indica and japonica accessions, respectively) (Table S2). These clean reads were then mapped to the reference japonica rice genome (Nipponbare; IRGSP v7.0), with at most two mismatches allowed for each read, while ignoring the reads that were mapped to more than two locations in the reference genome (multi-mapped reads). According to these criteria, we filtered out (~ 15%) of the clean reads and mapped 70.36–89.88% of the clean reads to the reference genome (Fig. 1A; Table S3). Among the mapped clean reads, 62.47–78.92% were mapped uniquely to one genomic location (Table S3). In addition, the rates of non-splice genes were 41.08–56.60% for indica accessions and 44.68–60.83% for japonica accessions (Fig. 1B; Table S3). We then calculated the mapping rates for each sample and the average mapping rates for the two subspecies. The results showed a significant difference in the average mapping rate between the two subspecies. The average mapping rate for indica accessions was 77.66%, whereas that for japonica was 83.88% (t = 19.275 and p < 0.001). Due to the fact that we used the japonica genome as the reference, we suspect that this difference may have been caused by mapping biases. Therefore, we decided to construct a pseudo-transcriptome of indica rice referring to the method in the tomato34 as the reference genome for the reads obtained from the indica rice samples. As a result, there was no significant difference in the average mapping rate between the two subspecies. The newly calculated average mapping rate for indica accessions was 84.01%, whereas that for japonica remained 83.88% (t = 0.12 and p = 0.91).
The mapping rates of the clean data in 26 indica and 25 japonica plants. (A) The mapping rates of the clean data. The white block represents the rate of multi-mapped reads, and the grey block indicates the rate of uniquely mapped reads. (B) The splice rate in 51 rice plants. The white block represents the splice rate, whereas the grey block indicates the non-splice rate. The numbers associated with the x-axis correspond to the sample name (see Table S1 for sample information). This figure was created using Microsoft Excel 2013 and Adobe Illustrator CS6.
RNA-Seq read assembly and identification of novel transcriptionally active regions
We subsequently assembled the RNA-Seq clean reads into 97,005 transcribed fragments with a mean length of 884 bp (ranging from 50 to 20,674 bp) (Fig. 2A). These fragments were aligned to the sequences of known rice genes (MSU 7.0). The sequence alignment results are shown in Fig. 2B. Among the assembled fragments, 26.31% overlapped with exons, 36.31% fell into annotated exons, 16.57% fell into introns, and the remaining 20.77% were non-gene sequences (Fig. 2B). We also found that 42% of rice genes had alternative transcripts. This percentage was much lower than that previously estimated based on microarray results18.
Summary of the assembled transcribed fragments. (A) Length distribution of the assembled transcribed fragments. (B) Classification of the assembled transcribed fragments. The transcribed fragments overlapping with annotated exons, exons, introns and non-gene regions are shown in blue, green, red and purple, respectively. This figure was created in Microsoft Excel 2013 and Adobe Illustrator CS6.
In total, we identified 4,579 novel transcriptionally active regions based on the sequences of rice genes and non-coding RNAs (ncRNAs) deposited in the Ensemble ncRNA and National Center for Biotechnology Information (NCBI) EST databases (E-value < 1e-6, Table S4). Among these regions, 61.7% were unknown protein-coding regions, 67.2% could be transcribed into single-exon transcripts, 42.71% shared more than 90% sequence identity with at least one known EST, and 12.14% were likely to encode non-coding RNAs. In addition, our RNA-Seq data expanded the untranslated regions (UTR) of 11.23% of rice genes.
Identification of DEGs between the two rice subspecies
According to the MSU rice genome annotation database, 74.71–88.49% of the mapped reads overlapped with annotated genes for each sample. The number of reads mapped to a certain annotated gene ranged from 2 to more than 300,000, with a median number of 23 and 32 for indica and japonica accessions, respectively. There were 40,580 genes mapped by two reads in at least two samples. In addition, we were able to establish linear relationships between the number of reads mapped to a certain gene and the gene expression level (FPKM) in all the samples (0.77 < R2 < 0.94; Fig. 3A). Given that we found significant differences in the number of reads mapped to a certain gene between the indica and japonica accessions (t-test; P = 0.012). Furthermore, principal variance component analysis also revealed significant differences in gene expression levels between japonica and indica individuals (Fig. 3B).
Significant differences in gene expression levels between indica and japonica. (A) Linear relationships in gene expression level between each sample. The R2 ranges from 0.75 (red) to 0.95 (blue). (B) The plot for principal variance component analysis of expression variations between indica and japonica plants. This figure was created using Microsoft Excel 2013 and Adobe Illustrator CS6.
We then employed fragments per kilobase of transcript per million mapped reads (FPKM) values to estimate the expression level of various genes. To further confirm gene expression validation, 29 genes with different expression patterns revealed by RNA-seq were randomly selected for validation by quantitative real-time RT-PCR (qPCR). Three technical replicates were performed for each gene, and their specific primers were listed in Table S5. All of the 29 selected genes, only three genes were inconsistent with the results by the Illumina sequencing technology (Fig. 4). Taken together, most of the genes showed consistent expression patterns those were consistent with the RNA-Seq data, indicating that our experimental results were valid. Among the 41,080 expressed genes, 38,911 (94.72%) were expressed in both rice subspecies, 623 genes were expressed only in indica accessions and 1,546 genes were expressed only in japonica accessions. Thereafter, we analyzed our RNA-Seq data using a generalized linear model and identified 1,357 (3.3% of all the expressed genes) DEGs between indica and japonica rice (false discovery rate [FDR] < 0.05; Fig. 5A). 576 and 781 of these DEGs were expressed at lower and higher levels in indica rice than in japonica rice, respectively. (Fig. 5A). To identify biological functions of the differentially expressed genes (DEGs) (Table S6), we performed a literature search and found nine DEGs with important known functions including GW2 (LOC_Os02g14720) and OsBISERK1 (LOC_Os08g07760) that regulate grain shape in rice13,35. These two genes exhibit the same trend of expression changes, with higher expression levels in indica rice. OsARG (LOC_Os04g01590) and OsNRT1 (LOC_Os03g13274), which are involved in nitrate (N) and ammonium absorption in indica rice36,37, may be related to the higher uptake of N in indica rice. One leucine zipper protein OsbZIP38, and Proline-rich protein OsPRP3, enhance cold stress responses in japonica rather than in indica rice38,39. The Wx (LOC_Os06g04200) gene encodes a granule-bound starch synthase (GBSS) and plays a key role in determining rice eating and cooking qualities (ECQs). In our results, the expression level of Wx in indica rice was up to 3-folds higher than in japonica rice, as well as OsSSI (LOC_Os06g06560) and OsAGPL1 (LOC_Os03g52460) genes, which is consistent with the observation that the swelling of indica is stronger than japonica rice. We then mapped these DEGs to the rice genome and found they are distributed throughout the 12 chromosomes (P > 0.05; Fig. 5B). For better presentation of the results, these eight genes also be mapped on Fig. 5B. Furthermore, using REEF software (sliding window size was set to 500 kb to 1 Mb and the step size was set to 10 kb), we found that the DEGs tended to group in close proximity along the chromosomes (FDR < 0.05)40. However, we could not conclude that the DEGs were clustered.
Summary of DEGs between indica and japonica rice. (A) Genes that were expressed at significantly different levels between indica and japonica rice. Red dots represent the up-regulated genes and green dots show the down-regulated genes. The blue dots represent non-DEGs. The volcano spots show 1357 unigenes, including 781 up-regulated unigenes and 576 down-regulated unigenes, which were identified as DEGs. (B) Distribution of the DEGs along rice chromosomes. The maps (A, B) were created in R (v.3.2.1; https://www.r-project.org/).
Functional annotation of the DEGs
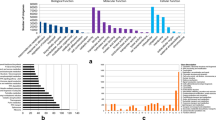
We annotated the DEGs using enrichment analysis tool. According to the annotations, 21 Gene Ontology (GO) terms were significantly enriched (P < 0.05; Fig. 6). These terms could be classified into two groups. The first group consisted of reproduction-related terms, including “reproduction”, “recognition of pollen”, “multi-organism reproductive process” and “cell recognition”. The second group consisted of cell wall biosynthesis-related terms, including “cell wall organization”, “cellulose biosynthetic process”, “cellulose metabolic process”, “cellulose synthase activity” and “UDP-glucosyl transferase activity”. The GO analysis showed that the genes encoding the binding proteins were enriched in these co-expressed genes (Fig. 6). Interestingly, genes expressed at higher levels in indica rice than in japonica rice were annotated with reproduction-related terms, whereas those expressed at lower levels in indica rice than in japonica rice were annotated with cell wall biosynthesis-related terms. Consistent with these findings, among the ten most significant morphological differences between the two subspecies, six were reproduction-related traits.
Histogram of Gene Ontology classifications. The letters associated with the x-axis indicate the GO categories, and the y-axis indicates the number of unigenes in each category. (A) GO analysis of all the DEGs. (B) GO analysis of the down-regulated genes. (C) GO analysis of the up-regulated genes. This figure was created using Goseq software.
To explore candidate pathways and genes that might be associated with the domestication of indica and japonica rice, we analyzed the enriched KEGG pathways (P < 0.05). Our results showed that the top four enriched pathways were regulation of autophagy (dosa04140), arginine and proline metabolism (dosa00330), pyrimidine metabolism (dosa00240), and starch and sucrose metabolism (dosa00500). It has been reported that the amylose content is usually higher in endosperm starch of indica rice than that of japonica rice41. In our results, there were 184 genes annotated being involved in the starch and sucrose metabolism pathway, with 15 DEGs (5 up- and 10 down-regulated genes in japonica rice) between the two subspecies. Overall, these results indicate that rice domestication has significant effects on starch and sucrose metabolism.
Effect of selection pressure on the regulation of the DEGs
To explore whether artificial selection may have affected the expression of the DEGs, we investigated the gene regulatory regions (i.e., 5′ and 3′ flanking regions) and gene-coding regions of the DEGs (Fig. 7, Table S6). Specifically, we searched for genomic regions with polymorphism to divergence (π/Dxy) ratios ranked in the lowest 5% (P < 0.001), because such regions may have a greater chance of experiencing positive selection. Compared with non-DEGs, DEGs from both subspecies had more 5′ flanking regions with low π/Dxy ratios (Fisher’s exact test, P < 0.05). These results were then confirmed by a resampling test (P < 0.05). Taken together, these results suggest that the 5′ regulatory regions of the DEGs experienced positive selection during rice domestication, and that gene regulation plays an important role in the evolution of the two subspecies.
Polymorphism and divergence in gene-coding and regulatory regions of genes in indica and japonica rice. Y-axis indicates the ratio of polymorphism to divergence (π/Dxy), and bars in the x-axis represent the 5′ regulatory regions, gene-coding regions and 3′ regulatory regions of genes. The grey blocks indicate the DEGs, and the black blocks represent the non-DEGs. ** indicates P-value < 0.05. This figure was created using Microsoft Excel 2013 and Adobe Illustrator CS6.
Discussion
In this study, the transcriptomes of two rice subspecies, indica and japonica, were characterized by the high-throughput RNA-Seq approach. The 31.58 billion RNA-Seq data provided us with valuable resources to discover novel transcriptionally active regions, identify DEGs and investigate the effect of selection pressure on the expression of the DEGs. According to the read mapping results, 25% of the clean reads were mapped to intergenic regions in the reference rice genome, suggesting the identification of 4,579 novel transcriptionally active regions. This speculation was then confirmed by RT-PCR, which validated the expression of some of these transcripts in all the samples tested. Therefore, in addition to characterizing the transcriptome of annotated rice genes, we also identified 4,579 novel transcriptionally active regions. More than 53% of these novel transcriptionally active regions may have been transcribed into ncRNAs because: (1) the transcribed fragments could be processed into mature transcripts with a mean length of 200 bp, which is the general length of long noncoding RNAs42; (2) 98.76% of the interval sequences between these transcribed regions were less than 5 kb in length, which is consistent with the distribution characteristics of intergenic pre-miRNAs42; and (3) the sequences of these transcribed regions shared high similarity with 37.2% of the ncRNAs deposited in the Ensembl ncRNA database. These results also highlight the significance of RNA-Seq in identifying the boundaries between sense-antisense gene pairs and quantitatively characterizing transcriptomes at a high resolution. Taken together, these results improve our understanding of the rice genome.
The expression patterns of different rice genes may vary during different developmental stages and in different plant individuals. Therefore, it is important to obtain RNA samples from a large number of plant individuals to reveal statistically significant differences in gene expression levels between rice subspecies. In this study, we obtained RNA samples from three young panicles of each of the 26 indica and 25 japonica accessions and searched for DEGs between the two rice subspecies based on the RNA-Seq data. In a previous study, the percentage of DEGs between closely related species in all detected genes varied greatly from 2 to 25%43. In extreme situations, this percentage can reach up to 78%22. The big differences in the percentage of DEGs among these studies may have been due to the fact that they use different gene expression quantification (e.g., qRT-PCR, microarray, and RNA-Seq) and data analysis methods26. Nonetheless, these studies have demonstrated that the percentages of DEGs between two species are generally higher than those between two subspecies. For example, a previous transcriptomic study found that 3.3% of the 18,242 genes detected were expressed at significantly different levels between maize and its progenitor teosinte44, whereas in another study, the percentage of DEGs between wild and weedy sunflowers (Helianthus annuus) was 5%45. In this study, we found that 3.3% of all the genes detected were expressed at significantly different levels between indica and japonica rice, suggesting that the strong artificial selection altered the expression of a small proportion of rice genes.
During artificial selection, cultivated rice has undergone significant changes in agronomically important traits, such as grain yield and traits that associated with abiotic stress resistance. We analyzed DEGs in depth and found that the expression levels of GW2 (LOC_Os02g14720) and OsBISERK1 (LOC_Os08g07760), which control grain width and length, respectively, were significantly different between the two rice subspecies. The expression of GW2 was higher in japonica than in indica, whereas that of OsBISERK1 was higher in indica. These results are in agreement with the grain shape of indica and japonica rice. Nitrogen is a crucial nutrient for plant growth and development. Indica has higher nitrate-absorption activity than japonica46. Here we show that the expression levels of OsARG (LOC_Os04g01590)36 and OsNRT1 (LOC_Os03g13274)37 were both higher in indica than in japonica, which may contribute to this difference in nitrate use between these two subspecies. Due to the remarkable adaptability to different ecological and geographical environments, the two major rice subspecies, indica and japonica are distinct in the ability to tolerate cold stress. In general, japonica cultivars are more cold-tolerant than indica47,48. Proline-rich protein (PRP) genes, OsPRP3 (LOC_Os10g05750), is positive regulators that control the cold tolerance39, Our RNA-seq data showed the expression levels of the two genes were much higher in japonica than in indica rice, which is consistent with the previous conclusion. Starch, which is composed of amylose and amylopectin, is the key determinant of rice quality. There are great differences in ECQs between japonica and indica during rice domestication49,50. In this study, three genes related to starch synthesis, Wx (LOC_Os06g04200), OsSSI (LOC_Os06g06560), and OsAGPL1 (LOC_Os03g52460) had the same expression trend, with higher expression levels in indica rice, which potentially affect rice quality.
According to our GO analysis, genes that were expressed at lower levels in indica rice than in japonica rice were annotated with reproduction-related terms, whereas those that were expressed at higher levels in indica rice than in japonica rice were annotated with cell wall biosynthesis-related terms. Therefore, it is reasonable to speculate that differences in gene expression patterns are one of the reasons for the reproductive isolation between the two rice subspecies.
The contribution of genetic drift and selection pressures to the genetic and phenotypic differences between species has become a research hotspot in evolutionary biology in the past few decades. During rice domestication, artificial selection has been reported to have a major impact on rice gene expression42. According to previous studies on domesticated plants, the 5′ regulatory regions of many genes evolved under the pressure of artificial selection and that the 5′ regulatory regions of DEGs evolved faster those of non-DEGs22. Consistent with these results, multiple studies in model fish species found that nucleotide diversity in gene regulatory regions, rather than gene-coding regions, are important for speciation22,51. The relationships between nucleotide diversity and gene expression during species evolution have also been investigated by transcriptomic studies in Drosophila, fire ants, Arabidopsis and maize, and by comparative transcriptomic studies in humans and chimpanzees, and humans and mice. Taken together, these results support the hypothesis that gene regulatory regions and gene-coding regions evolve independently during speciation. Meanwhile, divergence in 5′ regulatory regions of DEGs is a strong indication for the involvement of positive selection during speciation. In this study we looked at the role of RNAs transcript in shaping the changes in cell wall biosynthesis and reproductive processes that occurred during rice domestication. We’ve found that they play an important role in this process. Although the research on crop domestication using genomics and genomic technology has been carried out for nearly 20 years, we still know very little about the genetic basis of most domestication traits in crop varieties. Early research tended to the simple traits controlled by only one or two genes and mutations could be easily identified. It is more difficult to identify more subtle developmental changes that are critical to many changes during the domestication. This research is a step by studying a regulatory mechanism that is essential for regulating changes related to rice domestication.
Methods
Sample collection and total RNA isolation
In a previous study, we collected seeds from 825 Oryza sativa ssp. japonica and indica accessions in a rice cultivation area and assessed the morphological and genetic differences between the two subspecies5. Based on this previous study, we selected 26 indica and 25 japonica accessions and cultivated them in an artificial climate chamber under conditions of 12 h light and 12 h dark (Table S1).
For total RNA isolation, three 40-mm-long young panicles were collected from each rice plant. These panicles were subjected to RNA isolation using TRIzol reagent (Invitrogen, USA), according to the manufacturer’s instructions. The purity and integrity of the RNA were determined by the NanoPhotometer® spectrophotometer (IMPLEN, USA) and the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, USA), respectively. Thereafter, rRNA was removed from the total RNA samples and the rRNA-depleted total RNA was subjected to library construction using the NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina® (NEB, USA), according to the manufacturer’s instructions. First strand cDNA was synthesized using random oligonucleotides and SuperScript II. Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H.
RNA-Seq data filtering and assembly
Quality control of the raw data was performed with an in-house-developed PERL script. Low quality reads, reads with adaptor sequences and reads containing poly-N stretches were removed. The number of remaining clean reads for each sample is shown in Table S2. At the same time, Q20, Q30 and GC content of the clean reads were calculated (Table S2). To further evaluate the quality of the RNA-Seq data, we mapped reads from japonica and indica accessions to a reference japonica genome (RGAP 7.0) and a pseudo-transcriptome of indica34, respectively. The reference index was built using Bowtie v2.2.652. In addition, paired-end clean reads were aligned to the reference genome with TopHat v2.1.053. Duplicate reads were removed using SAMtools v1.2.554,55 and Picard v1.39. All the results are summarized in Table S2.
The mapped reads of each sample were assembled using Scripture beta256 and Cufflinks v2.1.157. Cufflinks was also be used to estimate the expression of a transcript based on the mapping results58.
Identification of DEGs
The FPKM of each gene was calculated using Cufflinks v2.1.157. We performed principal variance component analysis to evaluate the differences in gene expression levels between the two subspecies, and the Pearson correlation coefficients between RNA-Seq data and RT-PCR results were also calculated. To identify a gene as differentially expressed, we used the Cuffdiff program within the Cufflinks. With the application of a model based on the negative binomial distribution57, Cuffdiff presents statistical routines for the determining of differential expression in the transcripts of gene expression data. To control the false discovery rate, we filtered out genes that were expressed at a level of less than 20 mapped reads in any of the 51 plants. We also calculated the Bayesian posterior P-value of each gene using a previously described method59.
Determination of the effect of selection pressure based on resequencing data
To identify polymorphisms in all the DEGs and non-DEGs, we explored the resequencing data obtained from 12 japonica, 12 indica and 5 O. rufipogon plants grown in China60. The genomic DNA from the 29 individuals was sequenced at an 18–20 bp resolution and approximately 5.23 million single-nucleotide polymorphism (SNP) loci were obtained. We calculated the nucleotide diversity for japonica (πj), indica (πi) and O. rufipogon (πw) populations using Vcftools v0.1.13. A rank-based method was adopted to determine the nucleotide diversity in the 5′ flanking regions (2000 bp from start coden), gene-coding regions and 3′ flanking (2000 bp from stop coden) regions of the DEGs and non-DEGs between O. rufipogon and japonica or between O. rufipogon and indica. The results were confirmed by a resampling test, which was repeated 1000 times.
GO and KEGG analysis
To annotate the functions of the DEGs, we performed Gene Ontology (GO) analysis using a GO Seq R package, in which the gene length bias was corrected. We also calculated the FDR-corrected P-values for the GO terms using hypergeometrical distribution. GO terms with corrected P-values less than 0.05 were considered to be significantly enriched. KEGG is a database resource for understanding genome sequencing and other high-throughput experimental technologies (http://www.genome.jp/kegg/)61,62. We used KOBAS software to test the statistical enrichment of DEGs in KEGG pathways.
Quantitative RT-PCR analysis
Quantitative RT-PCR was performed using the SYBR® Premix Ex TaqTM Kit (TaKaRa, Japan) on an ABI PRISM 7900HT platform (Applied Biosystems, USA), according to the manufacturer’s instructions. For each sample, two biological replicates were analyzed by three technical repeats. The rice ubiquitin-1 gene (LOC_Os03g13170) was used as the internal reference gene. The primers for qRT-PCR analysis are listed in Table S5.
Data availability
All the data generated or analyzed during this study are included in the published version of this paper and its supplementary information files. The sequences have been deposited into the NCBI Sequence Read Archive database (PRJNA 610458 and PRJNA 628160).
References
Khush, G. S. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 35, 25–34 (1997).
Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501 (2012).
Garris, A. J., Tai, T. H., Coburn, J., Kresovich, S. & McCouch, S. Genetic structure and diversity in Oryza sativa L. Genetics 169, 1631–1638 (2005).
Zhu, Q., Zheng, X., Luo, J., Gaut, B. S. & Ge, S. Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice. Mol. Biol. Evol. 24, 875–888 (2007).
Wang, C. H. et al. Genetic diversity and classification of Oryza sativa with emphasis on Chinese rice germplasm. Heredity 112, 489–496 (2014).
Wu, C. et al. Development of enhancer trap lines for functional analysis of the rice genome. Plant J. 35, 418–427 (2003).
Zheng, Y., Crawford, G. W., Jiang, L. & Chen, X. Rice domestication revealed by reduced shattering of archaeological rice from the lower Yangtze valley. Sci. Rep. 6, 28136 (2016).
Kato, S. On the affinity of rice varieties as shown by fertility of hybrid plants. Bull. Sci. Fac. Agric. Kyushu Univ. 3, 132–147 (1928).
Matsuo, T. Origin and differentiation of cultivated rice. In Science of the Rice Plant Vol. 3 (eds Matsuo, T. et al.) 69–88 (Tokyo, Food and Agriculture Policy Research Center, 1997).
Oka, H. I. Origin of Cultivated Rice (Japan Sci, Soc, 1988).
Yang, C. C. et al. Independent domestication of Asian rice followed by gene flow from japonica to indica. Mol. Biol. Evol. 29, 1471–1479 (2012).
Beukert, U. et al. Genome-based identification of heterotic patterns in rice. Rice 10, 22 (2017).
Yuan, H. et al. 08SG2/OsBAK1 regulates grain size and number, and functions differently in Indica and Japonica backgrounds in rice. Rice 10, 25 (2017).
Huang, X. et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42, 961–967 (2010).
Zheng, X. M. et al. Genome-wide association study of rice grain width variation. Genome 1–8 (2017).
Lu, T. et al. Function annotation of the rice transcriptome at single-nucleotide resolution by RNA-seq. Genome Res. 20, 1238–1249 (2010).
Liu, F. et al. Gene expression profiles deciphering rice phenotypic variation between Nipponbare (Japonica) and 93–11 (Indica) during oxidative stress. PLoS ONE 5, e8632 (2010).
Jung, K. H. et al. Genome-wide identification and analysis of Japonica and Indica cultivar-preferred transcripts in rice using 983 Affymetrix array data. Rice 6, 19 (2013).
Sato, Y. et al. RiceXPro version 3.0: expanding the informatics resource for rice transcriptome. Nucleic Acids Res. 41, D1206-1213 (2013).
Yang, Y., Zhu, K., Xia, H., Chen, L. & Chen, K. Comparative proteomic analysis of indica and japonica rice varieties. Genet. Mol. Biol. 37, 652–661 (2014).
Horiuchi, Y. et al. Global expression differences and tissue specific expression differences in rice evolution result in two contrasting types of differentially expressed genes. BMC genom 16, 1099 (2015).
Guo, J. et al. Widespread and adaptive alterations in genome-wide gene expression associated with ecological divergence of two Oryza species. Mol. Biol. Evol. 33, 62–78 (2016).
Jones, F. C. et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484, 55–61 (2012).
Nosil, P. & Feder, J. L. Genomic divergence during speciation: causes and consequences. Philos. Trans. R. Soc. B. 367, 332 (2012).
Hanikenne, M. et al. Hard selective sweep and ectopic gene conversion in a gene cluster affording environmental adaptation. PLoS Genet. 9, e1003707 (2013).
Guo, W. & Sarkar, S. Adaptive Controls of FWER and FDR Under Block Dependence. arXiv: 1611.03155v1 (2016).
Furutani, I., Sukegawa, S. & Kyozuka, J. Genome-wide analysis of spatial and temporal gene expression in rice panicle development. Plant J. 46, 503–511 (2006).
Li, L. et al. Genome-wide transcription analyses in rice using tiling microarrays. Nat. Genet. 38, 124–129 (2006).
Li, L. et al. Global identification and characterization of transcriptionally active regions in the rice genome. PLoS ONE 2, e294 (2007).
Satoh, K. et al. Gene organization in rice revealed by full-length cDNA mapping and gene expression analysis through microarray. PLoS ONE 2, e1235 (2007).
Zhang, Q., Li, J., Xue, Y., Han, B. & Deng, X. W. Rice 2020: a call for an international coordinated effort in rice functional genomics. Mol. Plant 1, 715–719 (2008).
Wang, J. et al. Evolutionary transients in the rice transcriptome. Genom. Prot. Bioinf. 8, 211–228 (2010).
Marioni, J. C., Mason, C. E., Mane, S. M., Stephens, M. & Gilad, Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 18, 1509–1517 (2008).
Koenig, D. et al. Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc. Natl. Acad. Sci. US 110, E2655-2662 (2013).
Song, X. J., Huang, W., Shi, M., Zhu, M. Z. & Lin, H. X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 39, 623–630 (2007).
Ma, X., Cheng, Z., Qin, R., Qiu, Y. & Heng, Y. OsARG encodes an arginase that plays critical roles in panicle development and grain production in rice. Plant J. 73, 190–200 (2013).
Lin, C. M. et al. Cloning and functional characterization of a constitutively expressed nitrate transporter gene, OsNRT1, from Rice. Plant Physiol. 122, 379–388 (2000).
Hidekazu, S. et al. LIP19, a basic region leucine zipper protein, is a Fos-like molecular switch in the cold signaling of rice plants. Plant Cell Physiol. 1623–1634 (2005).
Gothandam, K. M., Nalini, E., Karthikeyan, S. & Shin, J. S. OsPRP3, a flower specific proline-rich protein of rice, determines extracellular matrix structure of floral organs and its overexpression confers cold-tolerance. Plant Mol. Biol. 72, 125–135 (2010).
Coppe, A., Danieli, G. A. & Bortoluzzi, S. REEF: searching REgionally enriched features in genomes. BMC Bioinf. 7, 453 (2006).
Umemoto, T., Yano, M., Satoh, H., Shomura, A. & Nakamura, Y. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor. Appl. Genet. 104, 1–8 (2002).
Zheng, X. M. et al. Genome-wide analyses reveal the role of noncoding variation in complex traits during rice domestication. Sci. Adv. 5, eaax3619 (2019).
Pavey, S. A., Collin, H., Nosil, P. & Rogers, S. M. The role of gene expression in ecological speciation. Ann. N. Y. Acad. Sci. 1206, 110–129 (2010).
Swanson-Wagner, R. A. et al. Pervasive gene content variation and copy number variation in maize and its undomesticated progenitor. Genome Res. 20, 1689–1699 (2010).
Lai, Z., Kane, N. C., Zou, Y. & Rieseberg, L. H. Natural variation in gene expression between wild and weedy populations of Helianthus annuus. Genetics 179, 1881–1890 (2008).
Hu, B. et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47, 834–838 (2015).
Ma, Y. et al. COLD1 confers chilling tolerance in rice. Cell 160, 1209–1221 (2015).
Liu, C. et al. Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 9, 3302 (2018).
Yan, S. Map-based cloning proves qGC-6, a major QTL for gel consistency of japonica/indica cross, responds by Waxy in rice (Oryza sativa L.). Theor Appl Genet. 5 (2011).
Li, Q. et al. Rice Soluble Starch Synthase I: Allelic Variation, Expression, Function, and Interaction With Waxy. Front Plant Sci. 9 (2018).
Wang, H. et al. Analysis of non-coding transcriptome in rice and maize uncovers roles of conserved lncRNAs associated with agriculture traits. Plant J. 84, 404–416 (2015).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Lappalainen, T. et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501, 506–511 (2013).
Guttman, M. et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 28, 503–510 (2010).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511 (2010).
Cabili, M. N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011).
Storey, J. D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J. R. Stat. Soc. Ser. B Stat. Methodol. 66, 187–205 (2004).
Xu, X. et al. Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat. Biotechnol. 30, 105–111 (2011).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 31670211 and 31970237), the Hundred Talent Program of Shenyang Normal University (SSDBRJH) and Shenyang Youth Science and Technology Project (no. RC190223).
Author information
Authors and Affiliations
Contributions
H.P. and X.Z. wrote the manuscript, H.P. and X.Z. designed the experiment, H.P. and Q.C. did experiment, Y.L., Z.W., L.W. and Q.Y. revised the paper. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pang, H., Chen, Q., Li, Y. et al. Comparative analysis of the transcriptomes of two rice subspecies during domestication. Sci Rep 11, 3660 (2021). https://doi.org/10.1038/s41598-021-83162-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83162-8
- Springer Nature Limited
This article is cited by
-
Responsive Transcriptome Analysis of Senecio vulgaris L. Under Different Drought Stresses
Journal of Plant Growth Regulation (2023)
-
Genome-wide differences of alternative splicing between Oryza sativa ssp. indica and Oryza sativa ssp. japonica
Acta Physiologiae Plantarum (2023)