Abstract
Inflammatory cardiomyopathy diagnosed by endomyocardial biopsy (EMB) is common in non-ischemic heart failure (HF) and might be associated with adverse outcome. We aimed to identify markers predicting myocardial inflammation in HF. We screened 517 patients with symptomatic non-ischemic HF who underwent EMB; 397 patients (median age 54 [IQR 43/64], 28.7% females) were included in this study. 230 patients were diagnosed with myocardial inflammation, defined as ≥ 7.0 CD3+ lymphocytes/mm2 and/or ≥ 35.0 Mac1 macrophages/mm2 and were compared to 167 inflammation negative patients. Patients with myocardial inflammation were more often smokers (52.4% vs. 39.8%, p = 0.013) and had higher C-reactive protein (CRP) levels (5.4 mg/dl vs. 3.7 mg/dl, p = 0.003). In logistic regression models CRP ≥ 8.15 mg/dl (OR 1.985 [95%CI 1.160–3.397]; p = 0.012) and Troponin I (TnI) ≥ 136.5 pg/ml (OR 3.011 [1.215–7.464]; p = 0.017) were independently associated with myocardial inflammation, whereas no association was found for elevated brain natriuretic peptide (OR 1.811 [0.873–3.757]; p = 0.111). In prognostic performance calculation the highest positive predictive value (90%) was detected for the combination of Global longitudinal strain (GLS) ≥ -13.95% and TnI ≥ 136.5 pg/ml (0.90 (0.74–0.96)). Elevated CRP, TnI and GLS in combination with TnI can be useful to detect myocardial inflammation. Smoking seems to predispose for myocardial inflammation.
Similar content being viewed by others
Introduction
Heart failure (HF) is a global health problem which affects approximately over 37 million people world wide1. According to data from the Framingham Heart Study, the lifetime risk of developing HF is estimated to be 20% for the ages between 40 and 80 years2. It is caused by structural, but also functional cardiac abnormalities, which result in loss of myocardial function3. However, in the absence of coronary artery disease, which is the leading cause of HF4,5,6, inflammatory cardiomyopathy is common, particularly in HF with reduced ejection fraction (HFrEF). Thereby, according to the report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies, inflammatory cardiomyopathy is defined as cardiac dysfunction in co-prevalence with inflammatory disease of the myocardium established by immunological, histological and immunohistochemical criteria7. The exact incidence of inflammatory cardiomyopathy underlying HF is not known. However, according to postmortem analysis, inflammatory cardiomyopathy or myocarditis seems to account for approximately 40% of the sudden cardiac deaths in the young8. Regarding endomyocardial biopsy (EMB) studies myocardial inflammation can be diagnosed in 9 to 23% of the patients with non-ischemic cardiomyopathy9,10. According to the above mentioned definitions, inflammatory cardiomyopathy can only be diagnosed on basis of EMB results11,12. Although the results of studies addressing the immunosuppressive therapy of inflammatory cardiomyopathy remain controversial13,14,15, results of the TIMIC trial suggest that immunosuppressive therapy in patients with virus negative inflammatory cardiomyopathy may be an effective and safe option for recovery of cardiac function in addition to optimal medical therapy16. Inflammatory cardiomyopathy can progress rapidly and may require immediate immunosuppressive therapy to prevent adverse outcomes. Thus, we aimed to identify predictors of myocardial inflammation in HF patients in order to improve diagnostic and therapeutic management.
Methods
Study population
Patients presenting with symptoms of HF at the Heart Failure Outpatient clinic, the emergency department or chest pain unit of the University Medical Centre Mainz between 14/10/2012 and 18/12/2018, who underwent EMB were enrolled in this retrospective monocentric analysis. Decision to obtain EMB of the patients was based on guidelines published of the AHA, the ACC and the ESC in 200717. Ischemic HF, valvular HF and systemic disease with known cardiac involvement were ruled out prior to EMB. The analysis of the patients’ medical records involved the following data: personal medical history, clinical presentation, laboratory values, echocardiography and the finding of the EMB, including viral activity. The exclusion criteria are presented in Fig. 1 and comprise missing CD3+ and/or Mac1 cell counts or presence of relevant virus activity. All data were obtained from individuals enrolled between 2013 and 2018 in the retrospective monocentric Mainz Endomyocardial Biopsy in Heart Failure Study (My Biopsy-HF Study, DRKS #22178), which was approved by the Ethics Committee of Rhineland Palatinate to be in accordance with the legal regulations and the declaration of Helsinki. Informed consent was obtained from all included individuals to use EMB tissue samples for further scientific purpose.
Echocardiography
Transthoracic echocardiography was performed in our echocardiography lab by trained and certified specialists of our department using iE33 Philips, a General Electrics E9 or Siemens Acuson s2000 machines. All examinations were performed with the patient in the standard left lateral position while apnea or quiet breathing. Routinely 2–4 cardiac cycles were obtained at frame rates of 50–100 fps and digitally transferred into a picture archiving and communication system (Xcelera) for offline analysis. Left ventricular (LV) ejection fraction (LVEF) was assessed by biplane Simpson’s method in the apical four and two chamber view. For the assessment of cardiac structure, LV internal diameters were measured. LVEF was categorized into classes LVEF ≥ 50% (HF with preserved LVEF = HFpEF), LVEF 40–49% (HF with mid-range reduced LVEF = HFmrEF) and LVEF < 40% (HFrEF)3. Global longitudinal strain (GLS) was measured offline utilizing QLab 13.0 (Philips Healthcare, Hamburg, Germany) automatic speckle-tracking software. The tracking result was visually controlled and manually readjusted if necessary. In case of persistent poor tracking quality, datasets were excluded from analysis. GLS was automatically calculated by average of GLS in apical four, three and two chamber view.
Endomyocardial biopsy and myocardial inflammation
EMB was performed using a biopsy forceps (Medwork bioptom, 180 cm, 1.8 mm, Cat.-No. BIO-C4-18-180) by taking up to eight biopsies either from the right ventricular septum or from the lateral wall of the LV. In case of LV biopsy the procedure was carried out via the right or left femoral artery or via trans-radial cardiac catheterization as reported before18. In case of right heart biopsy, the femoral vein was used. Immediately after sampling the biopsy, the specimens were stabilized in a solution to preserve ribonucleic acid (RNA) integrity (RNAlater, Ambion Inc., Austin, Texas) and were sent for further examination to a specialized laboratory approved by Food and Drug Administration [Institut Kardiale Diagnostik und Therapie (IKDT), Berlin, Germany]. For immunohistological evaluation, heart muscle tissue probes fixated in RNAlater were embedded in Tissue Tec (SLEE, Mainz, Germany) and immediately snap‐frozen in methyl butane which had been cooled in liquid nitrogen, and then stored at – 80 °C until processing. Embedded specimens were cut serially into cryosections of 5 mm thickness and placed on 10% poly‐l‐lysine‐pre‐coated slides. As part of a routinely performed work up in case of unclear heart failure histological and immunohistochemical examination as well as virus detection was conducted. Using specific antibodies inflammatory processes were detected by identifying immune cell infiltration and expression of cell adhesion molecules. The specimens of the patients in this study were tested for CD3-positive lymphocytes (Dako; dilution 1:25), CD11a+/LFA-1+ lymphocytes (ImmunoTools; dilution 1:250), macrophages Mac-1 macrophages (ImmunoTools; dilution 1:500), Perforin positive (cytotoxic T Cells) (clone dG9, BD Bioscience; dilution 1:150) and the expression of adhesion molecules HLA-1 (Dako; dilution 1:2000) and ICAM-1 (ImmunoTools; dilution 1:800). Myocardial inflammation was defined as ≥ 7.0 CD3+ lymphocytes/mm2 and/or ≥ 35.0 Mac1 macrophages/mm2 in accordance with previous published studies19. Diagnosis of relevant myocardial virus activity was performed by polymerase chain reaction (PCR) detecting the genomic sequences of viruses that most commonly cause myocarditis. This included: enterovirus, adenovirus, human cytomegalovirus, herpes simplex virus, Epstein–Barr virus, human herpesvirus 6, parvovirus B19 and influenza A and B viruses. Virus load was calculated via quantitative PCR methods. In case of relevant myocardial virus activity patients were excluded from analysis.
Statistical analysis
Descriptive statistics for relevant baseline comparisons of symptomatic non-valvular, non-ischemic heart failure patients, who underwent EMB, stratified according inflammation (≥ 7.0 CD3+ lymphocytes/mm2 and/or ≥ 35.0 Mac1 macrophages/mm2) are provided as median and interquartile range (IQR) or absolute numbers and corresponding percentages. We tested the continuous variables of the groups (inflammation vs. no inflammation) using the Mann–Whitney-U test and categorical variables with the Fisher’s exact or the chi2 test, as appropriate.
Receiver operating characteristics (ROC) curves were calculated for TnI, BNP, CRP and GLS with regard to myocardial inflammation and the area under the curve (AUC) is presented with the corresponding 95% CI. Additionally, patient cohort-optimised cut-off values of these mentioned laboratory markers with regard to myocardial inflammation were calculated based on ROC analyses using Youden index quantification. The software SPSS (version 23.0; SPSS Inc., Chicago, Illinois) was used for computerized analysis. p values of < 0.05 (two-sided) were considered to be statistically significant.
Univariate and multivariate logistic regression models were analyzed to investigate predictors of myocardial inflammation in heart failure patients, thereby using patient-optimised cut-off values derived from the ROC analyses. Results are presented as odds ratio (OR) and 95%CI. The multivariate regression models were adjusted for (1) age and sex, obesity and (2) cardiovascular risk factors (CVRF) including history of smoking, arterial hypertension, diabetes mellitus and hyperlipoproteinaemia.
Results
Overall, 517 patients with symptomatic non-valvular, non-ischemic HF who underwent EMB between 2012 and 2018 were screened. Results from EMB with CD3+ and/or Mac1 cell count were available in 447 cases. 50 cases were excluded due to relevant virus activity (Fig. 1). Thus, 397 patients remained in this study and were analysed. Patient characteristics are displayed in Table 1. In brief, median age was 54 years [IQR 43/64] and 71.3% of the patients were males. Median left ventricular ejection fraction (LVEF) was calculated with 30.0% [20.0/40.0] and median left ventricular enddiastolic diameter (LVEDD) with 5.9 mm [5.2/6.6] accompanied by an elevated median BNP value of 447 pg/ml [137.0/1188.5]. GLS was reduced with − 8.8% [− 12.25/− 6.0]. Regarding symptoms, 71.7% patients were admitted with dyspnea (43.7% NYHA functional class III or IV), 32.4% complained about angina pectoris at time of admission and in 19.1% edema were detectable. The duration of symptoms differed: In 36.4% of the patients symptoms started not longer than 2 weeks, 30.3% had symptoms between 2 weeks and 3 months and 32.6% suffered from symptoms more than 3 months duration before admission. In immunohistochemistry, median counts of CD3+ and Mac1 were 6.470/mm2 [IQR 2.0/16.085] and 33.6/mm2 [16.9/58.1), respectively, defining a cut-off of ≥ 7.0 CD3+ lymphocytes/mm2 and/or 35.0 Mac1 macrophages/mm2.
Comparison of symptomatic non-valvular, non-ischemic heart failure patients, who underwent EMB, stratified for presence of myocardial inflammation
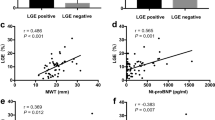
In 230 patients (57.9%), myocardial inflammation was diagnosed. Patients with and without inflammatory cardiomyopathy were of comparable age and revealed no differences regarding presented symptoms at admission. History of smoking (52.4% vs. 39.8%, p = 0.013) was more prevalent in HF patients with myocardial inflammation. While the groups did not differ regarding mean values of LVEF, GLS (Table 2, Fig. 2) and mean values of TnI and BNP (Table 2, Fig. 3), mean values of CRP (5.4 [2.1/19] vs. 3.7 [1.5/10], p = 0.003) were higher and median hemoglobin levels (14.1 [12.6/15.2] vs. 14.7 (13.5/15.7] g/dl, p = 0.001) were lower in patients with myocardial inflammation (Table 2, Fig. 3).
Predictors of myocardial inflammation
ROC curves demonstrated only a moderate prognostic performance of CRP for prediction of myocardial inflammation with acceptable specificity and positive predictive values (Table 3, supplementary Figure 1). The computed best cut-off (according Youden-Index calculation) for CRP was 8.15 mg/l. In contrast, TnI, BNP and GLS were accompanied by a low prognostic performance to predict myocardial inflammation shown in the ROC curves. The computed best cut-off (according Youden-Index calculation) for TnI was 136.5 pg/ml, for BNP 1030 pg/ml and for GLS -13.95% (Table 3). Nevertheless, when calculating best cut-offs (according Youden-Index calculation), the cut-offs were able to differentiate between myocardial inflammation vs. no cardiac inflammation.
In multivariate logistic regression analyses after adjustment for CVRF, elevated TnI values (≥ 136.5 pg/ml) (OR 3.011 [95%CI 1.215–7.464]; p = 0.017) as well as elevated CRP values (≥ 8.15 mg/l) (OR 1.985 [95%CI 1.160–3.397], p = 0.012) were independently associated with myocardial inflammation. No association was detected for elevated BNP (≥ 1030.5 pg/ml) values (OR 1.811 [95%CI 0.873–3.757]; p = 0.111). Regarding echocardiographic parameters, LVEF categories and reduced GLS (≥ − 13.95%) were not associated with myocardial inflammation (Table 4, Fig. 4). However when combining laboratory parameters with echocardiographic parameters, a strong association with myocardial inflammation was found for the combined variable consisting of reduced GLS and elevated TnI (OR 9.633 [95%CI 2.027–45.769]; p = 0.004). Additionally, but to a smaller extent, the combination of TnI and CRP was also independently associated with myocardial inflammation (OR 5.761 [95% 1.240–26.771], p = 0.025) (Supplementary table 1). Furthermore, supplementary table 2 illustrates the prognostic performance of combined parameters to predict myocardial inflammation. While all parameter-combinations had only moderate sensitivity (21–39%), the specificity was better: 0.71 (0.62–0.79) for GLS and elevated CRP, 0.95 (0.91–0.98) for positive TnI and elevated CRP and 0.96 (0.90–0.99) for the combination of GLS and TnI. Remarkably, highest positive predictive value was also detected for the combination of GLS and TnI 0.90 (0.74–0.96). The negative predictive values were only moderate in all of these combinations (41–46%).
Predictors for inflammation (Forest plot). OR (Odds Ratio) and 95% CI (Confidence Intervall) displayed in a Forest plot after adjustment for age/sex/CVRF. Absolute values are shown in Table 4.
Concerning the interrelation of cardiovascular risk factors with myocardial inflammation, only smoking was associated with myocardial inflammation in crude logistic regression analysis (OR 1.6687 [95%CI 1.113–2.500]; p = 0.013), but this association showed only a trend towards significance in the fully adjusted model (OR 1.659 [95%CI 1.000–2.753]; p = 0.050) (Table 4, Fig. 4).
Discussion
The key findings of the present study are that (I) elevated CRP and TnI levels are associated with inflammatory cardiomyopathy in patients with non-ischemic, non valvular heart failure; (II) TnI levels ≥ 136.5 pg/ml and CRP levels ≥ 8.15 mg/l are potential predictors of myocardial inflammation; (III) common heart failure biomarkers such as BNP and reduced LVEF were not predictive for myocardial inflammation and; (IV) GLS might add a benefit to predict myocardial inflammation when combined with TnI.
A growing body of evidence indicates that myocardial inflammation may lead to severe cardiac damage, deteriorate into dilated cardiomyopathy and be accompanied by poor prognosis. This implies that early diagnosis using predictive markers may avert poor outcome20, since immunosuppressive therapy can significantly improve the prognosis of HF patients with presence of myocardial inflammation16. Thus, readily available markers like the ones identified in our study might help to select patients for EMB and for shorter control follow-up examinations. Although cardiac MRI diagnostics has become increasingly important in the diagnosis of myocardial inflammation21,22, sensitivity and specificity of MRI findings are not unequivocal. Since EMB can be performed easily as part of coronary catheter examination18 and should be carried out as early as possible if there is clinical or laboratory suspicion of inflammatory myocardial disease, our study results might help to accelerate the diagnostic work-up of HF.
The association of elevated CRP and TnI with myocardial inflammation was independent of age and sex, supporting earlier findings by Lauer et al., who reported that patients with elevated troponin T values were more likely to have EMB-proven myocarditis23. Because cardiac troponins are detectable in the blood for approximately one week24 and have a half-life of 2h25, elevated troponins are suitable to indicate permanent myocardial cell injury26,27, which can be explained by inflammatory infiltration with consecutive cell damage documented in the EMB. Contrary to our results, Sramko et al. demonstrated that troponin was not able to distinguish between patients with idiopathic dilated cardiomyopathy or inflammatory dilated cardiomyopathy arguing that troponin indicates myocardial injury without indicating weather its due to myocardial inflammation or simply caused by myocardial wallstress related to heart failure28. However, the study sample size was small, and EMB was only performed on the right ventricle making a sampling error more likely.
Similar to positive troponin values, positive CRP values were associated with detection of inflammation in EMB. These results go in line with observation by Liu et al., who demonstrated that highly sensitive CRP levels were higher in patients with EMB-confirmed myocarditis than in patients without myocarditis29. However, it has to be pointed out that the sensitivity for highly sensitive CRP in detecting myocarditis was only 50.1% whereas the specificity was 80.7%. Since CRP is also increased in acute myocardial infarction30 or in rheumatic valve disease31. CRP should not be interpreted alone, but in conjunction with cardiac troponin. Moreover, as the present study indicates, cut-off values for TnI and CRP seem to be higher with regard to myocardial inflammation compared to cut-off values used for diagnosis of acute coronary syndrom. Further studies are needed to prove those cut-off values in patients with myocardial inflammation.
Interestingly GLS was not able to distinguish between EMB proven myocardial inflammation and no inflammation. Escher et al. demonstrated before, that patients with acute myocarditis had reduced GLS values. After a follow-up period of 6.2 months patients with persistent inflammation had worse GLS values than patients without inflammation32. However in contrast to our study GLS values were compared when LVEF had already been improved. Eventhough it has been shown that GLS is a superior predictor of adverse cardiac events compared with LVEF33,34, studies demonstrate that in general and irrespectively of outcome prediction, GLS correlates well with LVEF in heart failure patients35. Therefore the results of our study suggest that GLS alone is not able to identify patients with myocardial inflammation in the presence of reduced LVEF. Nevertheless, the combined evaluation of GLS and Troponin values were the second strongest predictor of myocardial inflammation in multivariate regression analysis. Additionally, the best prognostic performance was found for the combination of GLS and Troponin. In particular, the high positive prognostic value of 90% to predict myocardial inflammation is promising. Thus, the combination of GLS and Troponin may help to identify patients with myocardial inflammation in the future and could add a benefit in the diagnosis and follow up treatment of those patients.
Interestingly, smoking was associated with myocardial inflammation in our study. In a very small study sample, it was shown, that in patients hospitalized for acute myocarditis smoking was the most prevalent cardiovascular risk factor36. Recently, Detorakis et al. reported a significant correlation of smoking habits with late gadolinium enhancement extent in cardiac MRI in patients with clinically suspected myocarditis37. Prolonged exposure to tobacco smoke may cause cardiovascular cell damage, suggesting that increased myocardial cell necrosis and cell death would make myocardial inflammatory infiltration more likely38.
Another finding of the present study was that patients with myocardial inflammation had lower hemoglobin levels. It is well known that anemia of inflammation or anemia of chronic disease is primarily a disorder of iron distribution39 and even low grade inflammation is associated with lower hemoglobin levels40. As experimental models have demonstrated that low cardiac iron levels promote heart failure41,42 the link between the observed lower hemoglobin levels could be an effect of possibly local iron distribution disorders due to myocardial inflammation. Since hemoglobin levels in the present study were generally not significantly decreased, more data are needed to support this observation.
The primary stimulus for natriuretic peptide synthesis by myocardial cells is related to increased LV wall stress induced for example by acute myocardial infarction43, arterial hypertension44, muscle hypertrophy45 or increased pulmonary arterial pressure46. We expected that inflammation would also cause elevated BNP levels as reported earlier47, particularly in patients with myocarditis and dilated cardiomyopathy48,49,50. Increase of BNP has also been detected in systemic inflammation regardless of systolic function51. In the present study, no differences in BNP levels and LVEF were found in patients with and without myocardial inflammation, reflecting a rather compensated disease state of our study population with low rates of hydropic decompensation. Myocarditis and inflammatory cardiomyopathy are a dynamic processes; after inflammation has subsided, a reduced LVEF is due to secondary post-inflammatory fibrosis and myocardial cell death and scars52. As conditions with and without inflammation lead to changes of LVEF and BNP, the present study demonstrates that increase of BNP and the decrease of LVEF do not specifically reflect the presence of inflammation, but rather reflect a general loss of myocardial tissue with consecutive changes in hemodynamic conditions regardless weather inflammation can be detected or not.
Limitation
There are certain limitations of our study which need to be mentioned: Firstly, our study was conducted as a monocentric retrospective data analysis. Therefore, it has to be emphasized that the results of this study can only serve in terms of a hypothesis generating research and the results of the present study should be verified by prospective trials. Secondly, there is no generally applicable definition of myocardial inflammation. Thus, it might be difficult to adopt the results to other endomyocardial biopsy studies, but generating more information about inflammation process was one of the main aims of our study. Finally, a potential limitation regarding EMB interpretation might be the sampling error. Regarding this point, Hauck et al. demonstrated that sampling error was prevalent 45% in Ieft and up to 37% in right EMB53. The authors conclude that only positive results of EMB can be considered diagnostic. However, the cited study included a very small sample of only 36 patients.
Conclusion
Elevations of readily available markers like cardiac TnI and CRP as well as the combination of GLS and TnI may predict inflammatory cardiomyopathy and might be useful to select patients for EMB and for shorter control follow-up examinations. In the diagnostic approach to detect suspected myocardial inflammation, higher cut-off values concerning cardiac and inflammatory biomarkers may have to be applied. Smoking is associated with inflammatory cardiomyopathy in non-ischemic, non valvular HF patients and may indicate individuals at risk to develop inflammatory cardiomyopathy.
Ethics approval
All data were obtained from individuals enrolled between 2013 and 2018 in the retrospective monocentric Mainz Endomyocardial Biopsy in Heart Failure Study (My Biopsy-HF Study, DRKS #22178), which was approved by the Ethics Committee of Rhineland Palatinate to be in accordance with the legal regulations and the declaration of Helsinki.
Abbreviations
- ACC:
-
American College of Cardiology
- AHA:
-
American Heart Associaton
- AUC:
-
Area under the curve
- BMI:
-
Body Mass Index
- BNP:
-
Brain natriuretic peptide
- CI:
-
Confidence interval
- CRP:
-
C-reactive Proteine
- CVRF:
-
Cardiovascular risk factors
- EMB:
-
Endomyocardial biopsy
- ESC:
-
European Society of Cardiology
- GLS:
-
Global longitudinal strain
- Hb:
-
Haemoglobin
- HF:
-
Heart failure
- HFmrEF:
-
Heart failure with mid-range reduced ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- IQR:
-
Interquartile range
- LVEDD:
-
Left ventricular enddiastolic diameter
- LVEDP:
-
Left ventricular enddiastolic pressure
- LVEF:
-
Left ventricular ejection fraction
- NYHA:
-
New York Heart Association
- OR:
-
Odds ratio
- RNA:
-
Ribonucleic acid
- ROC:
-
Receiver operating characteristics
- TnI:
-
Troponin I
- VT:
-
Ventricular tachycardia
References
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 (2012).
Lloyd-Jones, D. M. et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 106, 3068–3072 (2002).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 37, 2129–2200 (2016).
Gheorghiade, M. & Bonow, R. O. Chronic heart failure in the United States. Circulation 97, 282–289 (1998).
Cowie, M. R. et al. Incidence and aetiology of heart failure; a population-based study. Eur. Heart J. 20, 421–428 (1999).
Uretsky, B. F. et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation 102, 611–616 (2000).
Richardson, P. et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 93, 841–842 (1996).
Basso, C., Calabrese, F., Corrado, D. & Thiene, G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc. Res. 50, 290–300 (2001).
Felker, G. M. et al. Idiopathic cardiomyopathy is less idiopathic than you think: An endomyocardial biopsy study of 1278 patients. J. Card. Fail. 5, 63 (1999).
Sotiriou, E. et al. Therapeutic implications of a combined diagnostic workup including endomyocardial biopsy in an all-comer population of patients with heart failure: a retrospective analysis. ESC Heart Fail 5, 630–641 (2018).
Holzmann, M. et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach. Circulation 118, 1722–1728 (2008).
Caforio, A. L. P. et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 28, 1326–1333 (2007).
Cooper, L. T. et al. Usefulness of immunosuppression for giant cell myocarditis. Am. J. Cardiol. 102, 1535–1539 (2008).
Mason, J. W. et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 333, 269–275 (1995).
Wojnicz, R. et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation 104, 39–45 (2001).
Frustaci, A., Russo, M. A. & Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur. Heart J. 30, 1995–2002 (2009).
Cooper, L. T. et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a Scientific Statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 28, 3076–3093 (2007).
Schulz, E. et al. Feasibility and safety of left ventricular endomyocardial biopsy via transradial access: technique and initial experience. Catheter. Cardiovasc. Interv. 86, 761–765 (2015).
Escher, F. et al. Presence of perforin in endomyocardial biopsies of patients with inflammatory cardiomyopathy predicts poor outcome. Eur. J. Heart Fail. 16, 1066–1072 (2014).
Feldman, A. M. & McNamara, D. Myocarditis. N. Engl. J. Med. 343, 1388–1398 (2000).
Lurz, P. et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J. Am. Coll. Cardiol. 67, 1800–1811 (2016).
Cheitlin, M. D. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis. Yearb. Cardiol. 2006, 376–378 (2006).
Lauer, B. et al. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll. Cardiol. 30, 1354–1359 (1997).
Cummins, B. & Cummins, P. Cardiac specific troponin-I release in canine experimental myocardial infarction: development of a sensitive enzyme-linked immunoassay. J. Mol. Cell. Cardiol. 19, 999–1010 (1987).
Katus, H. A., Remppis, A., Scheffold, T., Diederich, K. W. & Kuebler, W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am. J. Cardiol. 67, 1360–1367 (1991).
Park, K. C., Gaze, D. C., Collinson, P. O. & Marber, M. S. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc. Res. 113, 1708–1718 (2017).
Hoff, J., Wehner, W. & Nambi, V. Troponin in cardiovascular disease prevention: updates and future direction. Curr. Atheroscler. Rep. 18, 12 (2016).
Šramko, M. et al. Utility of combination of cardiac magnetic resonance imaging and high-sensitivity cardiac troponin T assay in diagnosis of inflammatory cardiomyopathy. Am. J. Cardiol. 111, 258–264 (2012).
Liu, Y. et al. Application value of hypersensitive C-reactive protein, lactic acid and myoglobin in the combined detection of myocarditis. Exp. Ther. Med. 17, 4471–4476 (2019).
Berton, G. et al. C-reactive protein in acute myocardial infarction: association with heart failure. Am. Heart. J. 145, 1094–1101 (2003).
Gölbasi, Z. et al. Increased levels of high sensitive C-reactive protein in patients with chronic rheumatic valve disease: evidence of ongoing inflammation. Eur. J. Heart Fail. 4, 593–595 (2002).
Escher, F. et al. New echocardiographic findings correlate with intramyocardial inflammation in endomyocardial biopsies of patients with acute myocarditis and inflammatory cardiomyopathy. Mediat. Inflamm. 2013, 875420 (2013).
Iacoviello, M. et al. Independent role of left ventricular global longitudinal strain in predicting prognosis of chronic heart failure patients. Echocardiography 30, 803–811 (2013).
Stanton, T., Leano, R. & Marwick, T. H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ. Cardiovasc. Imaging 2, 356–364 (2009).
Delgado, V. et al. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J. Am. Soc. Echocardiogr. 21, 1244–1250 (2008).
Antit, S. et al. Diagnostic characteristics of acute myocarditis. Tunis. Med. 97, 789–794 (2019).
Detorakis, E., Illing, R., Lasithiotaki, I., Foukarakis, E. & Raissaki, M. Role of smoking in the evolution of cardiovascular magnetic resonance and laboratory findings of acute myocarditis. Heart Views Off. J. Gulf Heart Assoc. 21, 22–30 (2020).
Leone, A. Jr., Biadi, O. & Balbarini, A. Smoking and cardiovascular system: cellular features of the damage. Curr. Pharm. Des. 14, 1771–1777 (2008).
Ganz, T. Anemia of inflammation. N. Engl. J. Med. 381, 1148–1157 (2019).
Kotzé, S. R. et al. Low-grade inflammation is associated with lower haemoglobin levels in healthy individuals: results from the Danish blood donor study. Vox Sang 111, 144–150 (2016).
Lakhal-Littleton, S. et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife 5, e19804 (2016).
Xu, W. et al. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 13, 533–545 (2015).
Morita, E. et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 88, 82–91 (1993).
Buckley, M. G., Markandu, N. D., Miller, M. A., Sagnella, G. A. & MacGregor, G. A. Plasma concentrations and comparisons of brain and atrial natriuretic peptide in normal subjects and in patients with essential hypertension. J. Hum. Hypertens. 7, 245–250 (1993).
Lukowicz, T. V. et al. BNP as a marker of diastolic dysfunction in the general population: importance of left ventricular hypertrophy. Eur. J. Heart Fail. 7, 525–531 (2005).
Nagaya, N. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J. Am. Coll. Cardiol. 31, 156A (1998).
George, J., Mackle, G., Manoharan, A., Khan, F. & Struthers, A. D. High BNP levels in rheumatoid arthritis are related to inflammation but not to left ventricular abnormalities: a prospective case-control study. Int. J. Cardiol. 172, e116–e118 (2014).
Ogawa, T., Veinot, J. P., de Bold, M. L. K., Georgalis, T. & Bold, A. J. Angiotensin II receptor antagonism reverts the selective cardiac BNP upregulation and secretion observed in myocarditis. Am. J. Physiol. Heart Circ. Physiol. 294, H2596–H2603 (2008).
Grabowski, M. et al. Diagnostic value of BNP in suspected perimyocarditis–a preliminary report. Kardiol Pol 61, 451–458 (2004).
Miller, W. L., Hartman, K. A., Burritt, M. F., Burnett, J. C. & Jaffe, A. S. Troponin, B-type natriuretic peptides and outcomes in severe heart failure: differences between ischemic and dilated cardiomyopathies. Clin. Cardiol. 30, 245–250 (2007).
Shor, R. et al. BNP in septic patients without systolic myocardial dysfunction. Eur. J. Intern. Med. 17, 536–540 (2006).
Imanaka-Yoshida, K. Inflammation in myocardial disease: from myocarditis to dilated cardiomyopathy. Pathol. Int. https://doi.org/10.1111/pin.12868 (2019).
Hauck, A. J., Kearney, D. L. & Edwards, W. D. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin. Proc. 64, 1235–1245 (1989).
Acknowledgements
This manuscript contains results that are part of the medical doctoral theses work of Friederike Fueting and Carolin Klank.
Funding
Open Access funding enabled and organized by Projekt DEAL. P.W. was supported by grants from the German Federal Ministry for Education and Research (BMBF 01EO1503). T.M. and P.W. are Principal Investigators of the German Center for Cardiovascular Research (DZHK).
Author information
Authors and Affiliations
Contributions
S.S.-T.: designed the study, analyzed and interpreted data, wrote the manuscript. P.W.: designed the study, analyzed and interpreted data, edited the manuscript. K.K.: designed the study, analyzed and interpreted data, edited the manuscript. S.G.: designed the study. E.T.: analyzed and interpreted data. V.H.S.: critically commented and edited the manuscript. F.F.: analyzed and interpreted data. C.K.: analyzed and interpreted data. F.E.: critically commented and edited the manuscript. H.P.S.: critically commented and edited the manuscript. T.M.: critically commented and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwuchow-Thonke, S., Göbel, S., Emrich, T. et al. Increased C reactive protein, cardiac troponin I and GLS are associated with myocardial inflammation in patients with non-ischemic heart failure. Sci Rep 11, 3008 (2021). https://doi.org/10.1038/s41598-021-82592-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82592-8
- Springer Nature Limited
This article is cited by
-
Applying global longitudinal strain in assessing cardiac dysfunction after radiotherapy among breast cancer patients: a systemic review and meta-analysis
Clinical and Translational Imaging (2022)