Abstract
The present study evaluated the effects of exogenous hemin on cadmium toxicity in terms of metal accretion and stress resilience in Vigna radiata L. (Wilczek). One-week-old seedlings were treated with CdCl2 (50 μM) alone and in combination with hemin (0.5 mM) in half-strength Hoagland medium for 96 h. The optimum concentrations of Cd and hemin were determined on the basis of haem oxygenase-1 activity. The results demonstrated that under Cd stress, plants accumulated a considerable amount of metal in their tissues, and the accumulation was higher in roots than in leaves, which significantly reduced the plant biomass and chlorophyll content by increasing the oxidative stress (MDA and H2O2 content). However, hemin supplementation under Cd,-stress improved plant growth by enhancing the harvestable biomass and photosynthetic pigments, increasing antioxidant activities (SOD, APX, POD, HO-1 and proline), lowering oxidative damage and increasing Cd tolerance in plants. Furthermore, the application of hemin enhances the removal efficiency of Cd in V. radiata by increasing the uptake of Cd via roots and its translocation from roots to foliar tissues. Thus, the study suggests that hemin has the potential to improve the stress tolerance and phytoremediation ability of heavy metal-tolerant plants so that they can be used instead of hyperaccumulators for remediation of Cd-contaminated environments.
Similar content being viewed by others
Introduction
Heavy metal contamination is one of the key environmental issues, particularly for agricultural areas, due to the continuous increase in human activities, such as intense usage of phosphate fertilizers, swift urbanization, industrialization and increased utilization of sewage sludge, that accelerate the discharge of noxious elements into the surroundings1,2. Among heavy metals, cadmium (Cd) is the most dangerous environmental pollutant that severely restricts the growth and development of plants3. Cadmium is readily taken up by plant roots and transferred to foliar tissues, where it starts to accumulate in the esculent parts. This facilitates the entry of Cd into the food chain when consumed by living organisms and causes severe threats to their health3,4,5. Moreover accrued Cd in crop tissues results in diverse structural, biochemical and physiological transformations. Cd2+ disturb the nutrients and water uptake in plants, hinder opening and closing of stomata, inhibits the activity of Calvin cycle enzymes (particularly RUBISCO), affects photosynthesis [via hampering photoactivation of photosystem II (PS II)], respiration, carbohydrate metabolism, alters antioxidants activity and declines the yield and productivity of crop plants6. Therefore, an efficient approach for Cd remediation from contaminated environments is essential to prevent severe damage in plants as well as to inhibit Cd accumulation in edible plant tissues. Conventional physical and chemical remediation techniques are costly and lead to secondary contamination of the surrounding areas. Conversely, phytoremediation is a promising green approach that utilizes the ability of hyperaccumulator crops,- to take up pollutants from contaminated environments7. Phytoremediation has been gaining importance as an efficient, economically feasible, eco-friendly and highly stable technology7. However, hyperaccumulators are not particularly abundant, and their slow growth rate as well as reduced biomass limits their phytoremediation efficiency3. Therefore, an alternative approach is needed that strengthens the phytoremediation efficiency of metal-tolerant plants so that they can be used as an efficient biological tool to remediate heavy metal pollution. Several chemical reagents and bioregulators, such as ethylene, melatonin, nitric oxide and plant growth regulators (PGRs), are known to enhance Cd stress tolerance by modulating various physiochemical processes that improve crop biomass and further stimulate the uptake and translocation of heavy metals2,8,9.
Numerous reports have demonstrated that the application of hemin as a potent biostimulator mitigates metal-induced oxidative stress by enhancing the stress tolerance of plants1,10. For example, the application of exogenous hemin ameliorates Al and Cd toxicity in Medicago sativa L.11,12, induces ammonium tolerance in Oryza sativa L.10 and enhances salinity stress adaptations in Triticum aestivum L.13. Hemin, chemically known as ferroprotoporphyrin IX, is a derivative of haem that shares similar chemical properties with natural haem. Since hemin acts as haem, it greatly enhances the catalysis of the haem oxygenase-1 enzyme by overexpressing its mRNA and increasing protein abundance; hence, hemin is regarded as a proficient activator of haem oxygenase-1 that exerts protective effects against various abiotic stresses in an enzyme-dependent manner5. Therefore, it is possible to implicate hemin as an effective and efficient biostimulator to improve the phytoremediation potential of heavy metal-tolerant plants.
Mung bean (Vigna radiata L. Wilczek) is an eco-friendly traditional legume that is consumed by the majority of the human population, particularly in Asia and Africa, after cereals14. It is a good source of proteins, vitamins and minerals and serves to provide a balance in diets mostly composed of lysine-deficient cereal grains15. Moreover, V. radiata has high tolerance against metals and can gather a substantial quantity of chromium, cadmium, copper, zinc and nickel in its tissues when subjected to polluted environments16,17. Hence, it could be advantageous to study the enhancement of this crop’s potential for cadmium remediation. To the best of our knowledge, the influence of hemin on the cadmium resilience and removal potential of V. radiata has not yet been evaluated. Therefore, the aims of the present work were (1) to investigate the effect of exogenous hemin on the biomass production and chlorophyll content of V. radiata; (2) to analyse the mechanism by which hemin affects Cd tolerance of V. radiata; and (3) to verify the influence of exogenous hemin in improving the Cd phytoremediation efficiency of V. radiata.
Results
Impact of Cd and hemin on crop morphology
The length and biomass of V. radiata seedlings were studied to evaluate the adverse impact of cadmium on crops and the role of hemin against Cd toxicity. In the current work, cadmium negatively affected plant morphology. Compared with the control, the plant biomass (fresh and dry weight) in the Cd-treated plants decreased by 33.33% and 42.86%, respectively, whereas the dose of hemin, under Cd stress conditions (T3), recovered the biomass by 1.17- (fresh weight) and 1.13 (dry weight)-fold more than that under the Cd treatment (T2) (Table 1). Similar trends were observed for plant height and the tolerance index. A 22.86% increase in root length and 24.14% increase in shoot length was noted when seedlings of V. radiata were exposed to the combined treatment of hemin + Cd compared with the Cd treatment alone (Fig. 1). The tolerance index decreased by 33.3% under Cd stress, which improved by 17.2% under an exogenous supply of hemin. However, the improvements in biomass and height of the plants in the combined treatment were lower than those of untreated seedlings (Table 1).
Chlorophyll contents
The level of photosynthetic pigments (chlorophyll a, chlorophyll b and total chlorophyll) decreased markedly under cadmium stress. In comparison to the control, decreases of 18.8% (chlorophyll a), 32.3% (chlorophyll b) and 25.7% (total chlorophyll) were recorded when crop seedlings were subjected to cadmium stress. The exogenous application of hemin increased the chlorophyll content under both control and stress conditions. Under control conditions, the hemin treatment (T1) increased the photosynthetic pigments by 1.03 (Chl a), 1.29 (Chl b) and 1.16 (total Chl) times that of the control. Moreover, the cotreatment of 50 µM Cd and 0.5 mM hemin elevated the chlorophyll contents by 1.4 (Chl a), 1.68 (Chl b) and 1.52 fold in comparison with the respective Cd-only treatments (Table 1).
ROS generation and oxidative damage
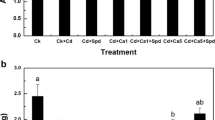
Cadmium addition to the liquid medium, increased H2O2 production and oxidative damage in plants. The malondialdehyde content (indicator of membrane damage) was found to increase under Cd stress, with levels 3.05 (leaves) and 1.22 (roots) times than those of untreated seedlings (Fig. 2A). Moreover, there was a 41.6% (leaves) and 130.9% (roots) rise in the H2O2 content of Vigna radiata seedlings treated with 50 µM CdCl2 (Fig. 2B). The increase in H2O2 was responsible for the rise in lipid peroxidation (LPX). However, the application of hemin reduced both the MDA (25.9% in leaves and 10. 7% in roots) (Fig. 2A) and H2O2 (by 27.5% in leaves and 50.4% in roots) (Fig. 2B) contents under CdCl2 compared to those cadmium-treated plants without hemin.
Effect of CdCl2 (50 μM) alone or in combination with hemin (0.5 mM) on MDA (A) and H2O2 (B) content in seedlings of Vigna radiata. CK, T1, T2 and T3 indicate control, 0.5 mM hemin, 50 μM CdCl2 and 50 μM CdCl2 + 0.5 mM hemin respectively. Values are mean ± SE (n = 3) and are statistically significant according to DMRT test (p < 0.05). Data points marked with the different letters show significant differences (p < 0.05) within treatments.
Proline content
To overcome the harmful effects of the cadmium treatment, plants accumulated a considerable amount of proline as a compatible osmolyte in their tissues. In the present study, under Cd stress, proline accumulation was approximately twice and 1.07 times in leaves and roots, respectively, that of control plants. However, the accretion of proline was further found to be increased by the application of exogenous hemin. In contrast to individual cadmium treatments, 0.5 mM hemin supplementation improved the proline content by 36% (leaves) and 40% (roots) in the Cd-treated seedlings of V. radiata (Fig. 3E).
(A) Peroxidase (POD) (B) Catalase (CAT) (C) Ascorbate peroxidase (APX) (D) Superoxide dismutase (SOD) (E) Proline conent and F Haem oxygenase-1 activity in seedlings of Vigna radiata. Seedlings were treated with 50 μM CdCl2 independently and in combination with 0.5 mM hemin for 4 days. Values are mean ± SE of three replicates (n = 3) and are statistically significant according to DMRT test (p < 0.05). Data points marked with the same letters show insignificant differences (p < 0.05) within treatments.
Activity of antioxidant enzymes
SOD catalysis increased by 20% and 28.6% in leaves and roots, respectively, after CdCl2 treatment in comparison to the control levels. Supplementation of 0.5 mM hemin with CdCl2 significantly improved SOD activity, which was 1.17 (leaves) and 1.22 (roots) times higher than that under Cd treatment alone (Fig. 3D).
Conversely, catalase activity in seedlings increased progressively upon Cd treatment, showing levels 2.71 and 3.49 times higher than those of the control. However, a sudden drop of 37.9% and 14.3% was recorded with the addition of hemin to cadmium-affected crops in comparison to the levels of plants under stress conditions only (Fig. 3B).
Peroxidase (POD) activity increased gradually by 24% (leaves) and 30% (roots) in seedlings of V. radiata treated with CdCl2 stress. However, the addition of 0.5 mM hemin resulted in a subsequent enhancement in enzyme catalysis compared to that in plants under CdCl2 stress alone (Fig. 3A). Similar trends of results were obtained for ascorbate peroxidase (APX). An increase in APX catalysis was recorded in both parts, with levels 1.45-(leaves) and 1.2- (roots) fold higher than those of the control upon exposure to Cd at a 50 μM concentration. Cd treatment with hemin further enhanced APX activity by 21% and 48% in roots and leaves, respectively (Fig. 3C).
The impact of various treatments on HO-1 catalysis is depicted in Fig. 3F. A considerable rise in HO-1 activity was observed upon treatment of V. radiata seedlings with CdCl2. However, the application of exogenous 0.5 mM hemin in the Cd treatment group restored HO-1 activity by 15.7% and 9.1% in leaves and roots, respectively, compared with Cd treatment alone (Fig. 3F).
Effect of exogenous hemin on the uptake, accumulation and translocation of Cd2+
Vigna radiata seedlings treated with 50 µM CdCl2 showed higher accumulation of Cd2+ in roots than leaves, as depicted in Table 2. In terms of phytoremediation, the addition of 0.5 mM hemin progressively increased the intracellular cadmium content by 40% and 17% in leaves and roots, respectively, in comparison to those of the seedlings subjected to CdCl2 treatment only (Table 2).
Cadmium uptake from the liquid medium via roots and its translocation to the foliar tissue affected the BCF and TF. For the seedlings of V. radiata subjected to Cd stress, hemin increased the uptake and translocation of cadmium in the plants. Supplementation of Cd-treated plants with 0.5 mM hemin improved the BCF by 2.0-and 1.6-fold in leaves and roots, respectively. A similar pattern of results was obtained for the TF, and the transfer of Cd ions from roots to aerial parts increased by 18.8% upon the addition of hemin to the stressed plants (Table 2).
Principal component analysis (PCA)
Principal component analysis was performed to determine the correlation between different evaluated parameters (morphological and physiological) in response to various treatments and to understand their importance in enhancing Cd tolerance in V. radiata seedlings supplemented with a low dose (0.5 mM) of hemin. The results showed that all the parameters (growth, total chlorophyll, tolerance index, stress and antioxidants) were grouped into two components (PC 1 and PC 2) and accounted for 97.5% of the total variance (Fig. 4). The first principal component showed 80.15% variance and correlated with the biomass (Fwt: fresh weight, Dwt: dry weight), plant height (SL: shoot length, RL: root length), leaf water content (LWC), total chlorophyll (Total Chl), tolerance index (TI) and antioxidants (SOD, POD, APX, proline), including HO-1. The second principal component contributed to 17.35% of the variance and was grouped with stress parameters (LPX and H2O2), the LWC and antioxidants (SOD, POD, APX, proline, HO-1, CAT) (Fig. 4).
Principal component analysis of combined data sets, growth parameters: fresh weight (Fwt), dry weight (Dwt), root length (RL), shoot length (SL), leaf water content (LWC), chlorophyll content (Total Chl), stress parameters: lipid peroxidation (LPX), hydrogen peroxide content (H2O2), tolerance index and antioxidants: proline content, activity of SOD, POD, APX, CAT and HO-1 in response to various combination of treatment.
Discussion
As a serious environmental pollutant, cadmium causes severe damage to plants by negatively influencing its morphology and physiology2,5,17. The outcome of the work demonstrated that metal stress hinders overall crop development by reducing plant biomass and chlorophyll contents. However, hemin supplementation markedly alleviated the adverse impact of cadmium by improving the growth and physiology of the plants. This is in accordance with earlier reports on hemin-induced alleviation of the inhibitory impact of cadmium in Medicago sativa L.11 and Brassica chinensis L.5. The morphology of root tissues is an imperative marker to assess the toxic effects of Cd2+ as roots are the initial tissues that are associated with metal ions17. In this study, root length was significantly affected by Cd stress, which disturbs the uptake of water and nutrients via roots and reduces crop growth and biomass5,18,19. Thus, root length affects the uptake and translocation of Cd by plants. Increases in root length and the number of root hairs were observed with hemin supplementation, which increases the absorption of water and nutrients and ultimately enhances plant biomass. This might be because exogenous hemin advances auxin-stimulated lateral root growth, which increases root numbers by stimulating cell division5,20. Hence, hemin favours the uptake of nutrients and metal ions from the liquid medium21,22.
In addition to enhanced root growth, the impact of hemin on crop improvement was mainly reflected in the alterations in photosynthetic pigments, as photosynthesis is the basis of plant growth and development. In our study, the chlorophyll content noticeably declined upon exposure of crop seedlings to Cd stress, which is probably due to damage to the photosynthetic apparatus, specifically photosystems I and II5,23. Cadmium blocks the photoactivation of photosystems by hindering electron transport in plastids24. Moreover, Cd inhibits the Calvin cycle (an important cycle of the dark reaction during photosynthesis) by affecting enzyme activities, which results in a lower photosynthetic rate25. However, hemin supply progressively improved the chlorophyll content in the stressed seedlings. The role of hemin in improving the chlorophyll concentration might be correlated with augmented haem oxygenase-1 activity. The results of this study are comparable with prior research results that demonstrated the role of haem oxygenase as an essential element in chlorophyll biosynthesis26,27,28,29.
The cadmium concentration in liquid medium changes the osmotic balance of plant cells by hindering water uptake through roots and reducing the leaf water content (LWC)2. Conversely, our study showed no noticeable reduction in the leaf water content under Cd stress, which might have been due to the progressive increase in the proline content in V. radatia seedlings. Proline is an effective quencher of ROS and works as a metal chelator in stress environments30,31. The addition of hemin to Cd-stressed plants further improved the proline content, which restored the leaf water content in plants. The improvement in the LWC due to the increased proline content was probably due to the antioxidant nature of proline, which alters plant metabolic activities to improve stress tolerance31,32. A simultaneous increase in the proline content was reported earlier in different plant species subjected to Cd stress2,33,34,35.
The toxic effects of cadmium in the present work were indicated by the overproduction of H2O2 and increased accumulation of MDA. The MDA content, a product of lipid peroxidation, is considered a signal of membrane destruction27. The study outcomes demonstrate an increase in toxicity in seedlings of V. radiata subjected to cadmium stress. Parallel effects of Cd in other plant species were also reported in earlier studies2,5,33,34. However, addition of hemin to metal-treated crops reduced the detrimental effect of cadmium in the seedlings and enhanced their physiology. These study results were in accordance with those of Zhu et al.5, who determined the mitigation of cadmium stress in Brassica chinensis L. with an application of exogenous hemin to the treated crop. The shielding impact of hemin might be associated with the increased activities of antioxidants, which were probably mediated by haem oxygenase-111.
SOD, CAT, APX and POD directly participate in ROS detoxification. ROS detoxification commences with the catalytic reaction of superoxide dismutase, the preliminary antioxidant that scavenges ROS by converting O2- to H2O2 and O236. Further H2O2 is converted to water through CAT, POD and APX. The accumulation of H2O2 inside the plant cell is prohibited by CAT, while APX and POD reduce it to H2O so that ROS deposition is efficiently controlled37. These antioxidant enzymes show regular activity under normal conditions, but their catalytic reaction is magnified under stress17. In our study, the activity of all antioxidants, including that of SOD, CAT, POD and APX, increased under Cd stress, which shows the clear response of V. radiata seedlings to metal stress and is likely one of the reasons for their survival along with Cd accumulation. Similar trends in antioxidant activity were recorded in different plant species exposed to Cd stress2,9,17,33,34.
However, exogenous application of 0.5 mM hemin further augmented the catalysis of APX, SOD and POD, which decreased the ROS concentration in V. radiata seedlings. These findings are comparable with those of Zhu et al.5, which verified the positive effect of hemin on antioxidant enzyme activity in seedlings of Brassica chinensis L. treated with Cd stress. Similarly, Chen et al.38 reported that supplementation with 1 μM and 5 μM hemin enhanced SOD and APX activity in Oryza sativa L. seedlings exposed to Zn (1 μM), Pb and Cr (5 μM)-treated hydroponic medium. The affirmative impact of hemin on antioxidant activities might be associated with the role of haem oxygenase-1. Hemin is a potent stimulator of HO-139, which oxidizes haem to biliverdin (BV), Fe2+ and carbon monoxide by employing NADH as a reducing equivalent40. Haem oxygenase-1 maintains the normal physiology of plants under stress environment by regulating antioxidant activities which neutralizes the negative effect of metal stress17,41. In recent findings, supplementation of 0.5 mM hemin to the Cd stress medium up-regulates the activity of haem oxygenase-1 enzyme to many folds (1.35 folds higher than control) which induces the activities of APX, SOD, POD and proline. The stimulated activity of antioxidants (APX, SOD, POD and proline) by HO-1 was clearly apparent in Fig. 4 which shows a linear co-relation with HO-1. The highest correlation of HO-1 was recorded with SOD and APX (Fig. 4). Additionally, CAT, POD and APX are haem enzymes with Fe in their structures, so Fe deficiency (due to competition between Cd and bivalent ions) is probably a limiting factor for their activities under Cd stress3. Hemin application in the Cd-treated medium elevates the activity of these antioxidants by increasing accessibility to iron (formed as a by-product during haem catalysis by HO-1)17. The study is supported by numerous reports that validate the shielding action of haem oxygenase towards cadmium treatment in different plant genotypes17,41,42,43. Furthermore, the PCA results revealed that augmented antioxidant activity improved the harvestable biomass and chlorophyll content of V. radiata under the combined treatment, and these parameters were negatively correlated with the H2O2 content and membrane damage (LPX) (Fig. 4).Thus our findings display the exact mechanism of hemin to tolerate Cd2+ by intensifying the activities of antioxidants which is mediated by hemin induced upregulated activity of HO-1.
To understand the uptake and translocation behaviour of metals in plants, it is important to select a suitable agent for improving the phytoremediation and stress tolerance of plants. Hemin, as a biostimulator, is known to enhance stress resilience in crops5,38. Similar to the results from other prior studies, the intracellular cadmium concentration in our study was far higher in roots than in leaves3,17,33,34. However, the intracellular Cd concentration in both tissues, viz. leaves and roots, was much lower than the critical concentration reported from Cd hyperaccumulators, which is 100 mg kg−1 dry weight of tissue3. Moreover, the BCF of leaves and roots as well as the TF were also less than 1. Thus, in our study, Vigna radiata did not display the characteristics of a cadmium hyperaccumulator according to the standard described by Baker and Brooks44 but was considered a cadmium eliminator that efficiently removes Cd from the contaminated environment.
In the current investigation, BCF and TF declined upon exposure of V. radiata seedlings to cadmium, as revealed by Mahmud et al.2 and Nabei and Amooaghaie3 in Brassica juncea L. and Catharanthus roseus (L.) G. Don; treated with Cd concentrations. This decrease may be due to the reduction in biomass and chlorophyll content of seedlings. Moreover, the decline can also be attributed to the saturation of metal uptake and root-to-leaf translocation45. Interestingly, exogenous application of hemin at a lower dose (0.5 mM) enhanced metal accretion in V. radiata tissues, which confirmed the efficiency of hemin for phytoremediation. Correspondingly, the uptake and transfer of metal from roots to leaves was noticeably amplified upon supplementation of hemin to Cd-stressed medium (Table 2). The effective promotion of the BCF and TF with the application of hemin was due to many reasons. The first probable reason is that hemin promotes auxin (specifically IAA)-induced lateral root development, which has already been reported in several studies conducted on different plant species46,47,48,49. Increased production of endogenous auxin stimulates the activity of membrane H+ ATPases that alter the transport of cation transporters50 and advances the absorption of Cd by V. radiata. A similar increase in the efficient absorption of Cd and U ions by an exogenous treatment of IAA was reported by Chen et al.9 in B. juncea. Moreover, auxin increases the solubility and bioavailability of Cd in the growing medium by decreasing the pH of the medium, which enhances metal absorption via roots and transfer to aerial tissues9,26. Second, hemin supplementation of Cd-stressed plants triggers a haem oxygenase-1 mediated antioxidant defence mechanism that improves the endogenous concentration of Fe2+ and CO (by-products of the HO-1 catalytic reaction) in the liquid medium. Improved Fe2+ and CO concentrations increase the uptake and accumulation of Cd while decreasing micronutrient uptake in plant tissues51. This might be understood on the basis of competition between Cd and micronutrients, particularly bivalent ions (Fe, Mg, Zn, Cu and Ca), for the transport channels that are involved in the translocation of these ions3.
Additionally, in the present study, hemin increased the tolerance index along with Cd accumulation in plant tissues, which suggests that the defensive role of hemin is not associated with the inhibition of uptake and transfer of metals but is possibly involved in activating the endogenous mechanism of cadmium detoxification in V. radiata. This assumption was subsequently verified by the finding that the application of hemin increased Cd translocation to the foliar parts, and despite the higher Cd concentration in the aerial tissues, the growth and physiology of the plant improved. This might be due to the release of some strong ligands by hemin to the Hoagland medium, which counterbalanced the Cd ion concentration in the plants. Moreover, hemin may also act as a metal chelator and efficiently translocate Cd over long distances through the xylem to avoid the high toxicity of Cd ions in the edible parts of plants, similar to other biostimulators3. Our results are in contrast with previous reports on Chinese cabbage5, rice38 and alfalfa11, where hemin application inhibited metal uptake and accumulation in plants to mitigate the noxious effects of cadmium ions. These discrepancies in the results are probably because the impact of hemin cotreatment on heavy metal accumulation depends on the type of plant species, concentration of the metal and hemin, exposure time and experimental conditions. Additionally, hemin might also increase metal accumulation in tolerant plants while decreasing metal accumulation in vulnerable species7,52 which is why diverse effects were observed based on the physiological nature of the plants.
Conclusion
The present investigation explores the role of hemin application in the advancement of Cd stress tolerance and remediation efficiency using Vigna radiata. Cadmium adversely affects plant growth by reducing the harvestable biomass and chlorophyll content and increasing oxidative stress (H2O2 and MDA content). However, exogenous supplementation of hemin in liquid medium that also contains Cd mediates the initiation of tolerance mechanisms in plants through upregulation of antioxidant activities. Moreover, this study presented strong evidence that hemin supports the removal efficiency of V. radiata by (1) improving the plant biomass and chlorophyll content and (2) enhancing Cd uptake and its translocation from roots to foliar tissues. Thus, our data concluded that hemin, as a biostimulator, has the potential to improve the phytoremediation efficiency of heavy metal tolerant plants so that they can be used as an alternative to hyperaccumulators to remediate Cd contamination. Future studies in the natural environment need to be carried out to further verify the significance of hemin for cadmium tolerance and remediation.
Materials and methods
Processing of experimental material and stress treatments
Vigna radiata var. PDM 54 seeds were collected from the National Bureau of Plant Genetic Resources, Jodhpur, India and disinfected with mercuric chloride (0.1% w/v) for one minute followed by rinsing with autoclaved deionized water (4–5 times) to remove the remaining traces of HgCl2. Disinfected seeds were germinated in sterile conditions in petri-plates with blotting paper soaked with distilled water at room temperature in the dark in a seed germinator. At the two-leaf stage, the germinated seedlings were transferred to half-strength Hoagland medium (pH 6.8–6.9) and placed under thermostatically controlled conditions (50% relative humidity and 25 ± 2 °C temperature). The liquid medium was replaced with fresh medium every other day and aerated twice daily to avoid nutrient and oxygen deficiency in the seedlings.
After 1 week, seedlings successfully adapted to hydroponic culture were treated with exogenous hemin at concentrations ranging from 0.1 to 20 mM (0.1, 0.5, 1.0, 5.0, 10, 20 mM) for 96 h53,54,55,56,57. Liquid medium without treatment was considered a control and utilized to evaluate the effect of hemin on haem oxygenase-1 catalytic activity. Maximum haem oxygenase-1 (HO-1) catalysis was observed under the 0.5 mM treatment (Fig. 5E); hence, this hemin concentration was selected for further analysis. Based on our previous report on the role of HO-1 in mitigating Cd stress in V. radiata seedlings17, we selected a 50 µM CdCl2 concentration for the present study because at this treatment level, the activities of all the antioxidants, including haem oxygenase-1, were maximal (Fig. 5A,B), which resulted in the lowering of oxidative damage at this concentration (Fig. 5C,D). Thus, the seedlings of V. radiata used in the present study were subjected to the following treatments:
-
1.
Hoagland nutrient solution without any treatment (Control, CK)
-
2.
0.5 mM hemin (T1)
-
3.
50 µM CdCl2 (T2)
-
4.
50 µM CdCl2 + 0.5 mM hemin (T3)
Dose dependent effect of CdCl2 (10 μM to 100 μM) (from A to D) and hemin (0.1 to 20 mM) (E) on antioxidants activity: HO-1 (A & E) and APX (B); Oxidative damage: H2O2 (C) and MDA content (D) in Vigna radiata. Seedlings were treated independently for a period of 96 h to select the optimum concentration of CdCl2 and hemin for further analysis. Values are mean ± SE (n = 3) and are statistically significant according to DMRT test (p < 0.05). Data points marked with the different letters show significant differences (p < 0.05) within treatments.
The treated plants were harvested after 96 h of stress for assessment of different physiological parameters17.
Morphological parameters
To study morphological parameters, freshly harvested crop seedlings were first washed with distilled water. Crop morphology was examined with regard to seedling height (root and shoot length), biomass (fresh and dry weight), a tolerance index (TI) and the leaf water content (LWC). The height of the seedlings was individually computed in centimetres. To calculate the dry weight, fresh seedlings were desiccated overnight at 65 ˚C in an oven, and the weight of dehydrated seedlings was measured. The TI was measured according to the Wilkins58 method and presented as percent tolerance. The LWC was calculated from seedling biomass by utilizing the equation (fresh weight − dry weight/fresh weight) × 10059.
Quantification of chlorophyll concentrations
Chlorophyll concentrations (Chl a, Chl b and total Chl) were quantified by the Arnon60 protocol. Freshly harvested young leaves were pulverized in 80% chilled acetone at 4 °C and centrifuged for 15 min at 10,000×g under cold conditions. The chlorophyll content (mg g−1 fresh weight of leaves) was determined from the optical density of the procured supernatant recorded at 645 nm and 663 nm60.
Determination of oxidative stress
Peroxidation of membrane lipids
The peroxidation of membrane lipids was determined by quantifying the malondialdehyde (MDA) content according to the protocol of De Vos et al.61. Freshly harvested seedlings were crushed in 2-thiobarbituric acid (0.25% w/v) prepared in trichloroacetic acid (10% v/v). The ground sample was incubated for 30 min at 95 °C in a water bath and centrifuged for 15 min at 10,000×g after cooling to room temperature. The MDA concentration (nanomolar per gram fresh weight of tissue) was quantified from the specific optical density (λ532–λ600) by utilizing 155 mM−1 cm−1 as a proportionality constant61.
H2O2 concentration
Fresh plant tissues were homogenized in TCA (0.1% v/v) under cold conditions. The pulverized sample was centrifuged for 15 min at 10,000×g. The H2O2 concentration (µM g−1 fresh weight of tissue) was determined by documenting the optical density of an assay compound [potassium phosphate buffer (10 mM, pH 7.0) + KI (1 M) + supernatant] incubated in the dark for an hour at 390 nm (molar absorption coefficient 0.28 µmol−1 cm−1)62.
Estimation of proline content
The proline content was determined by the procedure of Bates et al.63. Freshly harvested tissues were extracted in sulphosalicylic acid (3% w/v) and subsequently centrifuged for 20 min at 3000×g. The reaction mixture (equivalent volume of acid ninhydrin + supernatant + glacial acetic acid) was incubated for an hour at 60 °C in a water bath and transferred to an ice bath to terminate the reaction. The proline concentration (µg g−1 fresh weight of tissue) was estimated by documenting the optical density of an organic chromophore at 520 nm after adding toluene to the terminated reaction mixture63.
Extraction and assay of antioxidant enzymes
Freshly harvested crop seedlings were pulverized in NaPO4 buffer (50 mM, pH 7.0) in cold conditions. The homogenate was centrifuged for 20 min at 10,000×g at 4 °C. The procured supernatant was utilized for further analysis.
Superoxide dismutase (SOD) catalysis was assayed by the Beuchamp and Fridovich64, protocol. The catalytic reaction of SOD was determined by documenting the optical density of the assay compound [NaPO4 buffer (50 mM, pH 7) + methionine (13 mM) + nitroblue tetrazolium (NBT) (75 µM) + riboflavin (2 mM) + EDTA (0.1 mM) + enzyme extract] at 560 nm after 30 min of incubation under bright light, which indicates the capability of an enzyme to hinder the photolytic reduction of NBT (with a proportionality constant of 100 mM−1 cm−1).
The catalytic reaction of the catalase (CAT) enzyme was measured via the Aebi65, procedure. The decomposition rate of H2O2 (mM H2O2 degraded min−1 g−1 fresh weight of tissue) was evaluated by the decline in optical density of the assay mixture [NaPO4 buffer (50 mM, pH 7) + H2O2 (9 mM) + enzyme extract] at 240 nm by utilizing 0.039 mM-1 cm-1 as the molar absorption coefficient65.
The catalysis of ascorbate peroxidase (APX) was determined via Chen and Asada66. The oxidation rate of ascorbate (mM ascorbate oxidized min−1 g−1 fresh weight of tissue) was computed by the decline in optical density of an assay compound [NaPO4 buffer (50 mM, pH 7) + H2O2 (10% v/v) + enzyme extract + ascorbate (0.6 mM)] at 290 nm by employing 2.8 mM−1 cm−1 as a proportionality constant66.
The peroxidase (POD) catalysis was measured by using the method of Putter67. The rate of formation of tetraguaiacol (mM min−1 g−1 fresh weight of tissue) was calculated by the rise in optical density of an assay mixture [NaPO4 buffer (50 mM, pH 7) + guaiacol (20 mM) + H2O2 (3.7 mM) + enzyme extract] at 436 nm by employing 26.6 mM–1 cm–1 as a proportionality constant67.
The catalysis of the haem oxygenase-1 (HO-1) enzyme was evaluated according to the Balestrasse et al.68 method. Freshly harvested plant tissue was homogenized in chilled KPO4 buffer (50 mM, pH 7.4) containing PMSF (1 mM) + EDTA (0.2 mM) + sucrose (0.25 M). The procured supernatant, after centrifugation of the homogenate for 20 min at 10,000×g at 4 °C, was employed for HO-1 analysis. The concentration of HO-1 (µM biliverdin reduced mg−1 protein) was estimated by documenting the optical density of biliverdin (main product of HO-1catalysis) formed by the reaction between an assay compound [KPO4 buffer (50 mM, pH 7.4) + hemin (200 nm) + NADPH (60 nm)] and HO-1 extract at 37 °C for one hour at 650 nm (Molar absorption coefficient 6.25 µM–1 cm–1).
Intracellular Cd concentration
The Cd content accumulated in the plant cells was determined via the protocol of Bates et al.69. Fresh plant samples were cleansed with distilled water, and roots were separated from the aerial tissue. Separated tissues were kept in an oven overnight at 80 °C. Desiccated tissues were boiled in an acid mixture containing HNO3 (70% v/v) + H2O2 (30% v/v) + deionised water in the proportion of 1:1:3. The colourless remains of the mixture were diluted in HNO3 (2% v/v) and employed for estimation of the Cd content69.
Measurement of biological concentration factor (BCF) and translocation factor (TF)
The biological concentration factor reveals the ability of corresponding plant species to uptake the particular metal ion into tissues to its proportion in the related surroundings and was calculated by the formula17:
The translocation factor demonstrates the transfer ability of metal ion in particular plant70. The formula for estimating TF is:
Statistical analysis
For graphical representation of data Sigma plot version 12.0 (Chicago, IL, USA) software (http://www.sigmaplot.co.uk/products/sigmaplot/produpdates/prod-updates5.php) was used. Data were statistically examined by one way ANOVA and the correlation between different evaluated parameters were analyzed by principal component analysis using SPSS 16 version software. The data were considered as average (± standard error) of individual replicas (n = 3) of every test conducted separately. To check the reproducibility of results, three independent biological replicates were studied following completely randomized design (CRD). The significant variations among treated and untreated seedlings were illustrated at 0.05% significant level by applying Duncan’s Multiple Range test (DMRT)17,59.
References
Chen, B. et al. The effects of the endophytic bacterium Pseudomonas fluorescens sasm05 and IAA on the plant growth and cadmium uptake of Sedum alfredii Hance. Front. Microbiol. 8, 253 (2017).
Mahmud, J. A., Hasanuzzaman, M., Nahard, K., Bhuyana, M. H. M. B. & Fujitaa, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 147, 990–100 (2018).
Nabaei, M. & Amooaghaie, R. Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-019-07283-4 (2019).
Grant, C. A. Influence of phosphate fertilizer on cadmium in agricultural soils and crops. Pdeologist 54, 143–155 (2011).
Zhu, Z. et al. Increased antioxidative activity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol. Environ. Saf. 177, 47–57 (2019).
Asgher, M., Khan, M. I. R., Anjum, N. A. & Khan, N. A. Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252, 399–413 (2015).
Manoj, S. R. et al. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. https://doi.org/10.1016/j.jenvman.2019.109779 (2020).
Asadi, E., Maresca, V., Sorbo, S., Keramat, B. & Basile, A. Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium induced oxidative stress. Ecotoxicol. Environ. Safe. 144, 268–274 (2017).
Chen, L., Long, C., Wang, D. & Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere https://doi.org/10.1016/j.chemosphere2019.125112 (2020).
Xie, Y. et al. Heme-heme oxygenase 1 system is involved in ammonium tolerance by regulating antioxidant defence in Oryza sativa. Plant Cell Environ. 38, 129–143 (2015).
Cui, W. et al. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J. Exp. Bot. 63, 5521–5534 (2012).
Cui, W., Zhang, J., Xuan, W. & Xie, Y. Up-regulation of heme oxygenase-1 contributes to the amelioration of aluminum-induced oxidative stress in Medicago sativa. J. Plant Physiol. 170, 1328–1336 (2013).
Xie, Y., Cui, W., Yuan, X., Shen, W. & Yang, Q. Heme oxygenase-1 is associated with wheat salinity acclimation by modulating reactive oxygen species homeostasis. J. Integr. Plant Biol. 53, 653–670 (2011).
Nair, R. M. et al. Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J. Sci. Food Agric. 93, 1805–1813 (2013).
Sehrawat, N., Bhat, K. V., Sairam, R. K. & Jaiwal, P. K. Identification of salt resistant wild relatives of mungbean (Vigna radiata (L.) Wilczek. Asian J. Plant Sci. Res. 5, 41–49 (2013).
Diwan, H., Khan, I., Ahmad, A. & Iqbal, M. Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul. 61, 97–107 (2010).
Mahawar, L., Kumar, R. & Shekhawat, G. S. Evaluation of heme oxygenase 1 (HO 1) in Cd and Ni induced cytotoxicity and crosstalk with ROS quenching enzymes in two to four leaf stage seedlings of Vigna radiata. Protoplasma 255, 527–545 (2018).
Kubo, K. et al. Differences in cadmium accumulation and root morphology in seedlings of Japanese wheat varieties with distinctive grain cadmium concentration. Plant Prod. Sci. 14, 148–155 (2015).
Tai, Z. et al. Exogenous GR24 alleviates cadmium toxicity by deducing cadmium uptake in Switchgrass (Panicum virgatum) seedlings. Int. J. Environ. Res. Public Health 14, 852 (2017).
Xuan, W. et al. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol. 148, 881–893 (2008).
Aprill, W. & Sims, R. C. Evaluation of the use of prairie grasses for stimulating polycyclic aromatic hydrocarbon treatment in soil. Chemosphere 20, 253–265 (1990).
Teiri, H., Pourzamani, H. & Hajizadeh, Y. Phytoremediation of VOCs from indoor air by ornamental potted plants: A pilot study using a palm species under the controlled environment. Chemosphere 197, 375–381 (2018).
Küpper, H., Parameswaran, A., Leitenmaier, B., Trtilek, M. & Setlik, I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 175, 655–674 (2007).
Heyno, E., Klose, C. & Liszka, A. K. Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 179, 687–699 (2008).
Ying, R. R. et al. Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyper accumulator Picris divaricata. J. Plant Physiol. 167, 81–87 (2010).
Li, H. et al. Enhanced efficiency of cadmium removal by Boehmeria nivea (L.) Gaud. in the presence of exogenous citric and oxalic acids. J. Environ. Sci. 26, 2508–2516 (2014).
Mahawar, L. & Shekhawat, G. S. Haem oxygenase: A functionally diverse enzyme of photosynthetic organisms and its role in Phytochrome chromophore biosynthesis, cellular signalling and defence mechanisms. Plant Cell Environ. 41, 483–500 (2018).
Verma, K., Mehta, S. K. & Shekhawat, G. S. Nitric oxide [NO] counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species [ROS] in Brassica juncea: Cross-talk between ROS, NO and antioxidant responses. Biometals 26, 255–269 (2013).
Zhu, L. et al. Heme oxygenase 1 defects lead to reduced chlorophyll in Brassica napus. Plant Mol. Biol. 93, 579–592 (2017).
Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930 (2010).
Mahawar, L., Khator, K. & Shekhawat, G. S. Role of Proline in mitigating NaCl induced oxidative stress in Eruca sativa Miller: An important oil yielding crop of Indian Thar Desert. Vegetos Int. J. Plant Res. Biotechnol. 31, 55–63 (2018).
Yadav, S. K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 76, 167–179 (2010).
Nahar, K. et al. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense, and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 126, 245–255 (2016).
Nahar, K. et al. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 23, 21206–21218 (2016).
Rahman, A., Mostofa, M. G., Nahar, K., Hasanuzzaman, M. & Fujita, M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz. J. Bot. 39, 393–407 (2016).
Alscher, R. G., Erturk, N., Heath, L. S. & Alscher, R. G. Role of superoxide dismutases [SODs] in controlling oxidative stress in plants. J. Exp. Bot. 53, 1331–1341 (2002).
Sharma, S. S. & Dietz, K. J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 14, 43–50 (2009).
Chen, Q. et al. Hemin-mediated alleviation of Zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 81, 253–264 (2016).
Li, J. et al. ß-Cyclodextrin-hemin complex induced lateral root formation in tomato: Involvement of nitric oxide and heme oxygenase 1. Plant Cell Rep. 34, 381–393 (2015).
Shekhawat, G. S. & Verma, K. Heme oxygenase (HO): An overlooked enzyme of plant metabolism and defence. J. Exp. Bot. 61, 2255–2270 (2010).
Noriega, G. O., Santa-Cruz, D., Batlle, A., Tomaro, M. & Balestrasse, K. Heme oxygenase is involved in the protection exerted by jasmonic acid against cadmium stress in soybean roots. J. Plant Growth Regul. 31, 79–89 (2012).
Balestrasse, K. B., Yannarelli, G. G., Noriega, G. O., Batlle, A. & Tomaro, M. L. Heme oxygenase and catalase gene expression in nodules and roots of soybean plants subjected to cadmium stress. Biometals 21, 433–441 (2008).
Jin, Q., Zhu, K., Xie, Y. & Shen, W. Heme oxygenase-1 is involved in ascorbic acid-induced alleviation of cadmium toxicity in root tissues of Medicago sativa. Plant Soil 366, 605–616 (2013).
Baker, A. J. M. & Brooks, R. R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1, 81–126 (1989).
Milner, M. J. & Kochian, L. V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 102, 3–13 (2008).
Chen, Y. H., Chao, Y. Y., Hsu, Y. Y., Hong, C. Y. & Kao, C. H. Heme oxygenase is involved in nitric oxide- and auxin-induced lateral root formation in rice. Plant Cell Rep. 31, 1085–1091 (2012).
Fang, T., Cao, Z., Li, J. & Shen, W. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 76, 44–51 (2014).
Lin, Y. T. et al. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 171, 1–8 (2014).
Zhu, D. et al. Involvement of glutathione in β-cyclodextrin-hemin complex-induced lateral root formation in tomato seedlings. J Plant Physiol. 204, 92–100 (2016).
Altabella, T., Palazon, J., Ibarz, E., Pinol, M. T. & Serrano, R. Effect of auxin concentration and growth phase on the plasma membrane H+-ATPase of tobacco calli. Plant Sci. 70, 209–214 (1990).
Baldantoni, D., Cicatelli, A., Bellino, A. & Castiglione, S. Different behaviours in phytoremediation capacity of two heavy metal tolerant poplar clones in relation to iron and other trace elements. J. Environ. Manag. 146, 94–99 (2014).
Khan, M. S., Zaidi, A., Wani, P. A. & Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils: A review. Sustain. Agric. Rev. https://doi.org/10.1007/978-1-4020-9654-915 (2009).
Han, Y. et al. Exogenous hematin alleviates mercury-induced oxidative damage in the roots of Medicago sativa. J. Int. Plant Biol. 49, 1703–1713 (2007).
Su, N. et al. Hemin-decreased cadmium uptake in pak choi (Brassica chinensis L.) seedlings is heme oxygenase-1 dependent and relies on its by-products ferrous iron and carbon monoxide. Environ. Pollut. https://doi.org/10.1016/j.envpol.2020.115882 (2020).
Habib, N. et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 9(2), 285. https://doi.org/10.3390/plants9020285 (2020).
Elkelish, A. et al. Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 9(4), 431. https://doi.org/10.3390/plants9040431 (2020).
Soliman, M. et al. Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 8(12), 562. https://doi.org/10.3390/plants8120562 (2019).
Wilkins, D. A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 80, 623–633 (1978).
Mahawar, L. & Shekhawat, G. S. EsHO 1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO 1 in seedlings of Eruca sativa: Underutilized oil yielding crop of arid region. Physiol. Mol. Biol. Plants. 25, 895–904 (2019).
Arnon, D. I. Copper enzymes in isolated chloroplasts: Polyphenol oxidases in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
De Vos, C. H. R., Schat, H., Vooijs, R. & Ernst, W. H. O. Copper-induced damage to the permeability barrier in roots of silene cuiubalus. J. Plant Physiol. 135, 164–179 (1989).
Alexieva, V., Sergiev, I., Mapelli, S. & Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24, 1337–1344 (2001).
Bates, L. S., Waldren, R. P. & Tear, I. D. Rapid determination of free proline for water stress studies. Plant Soil 39, 205–207 (1973).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Aebi, H. Catalases. In Methods of Enzymatic Analysis (ed. Bergmeyer, H. U.) 680 (Academic Press Inc, New York, 1974).
Chen, G. X. & Asada, K. Ascorbate peroxidase in tea leaves: Occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 30, 987–998 (1989).
Putter, J. Peroxidase. In Methods of Enzymatic Analysis (ed. Bergemeyer, H. U.) 685–690 (Academic Press, London, 1974).
Balestrasse, K. B., Noriega, G. O., Batlle, A. & Tomaro, M. L. Involvement of Heme oxygenase as antioxidant defense in soybean Nodules. Free Radic. Res. 39, 145–151 (2005).
Bates, S. S., Tessier, A., Campbell, P. G. C. & Buffle, J. Zinc adsorption and transport by Chlamydomonas variabilis and Scenedesmus subspicatus [Chlorophyceae] grown in semicontinuous culture. J. Phycol. 18, 521–529 (1982).
Stoltz, E. & Greger, M. Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ. Exp. Bot. 47, 271–280 (2002).
Acknowledgements
Authors gratefully acknowledge University Grant Commission, New Delhi for providing financial assistance in the form of Centre for Advanced Study. The work is also supported by TECO "Technological Eco-Innovations for the Quality Control of Polluted Waters and Soils" funded by the European Union; Authors also extend their sincere appreciation to the Deanship of Scientific Research, King Saud University for its funding to the Research Group number (RGP-199).
Author information
Authors and Affiliations
Contributions
G.S.S. designed and conceptualized the study, L.M. and R.P. executed the experiments, M.N.A. and P.A. help in manuscript preparation, all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahawar, L., Popek, R., Shekhawat, G.S. et al. Exogenous hemin improves Cd2+ tolerance and remediation potential in Vigna radiata by intensifying the HO-1 mediated antioxidant defence system. Sci Rep 11, 2811 (2021). https://doi.org/10.1038/s41598-021-82391-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82391-1
- Springer Nature Limited
This article is cited by
-
Beneficial Effects of Hemin on Antioxidative Capacity and Anatomical Characters of NaCl-Stressed Rice Plants
Journal of Plant Growth Regulation (2024)
-
Novel insights into the mechanism(s) of silicon-induced drought stress tolerance in lentil plants revealed by RNA sequencing analysis
BMC Plant Biology (2023)
-
Understanding the Physiological Mechanism of Heme Oxygenase for Enhanced Tolerance and Phytoremediation of Cd2+ in Eruca sativa: Co-ordinated Function of Antioxidant Defense System
Journal of Plant Growth Regulation (2023)
-
Hemin protects against cell stress induced by estrogen and progesterone in rat mammary glands via modulation of Nrf2/HO-1 and NF-κB pathways
Cell Stress and Chaperones (2023)
-
Iron deficiency in plants: an update on homeostasis and its regulation by nitric oxide and phytohormones
Plant Growth Regulation (2023)