Abstract
The widely used rubber hand illusion (RHI) paradigm provides insight into how the brain manages conflicting multisensory information regarding bodily self-consciousness. Previous functional neuroimaging studies have revealed that the feeling of body ownership is linked to activity in the premotor cortex, the intraparietal areas, the occipitotemporal cortex, and the insula. The current study investigated whether the individual differences in the sensation of body ownership over a rubber hand, as measured by subjective report and the proprioceptive drift, are associated with structural brain differences in terms of cortical thickness in 67 healthy young adults. We found that individual differences measured by the subjective report of body ownership are associated with the cortical thickness in the somatosensory regions, the temporo-parietal junction, the intraparietal areas, and the occipitotemporal cortex, while the proprioceptive drift is linked to the premotor area and the anterior cingulate cortex. These results are in line with functional neuroimaging studies indicating that these areas are indeed involved in processes such as cognitive-affective perspective taking, visual processing of the body, and the experience of body ownership and bodily awareness. Consequently, these individual differences in the sensation of body ownership are pronounced in both functional and structural differences.
Similar content being viewed by others
Introduction
The growing body of neurocognitive findings surrounding self-location, agency, and body ownership has emphasized the role of multisensory processes that may contribute to bodily self-consciousness. Previous research on bodily self-consciousness have studied how bodily illusions can modulate the experience of one’s ownership over their own body, referring to the feeling that the body is an integral part of the self1,2,3.
The most commonly used experimental paradigm to manipulate body ownership is the rubber hand illusion (RHI). During the illusion, the synchronous stroking of a rubber hand and the participant’s own hand, the latter of which is separated from their visual field, leads the participants to perceive that the rubber hand is part of their own body1,3,4. However, the RHI does not occur when the rubber hand and the participant’s own hand are stroked asynchronously1,3, or when participants view the rubber hand in an anatomically implausible position (e.g., watching a right rubber hand while their left hand is being stimulated), or when viewing a neutral object, even though the stimulation is synchronous5. The RHI is a useful tool for measuring both the objective and the subjective aspects of body ownership. The objective (or implicit) measure of the RHI is the proprioceptive drift, which refers to the degree of displacement of the perceived position of the participant’s real hand towards the rubber hand. Conversely, the subjective (or explicit) aspect of the RHI refers to the phenomenological experience of the illusion and is usually measured by self-report questionnaires6.
With respect to the relationship between proprioceptive drift and subjective changes in body ownership during the RHI, however, the literature shows contradictory results. Initial studies of the RHI found that the proprioceptive drift was positively associated with the subjective feeling of owning the rubber hand, suggesting common brain mechanisms for illusory hand ownership and proprioceptive drift4,5,7. However, other studies revealed that the proprioceptive drift and the sense of body ownership are rarely correlated (e.g.8), suggesting there are distinct multisensory mechanisms underlying the phenomena. These findings have been further supported by functional magnetic resonance imaging (fMRI) studies demonstrating that there are at least two components of bodily self-consciousness: body ownership and self-location (i.e., the physical location of the body in a space)9. Furthermore, other functional brain imaging studies investigating various bodily illusions such as RHI1,10,11, full body illusion12, and body swap illusion13 also found convergent evidence for the notion that bodily self-consciousness is represented in a distributed neural network in the brain, including the premotor cortex (PMC), the parietal area, the somatosensory cortex, the occipitotemporal cortex, and the insula.
In line with this, a wide range of neurocognitive processes have been shown to be involved in the RHI. First, the body-related sensory information is processed in a bottom-up way in the sensory regions. With regard to the neurocognitive mechanism underlying bodily illusions, visual information relating to the body and body parts initially activate the occipital cortex, mainly the extrastriate body area (EBA14), which is selectively active when body parts are visually perceived15. The tactile sensation felt on the skin/body activates the primary somatosensory cortex in the postcentral gyrus16,17, while the proprioceptive information stimulates the precentral gyrus, the postcentral gyrus, and the anterior cingulate gyrus18. Although these unimodal visual, tactile, and proprioceptive body representations are essential components of the bodily experience, multisensory processes are also necessary. Multisensory areas in premotor, parietal, and insular cortices integrate sensory cues originating from various modalities and it has even been suggested that they provide the whole-body experience. One of the first fMRI studies targeting body ownership and the RHI1 postulated that the self-attribution of body parts arises as a result of multisensory integration processes in the PMC. More specifically, the increased activity in the bilateral ventral PMC (vPMC) has been associated with the feeling of ownership over the observed fake hand. Another fMRI study using the body swap illusion (viewing the body of a mannequin from its head’s point of view receiving tactile stimulation while the participant’s body is out of sight and is stimulated identically to the mannequin) also demonstrated that neural activity in the vPMC correlated with the strength of the experienced ownership of a virtual body, and also of a virtual hand13. Thus, these results suggest that the increased activation of vPMC reflects the subjective feeling of body ownership over a fake body part (e.g., a prosthetic hand) resulting from the integration of multisensory cues from various sources.
Previous studies showed that the temporo-parietal junction region (TPJ) involving the supramarginal gyrus (SMG) and the angular gyrus also contributes to the top-down process of the internal representation of the body, as it can affect the structural model thereof in order to facilitate the experience of ownership over one’s own body. Moreover, TPJ activity also modulates the converging visual, proprioceptive, vestibular, and tactile signals in order to construct a world-centered reference frame which provides stable self-location and visuo-spatial perspective19,20. The posterior parietal cortex (PPC) also plays a critical role in multisensory integration, transforming the sensory inputs from the body (i.e., information about the hands, arms, head, etc.) into a body map, which obtains a spatially represented body-centred reference frame. This mechanism contributes to the internal representation of self-location2,10. Perceiving a rubber hand as one’s own during an experimental illusion seems to result from a remapping process in PPC indicating changes in the sense of hand position, whilst the hand remapping in the PMC is related to the subjective feeling of body ownership10. Other studies investigating the neural basis of the bodily self have discovered that the activation in the right insula were positively correlated with the strength of the RHI3. The insula is known as a structure that integrates bodily signals with environmental information in order to increase homeostatic efficiency21; this plays a vital role in interoceptive and exteroceptive awareness (for a review, see22). Based on the converging findings, it is logical to hypothesize there exists a multicomponent neural network that is responsible for the modifications in bodily self-consciousness evoked by bodily illusion paradigms.
However, this neural model cannot explain the findings of previous studies demonstrating that 30% of the population do not experience („non-perceivers”) body ownership over the fake hand7,23. This suggests that individual differences exist in bodily self-consciousness. For example7, proposed that people who are better at relying on proprioceptive information (e.g., professional dancers) might be more resistant to the illusion than others. In line with this24, found evidence that subjects with lower interoceptive awareness were more susceptible to the experience of the ownership of corporeal objects (fake hands) in the RHI experiment. Similarly, sensory suggestibility is likely to contribute to the high response variability in RHI findings. A study by25 compared individuals with high and low sensory suggestibility and ascertained that highly suggestible individuals had a stronger experience of body ownership over the rubber hand, as measured by a subjective questionnaire, while the results of the implicit measures (proprioceptive drift) implied no differences between the two groups. For a deeper understanding of the individual differences found in this bodily illusion, a recent study attempted to investigate the relationship between brain anatomy and subjective reports of functional self-experience (measuring body ownership, narrative self and agency) in healthy individuals26. A significant association was observed between the insular cortex volume and ownership-related self-malfunctions (such as schizotypal, depersonalizing, and dissociative tendencies) in daily life. More specifically, the ownership subscale scores correlated with grey matter volumes in the postcentral gyrus, insular cortex, and angular gyrus, thus indicating that individual differences in cortical volume are related to ownership experiences, leading to the assumption that the morphology of certain brain structures could explain variabilities in body ownership experiences reported following the induction of a bodily illusion. However, this study assessed the body ownership and other self-related functions in an imagined daily situation. Furthermore, several studies showed altered cortical thickness, volume and cortical surface in body ownership-related brain areas in disorders where the representation of the body is impaired. In case of body integrity identity disorder (also known as xenomelia) a study found reduced cortical thickness in the superior parietal lobule and reduced cortical surface area in the anterior insular cortex, the somatosensory areas SI and SII, and the inferior parietal lobule27. In addition, several lines of evidence suggest that patients with anorexia nervosa have alterations in cortical thickness with respect to the stages of weight restoration. Comparing with healthy controls28, found globally lower cortical thickness in women with anorexia nervosa. In a longitudinal study it was shown that three months of weight restoration resulted in the normalization of cortical thickness in patients with anorexia nervosa29. Studies also reported increased gray matter volume of the medial orbitofrontal cortex and the insula30, and greater cortical thickness of the medial orbital sulcus and the insula31 in patients with anorexia nervosa.
To the best of our knowledge, no research has been conducted to shed light on the structural neuroanatomical differences that might explain the robust inter-individual differences in the RHI. Here, we aim to study whether the individual differences of body ownership experience in the RHI could be explained by structural brain differences in terms of cortical thickness. Cortical thickness was chosen primarily for methodological reasons. Firstly, it was validated in histological studies and it was found as a method with high accuracy (e.g.32). Secondly, Freesurfer’s thickness estimation method was tested on several clinical samples (e.g. schizophrenia33,34, Huntington’s disease35,36, epilepsy37,38), elderly people and young adults. These studies confirmed that this measurement tool provides a sensitive marker for clinical symptoms and psychological variables as well. We hypothesized that the strength of the proprioceptive drift and the subjective report of body ownership are associated with the cortical thickness of specific right hemispheric brain areas involved in the multisensory integration process: superior temporal gyrus (STG39,40), middle temporal gyrus (MTG41), inferior temporal gyrus42, precuneus39,40, SMG19,20, precentral and postcentral gyri18, superior parietal gyrus43,44, inferior parietal gyrus45, lateral and medial orbitofrontal cortices (OFC46), rostral anterior cingulate cortex18, pars opercularis45,47,48, lateral occipital cortex (LOC14), and insula3.
Methods
Participants
67 university students (36 were male) participated in this experiment, with a mean age of 22.25 years (SD: 2.29, range 19–29 years). All participants were right-handed according to the Edinburgh Handedness Inventory (i.e., the Handedness Laterality Quotient for each participant was higher or equal to 70%49). Participants did not have a history of neurological diseases or experience with the RHI. Informed consent was obtained prior to the experimental sessions. All participants received a small fee for taking part in the study. The study was conducted according to the principles of the Declaration of Helsinki and had been approved by the Regional Research Ethics Committee of the Medical Center, Pécs.
Experimental design and procedures
Rubber hand illusion paradigm
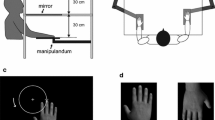
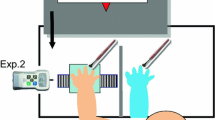
In this study, we used the original RHI paradigm introduced by4, supplemented with a proprioceptive drift baseline measurement before the stimulation. During the experiment, participants were sitting on a comfortable chair and their arms rested on a table with their palms facing down. A realistic artificial hand (left or right rubber hand, depending on which hand was stimulated) was placed on the table 20 cm from the participant’s real hand. In case of the non-stroked hand, we asked the participants to rest their hand on the table comfortably in a symmetrical position to the stroked hand. Only the rubber hand was visible to the participants, whereas both of their own hands were hidden behind a 40 cm × 65 cm wooden occluder. The RHI was performed by a trained experimenter who stroked both the real hand and the rubber hand with paintbrushes for two minutes while the subject was instructed to focus on the rubber hand. The experimenter stroked the upper and the lower part separately of all fingers (except the thumb) on the rubber and the unseen hand with two brushes at the same time. The illusion was elicited on both hands in a counterbalanced order across participants. The experimental block for both hands consisted of a baseline proprioceptive drift measurement (without stroking period) and two experimental conditions (synchronous and asynchronous stimulation). The experimental conditions were also counterbalanced across the blocks. One stroking period per condition was performed.
Procedure
Before the stimulations, participants underwent a baseline proprioceptive drift measurement in which the experimenter measured the pre-stroking difference between the actual and the perceived location of the hand. The experimenter asked the participant to close their eyes and point to the location of their real index finger on a ruler. A positive value for proprioceptive drift indicated that the participants perceived the location of their own index finger as drifting toward the rubber hand (toward the body midline). After the baseline measurement was taken, synchronous and asynchronous stimulations were delivered over a 2-min-long stroking period. The pattern and frequency of the stimulation (1 Hz) were the same in both conditions due to the use of a metronome that guided the experimenter through an earphone. Directly after the stimulation (both synchronous and asynchronous stroking), the proprioceptive drift and the subjective feeling of ownership were assessed. The strength of the proprioceptive drift was defined as the distance between the two reported locations of the index finger reported in the baseline and the stimulation trials50,51,52.
Analysis 1 (subjective ratings)
To assess participants’ subjective perception of ownership over the rubber hand, they were asked to complete the RHI questionnaire in both the synchronous and the asynchronous conditions6. The original questionnaire consists of 27 items, targeting the subjective feelings occurring during the illusion, ownership, and disownership, along with some control statements. Participants had to indicate their agreement or disagreement on a 7-item Likert scale. For the current study, four questions were adopted from the RHI questionnaire. Three questions measured body ownership (Q1. It seemed as if I were feeling the touch of the paintbrush in the location where I saw the rubber hand touched. Q2. It seemed as though the touch I felt was caused by the paintbrush touching the rubber hand. Q3 I felt as if the rubber hand was my hand) and one further question was a control item to test the response bias (Q4. It seemed as if I might have two right hands or arms). The 7-item Likert scale was changed to an 11-item scale as it was applied in several RHI studies (e.g.50,52). Participants responded to each statement by choosing a number ranging from 0 (‘strongly disagree’) to 10 (‘strongly agree’). The reliability of the three test questions was tested for both hands in the synchronous and asynchronous conditions (right hand synchronous condition: Crombach’s alpha = 0.877; right hand asynchronous condition: Crombach’s alpha = 0.672; left hand synchronous condition: Crombach’s alpha = 0.826; left hand asynchronous condition: Crombach’s alpha = 0.799). The scores on the questions assessing body ownership (Q1–Q3) were used to calculate an illusion index by adding up the differences between the synchronous and asynchronous conditions computed separately for the right and left hands (see the formula below):
Results greater than zero indicated that the participant experienced a greater RHI during the synchronous condition than the asynchronous condition. The illusion index was used in the statistical analysis to investigate which of the brain structures are related to body ownership.
Analysis 2 (proprioceptive drift)
A similar method was used to calculate a proprioceptive drift index:
MRI data collection and analysis
MRI data collection and the analysis of MRI measurements were performed on a 3 Tesla MR scanner (Siemens Magnetom Trio Tim System, SiemensAG, Erlangen, Germany) with a 12-channel head coil. Isotropic 3D T1-weighted sagittal magnetization-prepared rapid acquisition with gradient echo images were used for the volumetric analysis (repetition time = 2530 ms, echo time = 3.37 ms, inversion time = 1100 ms, slice thickness = 1 mm, slice number = 176, flip angle = 7°, receiver bandwidth = 200 Hz/pixel, field of view = 256 × 256 mm2, 256 × 256 matrix).
For the cortical reconstruction and volumetric segmentation of the images, Freesurfer v6.0 was used (http://surfer.nmr.mgh.harvard.edu/). This software allows us to assess the thickness of predefined brain structures in a large number of subjects53,54. Freesurfer’s semi-automatic anatomical processing scripts (autorecon 1, 2, and 3) were executed on all MR measurements. A visual check was performed for all subjects by an experienced MR expert who participated in our previous studies50,55. Error corrections were applied when indicated according to the recommended pipeline (https://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction) with either changing the thresholds or adding reference points to assist the software to conduct a more precise evaluation of the images. In total, four participants’ images required corrections. Since the participants were healthy and young university students, they all tolerated well the experimental situation and no motion was observed on the images. Along with this, no participants were excluded due to poor data quality. We conducted the analysis of 16 brain areas bilaterally, which were selected based on previous body ownership-related researches: STG39,40, MTG41, inferior temporal gyrus42, precuneus39,40, SMG19,20, precentral and postcentral gyri18, superior parietal gyrus43,44, inferior parietal gyrus45, lateral and medial OFC46, rostral anterior cingulate cortex18, pars opercularis45,47,48, LOC14, and insula3.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). A p value of less than 0.05 was considered statistically significant. Repeated measures ANOVAs (rANOVAs) with within-subject factors of Side (right vs. left) and Condition (synchronous vs. asynchronous) were performed for the mean questionnaire score (i.e., the mean of scores on Q1, Q2, and Q3) and the proprioceptive drift. Effect sizes were calculated using partial eta squared (ηp2).
The associations between body ownership (proprioceptive drift and body ownership) and cortical thickness were analyzed by a series of multiple linear regression analysis. We created separate models for each brain structure, in which body ownership indexes were the dependent variables and the cortical thickness of the brain structures were the independent variables. In addition, age, biological sex and head size (i.e.: intracranial volume (ICV)55) were entered as covariates into the models.
Proprioceptive drifts related to left and right hemispheres were evaluated separately. Since some of the regions of interest showed significant association with ICV, head size correction was carried out by entering participants’ ICV as an additional independent variable into the models55. To ensure the assumptions of linear regression were not violated, we followed the guidelines of56. Normality of residuals was assessed upon QQ plot inspection. Residuals were examined for the presence of outliers (i.e. residuals outside ± 3 SDs) too. Independence of errors was tested by the Durbin-Watson test. Multicollinearity was assessed using the variance inflation factor (VIF, i.e.: VIF values under 10 indicated the absence of multicollinearity). Homoscedasticity was tested by the Breusch-Pagan test. The Benjamini–Hochberg false discovery rate (FDR) procedure was used to correct for multiple testing57,58,59; the q was set at 10%. Based on previous results our main interests were the right hemispheric brain areas, but with an exploratory purpose we analyzed the left hemispheric areas as well. Therefore, we applied the Benjamini–Hochberg correction on the analyzes of the right and the left hemispheric areas separately (Supplementary material).
Results
Behavioral analysis
Analysis 1 (subjective ratings)
When investigating the mean questionnaire scores, the main effect for the condition was significant (F(1,66) = 92.492, p < 0.001, ηp2 = 0.584). Participants reported a significantly stronger illusion in the synchronous condition than in the asynchronous one. The mean questionnaire scores were higher in the synchronous condition on both the right (synchronous condition: mean = 13.112 SD = 8.28; asynchronous condition: mean = 5.88 SD = 5.65) and the left hands (synchronous condition: mean = 14.179 SD = 7.75; asynchronous condition: mean = 7.463 SD = 7.01). The main effect for Side was not significant (F(1,66) = 2.942, p = 0.091). The Condition x Side interaction was also not significant (F(1,66) = 0.258, p = 0.613).
We also analyzed if participants scored higher on the body ownership questions than on the control question using a paired sample t test. For the analysis, we used an average test question score which we calculated from the aforementioned illusion index divided by three; the scores on the control questions were integrated in a similar way:
The analysis revealed that participants scored significantly higher on the test questions than on the control questions (t(66) = − 6.313 p < 0.001 Cohen’s d = 0.771; test questions mean score = 4.652 SD = 3.96; control questions mean score = 1.015 SD = 3.27).
For the analysis between proprioceptive drift and the subjective feeling of ownership, we used Spearman’s Correlation as the data did not fulfill bivariate normality. The analysis showed a positive correlation between the two variables (r = 0.305, p = 0.013).
Analysis 2 (proprioceptive drift)
For the analysis of proprioceptive drift, we used a 2 × 2 rANOVA in which the main effect for the condition was significant (F(1,66) = 4.582, p = 0.036, ηp2 = 0.066). The mean drift was higher in the synchronous condition on both the right (synchronous condition: mean = 0.518 SD = 5.02; asynchronous condition: mean = − 0.503 SD = 4.17) and the left hands (synchronous condition: mean = 0.827 SD = 4.50; asynchronous condition: mean = 0.356 SD = 3.86, see Fig. 1.). The main effect for Side was not significant (F(1,66) = 1.516, p = 0.223). The Condition x Side interaction was also not significant (F(1,66) = 0.841, p = 0.363).
Cortical thickness and body ownership
Analysis 1 (subjective ratings)
Body ownership index was positively associated with the cortical thickness of the bilateral MTG, the bilateral STG, the bilateral postcentral gyrus, the left LOC, the bilateral precuneus, the bilateral SMG, the left medial OFC, and the right insula (see Table 1 and Fig. 2. for summary). All the left hemispheric brain structures (MTG, STG, postcentral gyrus, LOC, precuneus, SMG, and the medial OFC) survived the Benjamini–Hochberg correction.
Analysis 2 (proprioceptive drift)
Proprioceptive drift index was negatively associated with the cortical thickness of the left transverse temporal- and, the bilateral precentral gyri, and the right MTG; however, only the left transverse temporal gyrus survived the Benjamini–Hochberg correction (see Table 2 for summary).
Discussion
The aim of this study was to investigate whether the inter-individual differences of body ownership observed in RHI reflect the anatomical brain structure in terms of cortical thickness. We evoked the RHI in an experimental paradigm to measure both the subjective and objective aspects of body ownership. The behavioural analysis of the RHI, in line with previous studies1,3,4, illustrated that the subjective feeling of ownership was significantly stronger on both right and left hands after synchronous stimulation compared to asynchronous stimulation. With regard to the proprioceptive drift, we observed a significant difference between synchronous and asynchronous stimulations for both hands, as per our expectations. As suggested by the extant literature4,5,7 we also found a positive correlation between the subjective body ownership experience and the proprioceptive drift, although this was a weak association.
We found a positive relationship between the self-report of the subjective experience of body ownership during the RHI and the cortical thickness of the left LOC (considered as part of the EBA), bilateral MTG, and the bilateral precuneus. The EBA is a region selectively involved in the visual processing of the whole body14 and body parts60,61, but not faces14. It is suggested to be involved in the identification of individuals in situations in which the face is not visible or recognizable14. The EBA is also considered to be part of the left-lateralized praxis representation network (including regions of the parietal, frontal, and temporal cortex) and contributes to the semantic processing, as the EBA is sensitive to the meaning of symbolic gestures62,63. In more detail, the EBA with the left MTG shows activation for the visual processing of hand gestures providing the meaning of actions and representing the semantic ‘what’ of visually processed actions41.
Self-report scores on body ownership questions were positively correlated with the cortical thickness of the bilateral postcentral gyrus, SMG, STG, and the precuneus, which have previously been shown to be related to body ownership experience39,40. The subjective experience of body ownership is facilitated by the activation of the STG and SMG; these areas are known to be involved in the sense of agency as well, that is strongly related to body ownership64. The precuneus is also involved in self-other differentiation and is a part of a self-referential network, along with the superior frontal gyrus and the posterior cingulate46.
The subjective feeling of ownership positively correlated with the cortical thickness of the right insula, which has a pivotal role in multisensory integration21. This finding is in line with previous studies, which have indicated a positive correlation between the right insula and the strength of the RHI assessed by self-report (e.g.3). With the exception of its involvement in hand ownership, the right insula is part of a right-hemispheric predominant brain network which encodes two components of self-consciousness: self-location and first-person perspective65. In addition, we found the existence of a positive relationship between the self-reports of body ownership and the cortical thickness of the left medial OFC. The orbitofrontal region is part of an anatomical and functional unit, called the cortical midline structures (CMS)46. The CMS are involved in self-referential processing, which is suggested to play an intermediary role between sensory and higher-order cognitive processes66.
Our findings show that the individual differences of the two body ownership measurements induced by RHI are associated with the structural anatomy of different brain areas. More specifically, proprioceptive drift was negatively correlated with the cortical thickness of regions such as the precentral gyrus, the transverse temporal gyrus, and the MTG, although only the transverse temporal gyrus survived the Benjamini–Hochberg correction. The aforementioned areas have been associated with multisensory integration18, auditory processing, sensory processing, and higher-order self-related functions18,41. On the other hand, the subjective experience of body ownership intensity was correlated with the cortical thickness of areas that are linked to multisensory integration processing (STG, SMT)10,19, the visual processing of the body (LOC)14, and higher order self-related functions (OFC and MTG)41,46. The analysis of cortical thickness supports the dissociation of the subjective and objective aspects of the RHI.
Previous studies showed, that the precentral gyrus is an important contributor to the body ownership experience over the rubber hand67. In line with this the majority of fMRI studies focusing on bodily illusions emphasized the link between the precentral gyrus (mostly the premotor cluster of it) and the subjectively rated strength of ownership of a fake/virtual body or body parts1,13,68, thus suggesting that the activity in this area reflects changes in the subjective feeling of body ownership. Interestingly, however, we failed to identify a relationship between the thickness of the left precentral region and the strength of the illusion, as measured by the questionnaire. Instead, the thickness of the bilateral precentral area was negatively associated with the proprioceptive drift. The precentral gyrus is known to be part of a region where the number of “peripersonal neurons” is high, and it is usually referred to as the polysensory zone69. The neurons in this area respond to visual, tactile, and auditory stimuli, and also play a role in defensive behavior within the peripersonal space70,71.
Additionally, we found a negative correlation between the thickness of the right MTG and the proprioceptive drift. The MTG is known to have a role in the visual processing of hand gestures41. According to our results, it might be the case that those who have a smaller cortical thickness in the MTG have difficulties with the visual processing of hand movements, which would result in a larger proprioceptive drift. In the absence of more convincing evidence, however, this explanation should be treated with caution.
Besides its significant contributions, the current study has a few limitations. With respect to the subjective feeling of body ownership and the proprioceptive drift, we found significant correlations with the cortical thickness of multiple brain areas; however, very few of them survived the Benjamini–Hochberg correction. Moreover, this study was correlational in nature and provides no insight into the causal relationship between the measured factors. Furthermore, our participants were all young university students, hence these results cannot be generalized to the entire population. In addition, due to the high variability on our data it would be beneficial to expand our research and include more participants in the study. On this note, the results need to be interpreted with caution. Nonetheless, this study may still shed light on new perspectives for further considerations in body ownership research (e.g., studying clinical populations).
Conclusion
In sum, our findings support the view that the inter-individual differences of the body ownership experience may not just appear in differences in brain function but are also pronounced in the anatomical brain structure in terms of cortical thickness. In the present study, we investigated the objective and the subjective aspects of body ownership, both of which were found to be associated with the cortical thickness of areas previously shown to be functionally related to body ownership. The structural correlates of proprioceptive drift (i.e., the objective aspect) involved areas previously reported to be responsible for multisensory integration, action and movement perception, and body awareness; on the other hand, the subjective experience of body ownership correlated with the structural measures of areas involved in visual processing of the body, multisensory integration processing, somatosensory processing, and higher-order functions. Thus, our results are in line with the findings of functional neuroimaging studies and suggest that individual differences in body ownership experiences can also manifest in differences in the anatomic brain structure.
References
Ehrsson, H. H., Spence, C. & Passingham, R. E. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science (80-) 305, 875–877 (2004).
Guterstam, A., Björnsdotter, M., Gentile, G. & Ehrsson, H. H. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr. Biol. 25, 1416–1425 (2015).
Tsakiris, M., Hesse, M. D., Boy, C., Haggard, P. & Fink, G. R. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cereb. Cortex 17, 2235–2244 (2007).
Botvinick, M. & Cohen, J. D. Rubber hand ‘feels’ what eyes see. Nature 391, 756 (1998).
Tsakiris, M. & Haggard, P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 31, 80–91 (2005).
Longo, M. R., Schüür, F., Kammers, M. P. M., Tsakiris, M. & Haggard, P. What is embodiment? A psychometric approach. Cognition 107, 978–998 (2008).
Ehrsson, H. H. The concept of body ownership and its relation to multisensory integration. 775–792 (2011).
Rohde, M., Luca, M. & Ernst, M. O. The rubber hand illusion: Feeling of ownership and proprioceptive drift do not go hand in hand. PLoS ONE 6, e21659 (2011).
Serino, A. et al. Bodily ownership and self-location: Components of bodily self-consciousness. Conscious. Cogn. 22, 1239–1252 (2013).
Brozzoli, C., Gentile, G. & Henrik-Ehrsson, H. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. J. Neurosci. 32, 14573–14582 (2012).
Tsakiris, M., Longo, M. R. & Haggard, P. Having a body versus moving your body: Neural signatures of agency and body-ownership. Neuropsychologia 48, 2740–2749 (2010).
Ionta, S. et al. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 70, 363–374 (2011).
Petkova, V. I. et al. From part- to whole-body ownership in the multisensory brain. Curr. Biol. 21, 1118–1122 (2011).
Downing, P. & Kanwisher, N. A cortical area specialized for visual processing of the human body. J. Vis. 1, 341 (2001).
Limanowski, J. & Blankenburg, F. That’s not quite me: Limb ownership encoding in the brain. Soc. Cogn. Affect. Neurosci. 11, 1130–1140 (2016).
Giabbiconi, C. M., Trujillo-Barreto, N. J., Gruber, T. & Müller, M. M. Sustained spatial attention to vibration is mediated in primary somatosensory cortex. Neuroimage 35, 255–262 (2007).
Ploner, M., Schmitz, F., Freund, H. J. & Schnitzler, A. Differential organization of touch and pain in human primary somatosensory cortex. J. Neurophysiol. 83, 1770–1776 (2000).
Kenzie, J. M., Ben-Shabat, E., Lamp, G., Dukelow, S. P. & Carey, L. M. Illusory limb movements activate different brain networks than imposed limb movements: An ALE meta-analysis. Brain Imaging Behav. 12, 919–930 (2018).
Blanke, O. & Metzinger, T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 13, 7–13 (2009).
Lopez, C., Halje, P. & Blanke, O. Body ownership and embodiment: Vestibular and multisensory mechanisms. Neurophysiol. Clin. 38, 149–161 (2008).
Craig, A. D., & Craig, A. D. How do you feel--now? The anterior insula and human awareness. Nature reviews neuroscience. 10(1), 59–70 (2009).
Tsakiris, M. The multisensory basis of the self: From body to identity to others. Q. J. Exp. Psychol. 70, 597–609 (2017).
Hach, S. & Schütz-Bosbach, S. In (or outside of) your neck of the woods: Laterality in spatial body representation. Front. Psychol. 5, 1–12 (2014).
Tsakiris, M., Tajadura-Jiménez, A. & Costantini, M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. B Biol. Sci. 278, 2470–2476 (2011).
Marotta, A., Tinazzi, M., Cavedini, C., Zampini, M. & Fiorio, M. Individual differences in the rubber hand illusion are related to sensory suggestibility. PLoS ONE 11, 1–12 (2016).
Kanayama, N. et al. Subjectivity of the anomalous sense of self is represented in gray matter volume in the brain. Front. Hum. Neurosci. 11, 1–10 (2017).
Hilti, L. M. et al. The desire for healthy limb amputation: Structural brain correlates and clinical features of xenomelia. Brain 136, 318–329 (2013).
King, J. A. et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol. Psychiatry 77, 624–632 (2015).
Bernardoni, F. et al. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. Neuroimage 130, 214–222 (2016).
Frank, G. K., Shott, M. E., Hagman, J. O. & Mittal, V. A. Taste reward circuitry related brain structures characterise ill and recovered anorexia nervosa and bulimia nervosa. Am. J. Psychiatry 170, 1152–1160 (2013).
Lavagnino, L. et al. Cortical thickness patterns as state biomarker of anorexia nervosa. Int. J. Eat. Disord. 51, 241–249 (2018).
Cardinale, F. et al. Validation of freesurfer-estimated brain cortical thickness: Comparison with histologic measurements. Neuroinformatics 12, 535–542 (2014).
Schultz, C. C. et al. The visual cortex in schizophrenia: Alterations of gyrification rather than cortical thickness—A combined cortical shape analysis. Brain Struct. Funct. 218, 51–58 (2013).
Fujito, R. et al. Musical deficits and cortical thickness in people with schizophrenia. Schizophr. Res. 197, 233–239 (2018).
Nanetti, L. et al. Cortical thickness, stance control, and arithmetic skill: An exploratory study in premanifest Huntington disease. Park. Relat. Disord. 51, 17–23 (2018).
Johnson, E. B. et al. The impact of occipital lobe cortical thickness on cognitive task performance: An investigation in Huntington’s Disease. Neuropsychologia 79, 138–146 (2015).
Boutzoukas, E. M. et al. Cortical thickness in childhood left focal epilepsy: Thinning beyond the seizure focus. Epilepsy Behav. 102, 106825 (2020).
Kamson, D. O. et al. Cortical thickness asymmetries and surgical outcome in neocortical epilepsy. J. Neurol. Sci. 368, 97–103 (2016).
Blanke, O., Landis, T., Spinelli, L. & Seeck, M. Out-of-body experience and autoscopy of neurological origin. Brain 127, 243–258 (2004).
Tsakiris, M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia 48, 703–712 (2010).
Kubiak, A. & Króliczak, G. Left extrastriate body area is sensitive to the meaning of symbolic gesture: Evidence from fMRI repetition suppression. Sci. Rep. 6, 1–13 (2016).
Gross, C. G. Single neuron studies of inferior temporal cortex. Neuropsychologia 46, 841–852 (2008).
Grefkes, C., Ritzl, A., Zilles, K. & Fink, G. R. Human medial intraparietal cortex subserves visuomotor coordinate transformation. Neuroimage 23, 1494–1506 (2004).
Creem-Regehr, S. H. Sensory-motor and cognitive functions of the human posterior parietal cortex involved in manual actions. Neurobiol. Learn. Mem. 91, 166–171 (2009).
Naito, E. & Ehrsson, H. H. Somatic sensation of hand-object interactive movement is associated with activity in the left inferior parietal cortex. J. Neurosci. 26, 3783–3790 (2006).
Northoff, G. & Bermpohl, F. Cortical midline structures and the self. Trends Cogn. Sci. 8, 102–107 (2004).
Johnson-Frey, S. H. et al. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron 39, 1053–1058 (2003).
De Lussanet, M. H. E. et al. Interaction of visual hemifield and body view in biological motion perception. Eur. J. Neurosci. 27, 514–522 (2008).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Hegedüs, G. et al. The rubber hand illusion increases heat pain threshold. Eur. J. Pain 18, 1173–1181 (2014).
Kállai, J. et al. Multisensory integration and age-dependent sensitivity to body representation modification induced by the rubber hand illusion. Cogn. Process. 18, 349–357 (2017).
Horváth, Á. et al. Proprioception but not cardiac interoception is related to the rubber hand illusion. Cortex 132, 361–373 (2020).
Fischl, B. et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, 69–84 (2004).
Pardoe, H. R., Pell, G. S., Abbott, D. F. & Jackson, G. D. Hippocampal volume assessment in temporal lobe epilepsy: How good is automated segmentation?. Epilepsia 50, 2586–2592 (2009).
Perlaki, G. et al. Are there any gender differences in the hippocampus volume after head-size correction? A volumetric and voxel-based morphometric study. Neurosci. Lett. 570, 119–123 (2014).
Chan, Y. H. Biostatistics 201: Linear regression analysis. Singap. Med. J. 45, 55–61 (2004).
Glickman, M. E., Rao, S. R. & Schultz, M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 67, 850–857 (2014).
Verhoeven, K. J. F., Simonsen, K. L. & McIntyre, L. Erratum: Implementing false discovery rate control: Increasing your power (Oikos (2005) 108 (643–647)). Oikos 109, 208 (2005).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Downing, P. E. & Peelen, M. V. Body selectivity in occipitotemporal cortex: Causal evidence. Neuropsychologia 83, 138–148 (2016).
Peelen, M. V. & Downing, P. E. The neural basis of visual body perception. Nat. Rev. Neurosci. 8, 636–648 (2007).
Amoruso, L., Couto, B. & Ibáñez, A. Beyond extrastriate body area (EBA) and fusiform body area (FBA): Context integration in the meaning of actions. Front. Hum. Neurosci. 5, 1–3 (2011).
Lingnau, A. & Downing, P. E. The lateral occipitotemporal cortex in action. Trends Cogn. Sci. 19, 268–277 (2015).
Seghezzi, S., Giannini, G. & Zapparoli, L. Neurofunctional correlates of body-ownership and sense of agency: A meta-analytical account of self-consciousness. Cortex 121, 169–178 (2019).
Ionta, S., Martuzzi, R., Salomon, R. & Blanke, O. The brain network reflecting bodily self-consciousness: A functional connectivity study. Soc. Cogn. Affect. Neurosci. 9, 1904–1913 (2014).
Northoff, G. et al. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage 31, 440–457 (2006).
Grivaz, P., Blanke, O. & Serino, A. Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. Neuroimage 147, 602–618 (2017).
Ehrsson, H. H., Holmes, N. P. & Passingham, R. E. Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas. J. Neurosci. 25, 10564–10573 (2005).
Graziano, M. The spaces between us: a story of neuroscience, evolution, and human nature. (Oxford University Press., 2017)
Cooke, D. F. & Graziano, M. S. A. Sensorimotor integration in the precentral gyrus: Polysensory neurons and defensive movements. J. Neurophysiol. 91, 1648–1660 (2004).
Macaluso, E. & Maravita, A. The representation of space near the body through touch and vision. Neuropsychologia 48, 782–795 (2010).
Acknowledgements
The project has been supported by the European Union, co-financed by the European Social Fund. Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs. Grant number: EFOP-3.6.1.-16-2016-00004. This study was supported by the Hungarian Brain Research Program 20017-1.2.1-NKP-2017-00002 government-based fund. The study was supported by the NKFI K-120334 grant. Our research was partly financed by the National Research, Development and Innovation Fund of Hungary, financed under the 2020-4.1.1-TKP2020 funding scheme. SAN was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, ÚNKP-20-5-PTE-715 New National Excellence Program of the Ministry for Innovation and Technology and PTE ÁOK-KA-2020-08.
Author information
Authors and Affiliations
Contributions
T.M.B., B.L. and G.D. wrote the main manuscript text, A.M. prepared figures and conducted statistical analyses, J.J., G.P., G.O. and S.A.N. analysed MRI data, J.K. and T.S. designed the experiment, O.I. and A.N.Z. analyzed questionnaire and behavioural data, E.K. collected behavioural data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matuz-Budai, T., Lábadi, B., Kohn, E. et al. Individual differences in the experience of body ownership are related to cortical thickness. Sci Rep 12, 808 (2022). https://doi.org/10.1038/s41598-021-04720-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04720-8
- Springer Nature Limited