Abstract
Regional optimisation of malaria vector control approaches requires detailed understanding both of the species composition of Anopheles mosquito communities, and how they vary over spatial and temporal scales. Knowledge of vector community dynamics is particularly important in settings where ecohydrological conditions fluctuate seasonally and inter-annually, such as the Barotse floodplain of the upper Zambezi river. DNA barcoding of anopheline larvae sampled in the 2019 wet season revealed the predominance of secondary vector species, with An. coustani comprising > 80% of sampled larvae and distributed ubiquitously across all ecological zones. Extensive larval sampling, plus a smaller survey of adult mosquitoes, identified geographic clusters of primary vectors, but represented only 2% of anopheline larvae. Comparisons with larval surveys in 2017/2018 and a contemporaneous independent 5-year dataset from adult trapping corroborated this paucity of primary vectors across years, and the consistent numerical dominance of An. coustani and other secondary vectors in both dry and wet seasons, despite substantial inter-annual variation in hydrological conditions. This marked temporal consistency of spatial distribution and anopheline community composition presents an opportunity to target predominant secondary vectors outdoors. Larval source management should be considered, alongside prevalent indoor-based approaches, amongst a diversification of vector control approaches to more effectively combat residual malaria transmission.
Similar content being viewed by others
Introduction
Malaria elimination strategies have been described as demonstrating a ‘monolithic reliance’ on indoor vector control, based on classical preferences of major Anopheles mosquito vectors for feeding on humans indoors at night1. While six major anopheline vector species are responsible for 95% of transmission across sub-Saharan Africa2 (‘primary’ vectors), such control strategies may be compromised by the presence of other feeding and resting behaviours3 such as propensities of minor (‘secondary’) vector species for outdoor and/or early biting. This may facilitate residual malaria transmission despite the decline of primary vector species4,5; in some contexts, species deemed ‘secondary’ vectors when considered at a continental scale can act locally as primary vectors and can sustain comparable levels of malaria transmission4,6. Indoor-focused approaches such as long-lasting insecticide nets (LLINs) and indoor residual spraying (IRS) have been found to not only increase insecticide resistance7,8 but also to drive within-species shifts in behaviour9, plus shifts in vector community composition10,11,12,13 towards taxa whose behaviours enable them to elude such interventions. As well as responding to interventions, anopheline communities are subject to the influence of environmental conditions over various spatial and temporal scales. Potential future shifts in temperature14 and hydrological regimes15 may profoundly influence the distribution of malaria vectors and consequent transmission dynamics16,17. The twin threats posed by residual transmission and potential climate change effects on mosquito vector communities have been highlighted14,18 as major challenges to future malaria control, and emphasise the importance of entomological surveillance19.
Entomological surveillance is vital to monitor vector community responses to interventions and climate change, and to characterise the species assemblage to ensure that interventions are locally relevant20. The reliable identification of vector species is increasingly achieved through genetic barcoding alongside traditional morphology21, permitting the association of bionomic traits with individual species22,23,24 which enables an increasing diversity of interventions to be tailored to specific vector behaviours25. Genetic barcoding is also a powerful means for species identification of larval stages, which are frequently morphologically indistinguishable, with direct application to larval source management (LSM). Diverse LSM approaches26,27,28,29,30,31 have been successfully implemented over small scales of time and space, but operational deployment at large scale is challenging32. To optimise the timing and location of LSM implementation, it is vital to understand seasonal variations in mosquito community composition and distribution at the appropriate temporal and spatial scale33,34,35.
Cross et al.36 employed DNA barcoding of anopheline larvae sampled in an extensive spatial survey to reveal an unexpectedly high prevalence of secondary vector species in a region of persistent residual malaria transmission in western Zambia, with partitioning of species across ecological zones and stability of patterns observed over dry and wet seasons. The present study aims to build on that work by combining geographically extensive surveys of larval habitats and DNA barcoding of larvae sampled in the same region in the following year to assess inter-annual consistency of anopheline vector community composition, ecological zonation and seasonal patterns. Furthermore, barcode data for adults collected both by de novo sampling in this study, and previously by Orba et al. (201837 and 2021, pers. comm.) and Wastika et al.38 over a contemporaneous 5-year period in the same region of western Zambia are assessed for linking of adult and larval patterns. Finally, as the sampled period spanned a wet season in 2019 that was unusually early and dry, and followed by the most severe drought for at least 20 years39, we also discuss the results within a climate change context given the increased frequency and severity of droughts predicted for the region by climate change models.
Results
Larval habitat sampling
Sampling was undertaken in May–June 2019 after the peak of the wet season, comprising 32 transects totalling over 11 km (Fig. 1). Transects ranged from 68 to 647 m in length (mean ± standard deviation (SD): 348 ± 146 m); water was encountered in a similar proportion of transect points in 2019 as in the previous wet season (79.5% and 80.1%, respectively), and the average number of dips taken per wet transect point in 2019 was comparable to 2018 (22 ± 8.2 and 23 ± 5.4, respectively).
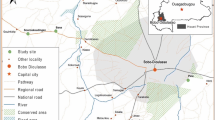
Ecological zones and entomological survey locations in Western Province, Zambia. 1 = Zambezi floodplain; 2 = Floodplain edge; 3 = Luena flats; 4 = Lui valley; 5 = dambos. Larval surveillance and adult mosquito trapping undertaken after the peak of the 2019 wet season. Pink rectangle in inset indicates study map extent. Map drawn in QGIS94 (v. 3.18.3-Zürich; https://www.qgis.org); basemap: ESRI Shaded Relief (2021).
Anopheles larvae were encountered across a diverse range of larval habitats, from flooded grasslands to seepage zones, and permanent water bodies in dambos to open, transient pools on sandy substrates. Water bodies were predominantly vegetated (see Fig. 3 in Hardy et al.40), with common vegetation types including grasses (e.g. Echinochloa spp. and Oryza spp.) and papyrus (Cyperus spp.); landscape and vegetation have been characterised in detail elsewhere40,41,42,43. Anopheles occurred in 74.1% of wet transect points in 2019, which was significantly lower than the previous wet season (85%; Odds Ratio (OR) 0.505, 95% CI 0.330–0.771, p < 0.001). An average of 1.31 (± 3.8) mosquito larvae were encountered per dip, of which approximately a quarter were anophelines (mean 0.32 ± 0.91 per dip). There was no significant difference between total anophelines per transect point between 2018 and 2019 wet seasons (median of 2 per 10 dips for both years; independent samples median (ISM) test 0.95, df 1, p = 0.33), although the proportion of larvae classified as late stage was significantly higher in 2019 than 2018 (36% and 23%, respectively; OR 1.9379, 95% CI 1.043–3.602, p = 0.036).
Ecological zone comparisons are made between total abundance values per transect point, standardised per 10 dips due to variation in water body size necessitating significant differences between ecological zones in total dips per transect point (ISM test 31.401, df 4, p < 0.001). Anopheline larvae were encountered in all ecological zones across the study area, with up to 20.5 per transect point (standardised per 10 dips). The median standardised total anophelines per transect point differed significantly between ecological zones (ISM test 21.536, df 4, p < 0.001), with fewer anophelines in dambos than in any other zone, and more in Lui valley transect points than in the Zambezi floodplain (stepwise step-down (SSD) post-hoc analysis; adjusted (adj) for multiple comparisons p < 0.05; Fig. 2).
Anopheline larval abundance within 5 defined ecological zones (see Fig. 1 and Methods) in western Zambia. 1 = Zambezi floodplain; 2 = Floodplain edge; 3 = Luena flats; 4 = Lui valley; 5 = dambos. Total value per transect point (standardised per 10 dips) represented by dots; boxplot indicates interquartile range and median (horizontal line), with whiskers extending to minimum and maximum values within 1.5 times IQR. Transect points n = 220; anophelines n = 1557. Median values for ecological zones that do not share a letter are significantly different (independent-samples median test with stepwise step-down comparisons; adjusted p < 0.001).
Genetic identification: larvae
Over 50% of field-surveyed anopheline larvae were collected for genetic analysis (n = 855/1557), and a viable DNA sequence from COI and/or ITS2 regions was obtained from 658 specimens (Table 1; the remainder did not amplify using COI or ITS2 primers, potentially due to sub-optimal preservation of DNA in the field). More than two-thirds of these specimens (n = 443) were identified to species based on both COI and ITS2 sequences with ≥ 95% similarity to GenBank accessions. An additional 20% of specimens (n = 129) matched with ≥ 95% identity with GenBank COI sequences, but their corresponding ITS2 sequences returned no match at ≥ 95% similarity with a GenBank sequence. Each remaining specimen (n = 86) was assigned a species identity based on an above-threshold match of either its COI or ITS2 sequence to GenBank accessions, with the exception of 18 specimens (2.7% of the total) whose closest matches were to Anopheles sequences but at similarities below the threshold; these 18 were subsequently designated ‘unknown Anopheles species’. Maximum likelihood (ML) tree analysis of the 119 resolved COI haplotypes corroborated the species identities assigned from matches to GenBank sequences by demonstrating statistically well-supported clusters nested with reference specimen sequences of known morphological identity (Fig. 3; Supplementary Fig. S1). An. gambiae complex species clustered together but were not resolved into species from COI phylogeny.
Maximum Likelihood phylogenetic tree of representative cytochrome c oxidase I (COI) sequences from Anopheles sampled in western Zambia. Consensus tree constructed in MEGA X from haplotypes of a 300 bp fragment of the COI region of mitochondrial DNA, using 100 bootstrap replicates. Non-singleton haplotypes preferred, where available; up to three haplotypes selected at random per taxon. Species identities assigned from > 95% BLAST similarity to COI and/or ITS2 sequences published on GenBank are indicated with coloured dots and prefixed ‘Hap’. Sequences from adult mosquitoes caught in the region (Orba et al., 2021, pers. comm.) are prefixed ‘ORBA’; species name indicates morphologically-derived identity, while coloured box indicates molecularly-derived identity. Published reference sequences labelled with GenBank accession number and species name; suffix denotes source paper. ‘–C’ denotes Ciubotariu et al.44; ‘–L’ denotes Lobo et al.22; ‘–SL’ denotes St Laurent et al.23. Species also indicated with coloured underline. Tree drawn to scale; branch lengths measured in number of substitutions per site. Full tree available in Supplementary Information as Fig. S1.
Within the sample of 658 sequenced larvae, a large majority were identified as An. coustani (80.1%; Table 1; Fig. 4). Two distinct genetic clades could be further separated within An. coustani; the first consisted of specimens with both COI and ITS2 sequences matching with ≥ 95% identity to accessions for the species, whilst the second clade (comprising 22% of the total sample) consisted of specimens with above-threshold matches to COI reference sequences but below-threshold matches to any GenBank ITS2 sequence. This second An. coustani clade also forms a well-supported cluster in the ML tree (Fig. 3; for full ML tree see Supplementary Fig. S1). An. pharoensis was the next most abundant species at 10.9% (Fig. 4), followed by small numbers of An. gambiae sensu lato (2.1%), An. squamosus (2.0%), An. species O/1523 (1.7%), and 3 specimens (0.5%) designated as An. species UG3, signifying ‘unknown group 3’ based on similarity to designations made in southern and central Africa by Ciubotariu et al.44. An. gambiae sensu lato could be further resolved into An. arabiensis (1.1%) and An. gambiae sensu stricto (1.1%, hereafter An. gambiae) based on ITS2 sequences.
Abundance and species composition of larval anopheline community in western Zambia in two consecutive wet seasons (2018 and 2019). (a) Estimated total abundance calculated by applying species proportions from DNA sequence-identified subset of sampled larvae (see b) at each transect point to the total surveyed larvae per transect point, and summing for all transect points. (b) Proportion of specimens assigned species identities from matches to COI and/or ITS2 DNA barcode sequences from GenBank (2018: n = 748; 2019: n = 658). Significant differences between years in proportion of of specimens represented by each individual taxon indicated by asterisk(s); *p < 0.01; **p < 0.001 (z-test for independent proportions). Groups represented by hatched bars were not resolved to individual member species in 2018. ‘Unknown An. spp’ indicates that match to Anopheles reference sequences fell below 95% similarity threshold.
Geographic distribution of larvae
An. coustani was distributed ubiquitously across ecological zones (Fig. 5c). A significantly lower median per transect point was found in dambos than in the Zambezi and Luena floodplains and the Lui valley, and a significantly higher median in the Lui valley compared to the Zambezi floodplain and its edge (Fig. 5a; ISM test 26.150, df 4, p < 0.001; SSD adj p < 0.05). Within An. coustani, however, clade 1 exhibited a significantly lower median in the Lui valley (and dambos; median = 0) than all other habitats (Fig. 6; 0.6–1.592; ISM test 20.036, df 4, p < 0.001; SSD adj p < 0.05). Conversely, the clade 2 median was significantly higher in the Lui valley than all other ecological zones (Fig. 6; median 1.822 compared to 0–0.369; ISM test 35.518, df 4, p < 0.001; SSD adj p < 0.05). The median proportion of estimated An. coustani populations composed of clade 2 individuals was significantly higher in the Lui valley than all ecological zones except dambos, and the proportion in dambo populations exceeded those of Zambezi floodplain and floodplain edge populations (Fig. 6; ISM test 34.057, df 4, p < 0.001; SSD adj p < 0.05).
Distribution of anopheline larvae across ecological zones after peak of 2019 wet season. Species identities assigned from matches to COI and/or ITS2 sequences from GenBank. (a–b) Species abundance across ecological zones. Ecological zones as per Fig. 1 and boxplot format as per Fig. 2. Ecological zones not sharing a letter are significantly different; lower case letters = independent samples median test; upper case letters = Kruskal–Wallis distribution test; stepwise step-down comparisons with adj p < 0.001. (c-e) Spatial distribution of species across ecological zones (see Fig. 1). Symbol area proportional to estimated total larvae of mapped species per transect point (product of total anopheline count and taxa proportions in subsample), standardized per ten dips. Map drawn in QGIS94 (v. 3.18.3-Zürich; https://www.qgis.org); basemap: ESRI Shaded Relief (2020).
Composition of An. coustani clade 1 and clade 2 larval populations in western Zambia, 2019. (a) Abundance of clade across ecological zones. Boxplot represents interquartile range and median (vertical line) estimated total of each clade per 10 dips; whiskers extend to minimum and maximum values within 1.5 × IQR. Ecological zones as per Fig. 1. (b) Median proportion of clade 2 in An. coustani specimens. Calculated for all transect points where at least one clade present (n = 128); median proportion indicated by circles, using secondary axis; whiskers indicate IQR. Ecological zones not sharing a letter within two separate comparisons in (a) (LHS and RHS) and in (b) are significantly different; independent samples median test; stepwise step-down comparisons with adj p < 0.001.
An. pharoensis was also encountered in every ecological zone (Fig. 5d), albeit in fewer transect points (21%) than An. coustani (65%). Its distribution in Zambezi and Luena floodplains was significantly different from dambo and floodplain edge habitats (Fig. 5b; Kruskal–Wallis H = 21.392, p < 0.001; mean rank former > latter, SSD adj p < 0.05). The primary vector complex An. gambiae s.l. was found in floodplain edge (n = 2 An. gambiae), Luena flats (n = 1 An. gambiae) and Lui valley ecological zones (n = 6 An. arabiensis and n = 4 An. gambiae; Fig. 5e). An. squamosus was found in all ecological zones except for dambos (Fig. 5d), and small numbers of An. species O/15 were present in every ecological zone, occurring in 5% of wet transect points; there was no significant difference in distributions between ecological zones for either of these species. Three specimens of An. species UG3 were found, from samples in floodplain edge and Luena flats ecological zones (Fig. 5e). The peri-urban anopheline larval community sampled along Kambule stream (n = 19 transect points; 55 anophelines) exhibited similar composition to the overall sample community, with An. coustani accounting for 76% of the estimated species total, An. pharoensis 18%, and An. arabiensis and unknown An. species making up the remainder (4% and 2%, respectively).
Adult mosquito sample
Paired BG-Malaria traps were deployed at a total of 63 households across 18 villages over 9 trap nights. Traps ran overnight for c. 18 h (mean duration 17h41 ± 1h00; mean start 16:29 ± 0h42; end 10:11 ± 0h40). One hundred and twenty-six trap nights yielded 58 anophelines which were predominantly female (93%), with a considerable majority trapped outside (71%), including all males (n = 3; excluded from subsequent analyses). The distribution of catches amongst the traps was highly clustered; 32 traps contained one or more anophelines, and a single outside trap accounted for 25% of the total catch. Although modest in extent and compromised by the unusually dry wet season, we report and interpret these results as they coincided geographically and temporally with larval surveys.
Fifty-four adult female specimens were identified to 6 species from above-threshold matches to COI and/or ITS2 DNA sequences (Table 2), confirmed by high bootstrap support of clustering within phylogenetic ML tree analysis (Supplementary Fig. S1). An. species UG3 dominated the adult sample (46%), with the majority found outdoors, although a single outdoor trap accounted for half of this outdoor total. Over half of An. species UG3 specimens were identified by matches of both COI and ITS2 sequences to accessions linked to Jones45 and Ciubotariu et al.44, respectively, whilst the remaining specimens were identified from matches to either these ITS2 (24%) or COI (20%) sequences. ITS2 sequences permitted differentiation between species within the An. funestus group that could not be resolved based solely on COI sequences. The typically anthropophilic primary malaria vector species An. funestus (24% of sample) was predominantly trapped indoors (77% of specimens). An. gambiae adults were found exclusively indoors, whilst An. coustani, An. arabiensis and An. rivulorum (An. funestus group member) were found exclusively outdoors. One specimen was assigned ‘unknown An. species’. None of the adults were bloodfed or reported positive amplifications from PCR aimed at detecting P. falciparum sporozoites.
Geographic distribution of adult mosquitoes
Adult anopheline catches exhibited a high degree of within-village similarity, whilst varying substantially between villages (Fig. 7). Although An. species UG3 numerically accounted for almost half of the adults sampled, this was a consequence of an unusually high catch in one dambo village; it was found in one-third of villages where Anopheles adults were caught (n = 4 of 12). Samples from two villages in the Luena flats and one in a dambo were exclusively An. funestus, while the species was also found on the floodplain edge and the Lui valley. An. coustani was found in 4 villages, whilst An. gambiae adults were confined to 2 villages in the Lui valley; An. arabiensis and An. rivulorum occurred solely in one floodplain edge and one Zambezi floodplain village, respectively (Fig. 7).
Distribution of adult Anopheles sampled using BGM traps in western Zambia. Specimens (n = 54) identified from above-threshold matches of COI and/or ITS2 sequences to GenBank accessions. Pie chart area proportional to total catch per trap pair (indoor + outdoor). Ecological zones as per Fig. 1. Map drawn in QGIS94 (v. 3.18.3-Zürich; https://www.qgis.org); basemap: ESRI Shaded Relief (2020).
Inter-annual comparisons: 2017–2019
Despite the considerable difference in the magnitude and timing of the wet season between these years, the relative abundance of all anophelines in each ecological zone in 2019 was more consistent with the 2018 wet season than the dry season of 2017 (Fig. 8). An. coustani comprised a larger proportion of the sample in the 2019 wet season than in 2018, whilst An. pharoensis and An. squamosus combined, An. species O/15, and unknown An. species occupied a larger proportion in 2018 than in 2019 (Pearson χ2 150.325; df 5; p < 0.001 and post-hoc z-tests for independent proportions; p < 0.001; Fig. 4b). Comparisons between anopheline species distributions in the 2017 dry season (larvae36), after the peak of the 2018 wet season (larvae36), and after the peak of the 2019 wet season (adults and larvae) revealed considerable inter-annual consistency in the spatial distribution of some anopheline species where sampling effort coincided. For example, An. coustani adults were caught in 6 outdoor traps in 2019 (Fig. 7), and larvae of this species were found in the nearest wet transect point to each of these traps (Fig. 5c). The presence of An. coustani on 2019 transects was always preceded by the presence of An. coustani group larvae on the corresponding transect in the previous 2018 wet season, and usually also in the dry season in 2017 (see Fig. 5c and Cross et al.36). Four larval transect points contained An. gambiae in 2019 (Fig. 5e); half of these occurred within 100 m of 3 of the 4 households where adult An. gambiae were caught indoors in the Lui valley (Fig. 7). An. arabiensis larvae were also found in the same Lui valley location in 2019 (Fig. 5e), within 250 m of three transect points which contained An. gambiae s.l. larvae in 2017 (2017 sample identities were resolved to species complex level only, as ITS2 sequences were not then available for all specimens36). In the floodplain edge ecological zone, adult An. arabiensis were trapped outdoors within 250 m of a transect point where An. gambiae s.l. larvae were found in 201836. Two An. gambiae larvae were encountered in floodplain edge habitat in 2019 analogous to two floodplain edge locations where An. gambiae s.l. larvae occurred in the same season in 201836. Similarly, although An. species O/15 larvae were comparatively fewer in 2019, they occurred in Zambezi floodplain habitats comparable to those surveyed by adjacent transects in 2018; several 2019 occurrences (Fig. 5e) were within 600 m of where the species was encountered in the preceding wet season36. In the Luena flats, locations positive for An. species O/15 larvae on overlapping 2018 and 2019 transects occurred within 100 m of each other (Fig. 5e and Cross et al.36).
Distribution of Anopheles larvae across ecological zones in western Zambia. Sampling undertaken in the dry season of 2017 (light grey) and after peak of the wet season in 2018 (mid grey) and 2019 (dark grey). Total anopheline larvae per transect point (standardised per ten dips) represented by dots; boxplot indicates interquartile range and median (white horizontal line), with whiskers extending to minimum and maximum values within 1.5 times IQR. 2017: n = 407 transect points; 2018: n = 340; 2019: n = 220. Ecological zones as per Fig. 1.
Adult data derived from previous studies
Trapping of adult mosquitoes in Mongu district between 2014 and 2017 yielded 1829 anophelines mainly outdoors37,38, and additional trapping in 2018–19 provided an additional 596 anophelines (Orba et al., 2021, pers. comm.). The majority of anophelines in each of six sampling periods from 2014 to 2019 were identified as An. coustani, ranging from 49 to 100% of the sample, whilst 13% of the specimens across all periods were An. squamosus (Fig. 8). An. gambiae s.l. mosquitoes were the sole primary vector species detected, occurring only in 2017 when they constituted < 0.5% of the sample. Although anopheline species count data from these studies37,38 were not published, the data collected during the studies and subsequently (Orba et al., 2021; pers. comm.) are provided in the Supplementary Information to this paper (Supplementary Table 1), along with six COI gene sequences (Supplementary Dataset S1).
There was strong support for the morphological identification of adult An. coustani as the DNA sequences obtained from reference samples within the independent dataset (Orba et al., 2021, pers. comm.) clustered with sequences on GenBank and An. coustani sequences from the present study (Fig. 3; Supplementary Fig. S1). The time series of data indicates the consistent dominance of An. coustani in the region in both dry and wet seasons (Fig. 9).
Species composition of adult anophelines trapped in multiple locations in Mongu district, Zambia. Samples collected mainly outdoors using CDC and BG-Sentinel traps, and identified morphologically, with a subset of identities confirmed by sequencing of COI. Data sources: Orba et al.34, Wastika et al.35, Orba et al. (2021, pers. comm).
Discussion
Geographically extensive larval mosquito sampling was undertaken across multiple ecological zones in western Zambia over three years, including one dry and two wet seasons, and data were integrated with adult mosquito catches from this study and an independent series of collections over a longer timeframe37,38 (Orba et al., 2021, pers. comm.). This combined dataset revealed a marked temporal consistency in the composition of the Anopheles mosquito community across western Zambia, and also consistency in geographic distribution of species across larval habitats in the region. This spatial consistency is evident both at landscape scale, across large ecological zones in a study area over 3500 km2, and also at fine scale whereby larvae were frequently encountered within 10–100 s of metres of congruent records from a preceding study in the region36. The community consistency was even more notable given the significant variation in ecohydrological conditions between the 2018 wet season and the 2019 season surveyed in the present study. Barotseland experienced an anomalously early and unusually dry wet season in 2019, such that widespread drought in the ensuing dry season was the most intense and severe for at least 20 years39, whilst Zambezi River discharge in 2018 was the second highest recorded for almost 30 years36. Extensive surveys of Anopheles larvae across diverse habitats in this study, coupled with objective molecular identification to species revealed a paucity of primary vector species, with support for this finding provided by an independent dataset of adult mosquitoes caught in the study area in the same time period37,38 (Orba et al., 2021, pers. comm.; see Supplementary Table S1 & Dataset S1).
An. coustani was found to be by far the most ubiquitous and abundant species across the region, constituting an estimated 78% of all surveyed anopheline larvae (Fig. 4), compared with 52% of surveyed anophelines in the preceding 2018 wet season and 65% in the dry season of 2017 which comprised An. coustani group larvae36. Although An. coustani is often rare in entomological surveys24, in recent studies it has been found to dominate local anopheline communities at locations in the western Kenyan highlands46 and Madagascar47, and was the second most prevalent species at locations in Ethiopia48 and rural southeast Zambia49. The species was distributed ubiquitously across our study area, but with higher abundance in the Luena flats corresponding to a significantly higher median in this ecozone in the previous wet season36. The lower abundance of An. coustani in dambo habitats in this study is likely to reflect the comparatively lower encounter rate of all anophelines in this ecological zone in 2019, whilst in 2018 anopheline abundance in dambos was comparable to that of habitats in the Luena flats and Lui valley36. Whilst An. coustani is relatively understudied22,44, typical larval habitat preferences have been broadly characterised as permanent water bodies with clear water and aquatic/semi-aquatic vegetation50,51; it has been found in vegetated aquatic habitats in association with long grass52 which are a feature of the survey landscape in western Zambia, particularly in waterlogged areas of the Luena flats which retain water year-round40. Adult An. coustani group mosquitoes maintain a year-round presence in Kenya53 and Ethiopia, although they reached peak density in the drier months following the rainy season in the latter48,54; An. coustani abundance may have been higher in the current study than in surveys following the 2018 wet season36 due to the drier conditions experienced in western Zambia in 201939. Although the long-term data from adult trapping in the centre of our study area (Fig. 9) have a limited seasonal resolution, they indicate the persistent presence of the An. coustani; whilst abundance fluctuated between years, An. coustani was consistently predominant, providing strong corroboration for the findings from larval surveillance.
The An. coustani group includes multiple species, including An. coustani, An. crypticus, An. tenebrosus and An. ziemanni 51,55,56; some are morphologically indistinguishable in some life stages, and the use of molecular methods are advocated particularly to differentiate the cryptic An. crypticus from An. coustani 57. While COI and ITS2 sequences have been published for the other species, none are available for An. crypticus. In the current study corresponding ITS2 sequences were obtained from 99% of specimens identified as An. coustani based on their COI sequences. This ITS2 dataset revealed two distinct genetic clades within An. coustani in western Zambia: one whose ITS2 sequences matched GenBank An. coustani sequences with > 95% similarity, and another with ITS2 similarity to GenBank accessions of < 95%. There was a high level of spatial overlap between clades, but significant differences in the proportion of the two An. coustani clades between ecological zones raises the possibility that the clades occupy different ecological niches within the study area. Ciubotariu and colleagues44 also report this phenomenon amongst morphologically-identified adults, reporting a clade whose closest match for known An. coustani ITS2 sequences was ~ 80%. Members of both clades in that study were also reported to overlap spatially in northern Zambia and neighbouring D.R. Congo, and contained specimens positive for human blood meals and P. falciparum confirming their roles as secondary malaria vectors44. It is possible that An. coustani clade 2 in this study represents An. crypticus, although we note that our sequences do not match the ITS2 sequences of two ‘coustani-like’ species reported by Lobo et al.22 which could also represent the species.
The presence of An. pharoensis in 2019 (reported here) despite its absence in 201836 is most likely attributable to a difference in genetic resolution between studies rather than a demographic/biological signal, as species assignment in the previous study was based almost entirely on COI which confers less discriminatory power between An. pharoensis and An. squamosus than ITS221,24,58. The more extensive ITS2 sequencing performed here therefore permitted identification of An. pharoensis, and was strongly supported by phylogeny (Fig. 3; Supplementary Fig. S1). This artefact does not affect our main findings, as pooling of An. squamosus with An. pharoensis in 2019 did not alter the ecological zone partitioning exhibited by An. pharoensis alone. Significantly higher An. pharoensis abundance in Zambezi and Luena floodplain ecological zones in 2019 corresponded with significantly higher prevalence of An. squamosus (potentially including An pharoensis individuals) in these habitats and along the floodplain edge in 201836.
Eight of the nine anopheline species identified in this study have previously been implicated as malaria vectors, whilst the vector status of An. species UG3 and of our An. coustani clade 2 is unknown. In Kenya at least half of 17 species in one study tested positive for P. falciparum or were known vectors23, while 12/21 species in a more recent study were potential vectors24, and 12/18 in eastern Zambia22 tested positive for P. falciparum. The number of adult anophelines sampled in the present study was severely limited by the unseasonally dry conditions, and the small sample did not contain bloodfed mosquitoes. Nonetheless, our adult samples were captured in traps baited with CO2 and lures emulating human odours, so it can be assumed that they were seeking bloodmeals. An. coustani has tested positive for P. falciparum in multiple settings4,22,46,47,59,60,61,62, and marked tendencies for exophagy46,53,54,59 (as evident in this study) and early biting48,54 may render it largely unaffected by indoor-focused interventions. Parasite-positive An. pharoensis is implicated in maintaining dry season malaria transmission in Ethiopia54 and this species also favours outdoor biting54,59, as does An. squamosus63 which was found to have a Plasmodium positivity rate equal to that of primary vectors on Madagascar47 and an unexpected degree of anthropophily in southern Zambia64. Recently An. species O/15 was shown for the first time to harbour malaria parasites following outdoor trapping in the western Kenyan highlands24. An. rivulorum is considered to be a less efficient vector than its sibling species An. funestus, but nonetheless carries the malaria parasite22,65, whilst An. arabiensis, An. gambiae and An. funestus are classical primary vector species56, and the latter two were caught predominantly indoors in the present study.
Comparatively few studies have examined the inter-annual dynamics of anopheline communities across the landscape scales at which larval source management would need to be implemented66, given the dispersal capacity of Anopheles vector species67. Studies frequently seek to quantify the effect of seasonality on vector communities48,53,54,68,69, examine a limited number of locations at high temporal resolution9,70 or investigate community change in response to an explicit intervention10,71. Spatially explicit descriptions of community composition and distribution at relatively large scales of space and time are scarce; previous studies have largely focused on adult mosquitoes and variously reported temporal shifts9 or consistency70,72,73. The current study reports a high degree of consistency in anopheline community composition and distribution over three consecutive years. An. coustani larvae are shown to be widely distributed and to predominate in the community in both dry and wet seasons, while the traditional primary vector species An. arabiensis and An. gambiae comprise a consistently low proportion of the community (< 3% of larvae) and manifest a distinct small scale geographic clustering which is consistent across years. Due to the limited numbers (n = 54), the adult sample is unlikely to be representative of the anopheline community, particularly given the absence of samples from 30% of larval sampling locations and the skewing of the sample by a single anomalous catch constituting 25% of sampled adults. Nonetheless, the adult sample corroborates the An. gambiae complex hotspot in the Lui valley. Despite the high prevalence of An. species UG3 in the adult sample, it represented < 0.5% of the larval sample. The adult sample also contained two species unrepresented in 2019 larval samples (An. funestus and An. rivulorum), perhaps due to the preference of the former species for heavily vegetated water bodies74 and tolerance of submergence75 reducing its representation in dipped larval samples76. However, an independent time series of extensive catches of adult mosquitoes from the centre of the study area (n > 2400; 37,38 and Orba et al., 2021, pers. comm.) provides substantial corroboration for the anopheline community composition described from the larval surveillance presented here and previously36. This dataset confirms the consistent dominance of An. coustani and the notable absence of significant numbers of primary vector species in both dry and wet seasons.
The anopheline community across Barotseland, western Zambia, experiences dramatic seasonal fluctuations in environmental conditions in a highly dynamic ecosystem driven by seasonal flooding and rainfall regimes40. Although anophelines were encountered in a lower proportion of water bodies after the 2019 wet season than after the preceding 2018 wet season, the larval community was composed of a higher proportion of late stage larvae, suggesting increased productivity in the drying down phase of the accelerated hydrological year in 2019. This has been reported in other settings, potentially as abundant habitats become smaller and warmer: larval abundance was higher in drying streams than other habitats in Kenya and Tanzania52, increased with falling river levels in Sudan77, and both larval abundance and adult productivity increased in the early dry season in Kenya78. Many of the species encountered in the present study area exhibit preferences for relatively permanent water bodies and community composition was consistent during three survey periods incorporating seasonal and inter-annual disparities in ecohydrological conditions. Nonetheless, nuanced intra-annual variations due to species-specific responses to seasonal changes and the changing importance of different water body types over the hydrological year may occur over finer temporal scales68. As climate change is predicted to increase the frequency of meteorological extremes in Zambia79, it is important to monitor the response of the Anopheles community to extreme conditions beyond the typical spectrum of seasonal variations. Analysis of historical data has revealed a downward trend in Zambezi river discharge since the 1950s80, and Zambia is projected to experience reduced rainfall and increased temperature in the twenty-first century81; a recent drought in southern Zambia reduced the abundance of An. arabiensis by an order of magnitude69. A significant and extreme drought in western Zambia followed the larval surveys presented in this study, and expanded entomological surveillance is advocated to characterise the effects of such events on the assemblage of anopheline species in this ecosystem.
In an area of persistent malaria transmission despite long-term indoor vector control efforts, extensive larval sampling of the anopheline community over consecutive years has revealed the numerical and spatial dominance of species widely accepted to be secondary vectors of malaria and a marked paucity of primary vectors. This finding underlines the importance of diversifying vector control approaches to counteract species whose behaviours may permit them to evade widespread use of indoor-centric interventions. The consistent dominance of these secondary vector species was strongly supported by independent surveillance of adult mosquitoes in the area over 5 years, and geographic distributions of candidate vector species were robust to seasonal and inter-annual variations in ecohydrological conditions. This temporal consistency of larval community structure in western Zambia suggests that this may be a setting where larval source management strategies could be highly effective, compared to other regions where the larval community fluctuates substantially over time.
Methods
Study area
The study area in Limulunga and Mongu districts in Western Province, Zambia has been characterised hydrologically40 and five main ecological zones (Fig. 1) described in detail36. Briefly, it is dominated by the Barotse or upper Zambezi floodplain (ecological zone 1) which is a heterogeneous grassland-wetland mosaic which receives overbank flow from the Zambezi River and its tributaries82,83. The floodplain edge (zone 2) along the base of the eastern escarpment contains persistently wet seepage zones43 fed by water from higher ground. Flooding from a highly branched tributary of the Zambezi River persists in wet grassland in the Luena flats43 (zone 3), whilst another tributary provisions distant seepage wetland42 habitats in the more defined Lui valley (zone 4) which dissects higher ground east of the Barotse floodplain. Shallow, frequently waterlogged depressions known as dambos (zone 5) form an important persistent agricultural and hydrological resource in the region in their own right84, as well as sustaining fertile regions along the floodplain edge below the scarp.
Mosquito sampling: larvae
Field surveys of anopheline larvae were undertaken in water bodies in the five ecological zones after the peak of the wet season in May–June 2019, following the sampling strategy employed in Cross et al. (2021)36. Two health facilities were selected in each ecological zone (except in the remote Lui valley, where n = 1) and selection of two sample villages adjacent to each facility was guided by local knowledge of current hydrological conditions. Previous sample locations of Cross et al.36 were prioritised for re-sampling where hydrological conditions permitted (almost 80% of 2019 transects), but some were unsuitable for entomological sampling in 2019 due to the earlier and lower peak of the wet season and were replaced by adjacent villages. Radial line transects were sited from each village and sampling points were located at 100 m intervals along each transect. Water bodies encountered within a 5 m radius of these pre-defined points or within 5 m of the transect line at intermediate locations were geolocated with a GPS handset (Garmin eTrex) and surveyed for mosquito larvae. Up to 40 dips per transect point were taken using standard 350 ml dippers (Bioquip, USA) by employing a purposive dipping strategy27,30,85 to search for larvae within suitable microhabitats. Dip contents were examined in a white plastic tray after a settling period, and counted after morphological differentiation into anophelines and culicines. Up to 12 Anopheles larvae per sampling point were collected and stored individually in 95% ethanol for genetic analyses.
In addition to sampling of ecological zones described above and previously36, larval surveys were undertaken along Kambule stream within Mongu town to characterise the anopheline community in this peri-urban setting.
Mosquito sampling: adults
Simultaneous indoor and outdoor sampling of adult mosquitoes was undertaken during the larval sampling period using an adaptation of the BG-Sentinel trap (Biogents, Germany86) optimised for anopheline mosquitoes and known as the BG-Malaria trap87. Each trap was suspended from a tripod and inverted with its opening 40 cm above ground level, baited with a cartridge of BG-Lure synthetic attractant (Biogents, Germany) and with CO2 produced by fermentation of yeast (40 g) and brown sugar (500 g) in 2 l of water and released within the trap. One indoor-outdoor pair of traps was deployed at each sample household to survey both endophagic and exophagic mosquitoes; the indoor trap was located close to the foot of an occupied bed and the outdoor trap positioned in the lee of the house or nearby vegetation, adjacent to outside sitting areas and away from sources of smoke or disturbance. Trap houses were preferentially located on the outer fringes of the village due to the ‘edge effect’ identified in some studies88,89,90, downwind of the village centre as female anophelines are postulated to fly upwind in search of human hosts91.
Traps were deployed in late afternoon and ran until the battery and fermentation mixture were disconnected the subsequent morning. Each trap catch bag was closed and labelled, and mosquitoes killed by freezing. Each catch was subsequently examined under a dissecting microscope and screened morphologically by genus, sex and bloodfeeding status. All anophelines were retained and stored individually in 95% ethanol.
Adult mosquito data derived from previous studies
Adult mosquitoes were sampled in several districts across Zambia between 2012 and 2017 by Orba et al.37 and Wastika et al.38. Outdoor trapping was undertaken between 2014 and 2017 in Mongu district, where additional trapping was also undertaken in 2018–19 (Orba et al., 2021; pers. comm.). Trapping was undertaken at several locations adjacent to Mongu town and Namushakende, on the floodplain edge c. 20 km south of Mongu, for an average of 5 consecutive nights per season using CDC and BG-Sentinel traps; see Orba et al.37 for details. Specimens were identified to species morphologically, with mtDNA COI gene sequences obtained from a subset of individuals for confirmation of identity (Orba et al., 2021; pers. comm.).
DNA barcoding species identification
Genetic analysis of larval and adult samples by mtDNA COI and nuclear ITS2 sequencing followed Cross et al.36. The species identity of each specimen was inferred from BLASTn search results yielding COI and/or ITS2 sequence similarity of ≥ 95% to sequences in GenBank (National Center for Biotechnology Information). Adoption of this standard sequence similarity threshold was vital to ensure comparability with previous results36 and those from other studies22,23. Species identities were further assessed by position of clustering within a maximum likelihood inferred phylogeny of the COI dataset and published confirmed species identity reference sequences22,23,44, constructed in MEGA v.10.0.592, with statistical support calculated using 100 bootstrap replicates. Additionally, adult female anophelines were screened for the presence of P. falciparum sporozoites by PCR amplification using primers for the protozoan93.
Data handling and statistical analyses
Field and molecular data were compiled in a database (MS Access) and linked to geographic locations in QGIS v. 3.18.3-Zürich94. Exploratory analyses were undertaken by plotting data points from individual dips together with aggregated summary measures (median values per year/ecological zone) using PlotsOfData95. The proportion of the genetically-identified subsample of anopheline larvae composed of each species individually was applied to the total anopheline count at each transect point to obtain the estimated total of each species at each transect point. Statistical analyses were undertaken in SPSS96; comparisons of field statistics (e.g. total anopheline larvae per transect point) and sequence-derived species data (e.g. estimated An. arabiensis per transect point) were made between consecutive wet seasons (201836 and 2019) using Odds Ratios97 and Pearson’s Chi-squared test. Sampling in 201736 is referenced as the dry season comparator. Within-year comparisons between ecological zones were made using non-parametric statistics: independent samples median (ISM) test and Kruskal–Wallis H, both with stepwise step-down (SSD) post-hoc comparisons and adjusted (adj) p value for multiple comparisons.
Ethical approval
An ethical approval waiver was provided by the University of Zambia’s Biomedical Research Ethics Committee (Ref 018–08-17) as the research did not involve human subjects. The Barotseland Royal Establishment granted their approval for entomological surveys to be conducted in and around villages in the study area. District Health Office staff accompanied the field survey team; at the beginning of each day’s fieldwork, the survey team checked in with the nearest health facility and sought permission from village chiefs to undertake fieldwork following introductory discussions. After full explanation of the adult trapping procedure in an appropriate language (usually siLozi), written informed consent was obtained from householders who volunteered to participate, and LLINs issued where absent from indoor trapping houses. All methods were performed in accordance with relevant guidelines and regulations.
Data availability
The COI and ITS2 sequence datasets generated by and analysed in the current study are available in the NCBI GenBank nucleotide archive with accession numbers OL619678-OL619792 and OL583776-OL583807 for larval and adult COI sequences, respectively and OL621257-OL621744 and OL621788-OL621838 for larval and adult ITS2 sequences. A full maximum likelihood phylogenetic tree of COI haplotypes from this study and from Orba et al. (2021, pers. comm.) is provided in Supplementary Information (Fig. S1). Anopheles count data and six COI sequences from the latter source are provided in Supplementary Table S1 and Supplementary Dataset S1.
References
Russell, T. L., Beebe, N. W., Cooper, R. D., Lobo, N. F. & Burkot, T. R. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 12, 56. https://doi.org/10.1186/1475-2875-12-56 (2013).
Mouchet, J. et al. Biodiversité du paludisme dans le monde. (Editions John Libbey Eurotext, 2004).
Sougoufara, S., Ottih, E. C. & Tripet, F. The need for new vector control approaches targeting outdoor biting anopheline malaria vector communities. Parasit Vectors 13, 295. https://doi.org/10.1186/s13071-020-04170-7 (2020).
Antonio-Nkondjio, C. et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J. Med. Entomol. 43, 1215–1221. https://doi.org/10.1093/jmedent/43.6.1215 (2006).
Afrane, Y. A., Bonizzoni, M. & Yan, G. in Current Topics in Malaria Ch. 20, (2016).
Goupeyou-Youmsi, J. et al. Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighboring villages of Madagascar. bioRxiv 13, 430, https://doi.org/10.1101/787432 (2019).
Ranson, H. & Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196. https://doi.org/10.1016/j.pt.2015.11.010 (2016).
Killeen, G. F. Control of malaria vectors and management of insecticide resistance through universal coverage with next-generation insecticide-treated nets. Lancet 395, 1394–1400. https://doi.org/10.1016/s0140-6736(20)30745-5 (2020).
Kreppel, K. S. et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci. Rep. 10, 14527. https://doi.org/10.1038/s41598-020-71187-4 (2020).
Chinula, D. et al. Proportional decline of Anopheles quadriannulatus and increased contribution of An. arabiensis to the An. gambiae complex following introduction of indoor residual spraying with pirimiphos-methyl: an observational, retrospective secondary analysis of pre-existing data from south-east Zambia. Parasit Vectors 11, 544, https://doi.org/10.1186/s13071-018-3121-0 (2018).
Lwetoijera, D. W. et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J 13, 331. https://doi.org/10.1186/1475-2875-13-331 (2014).
Russell, T. L. et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 9, 187. https://doi.org/10.1186/1475-2875-9-187 (2010).
Sougoufara, S., Harry, M., Doucoure, S., Sembene, P. M. & Sokhna, C. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med. Vet. Entomol. 30, 365–368. https://doi.org/10.1111/mve.12171 (2016).
Agyekum, T. P. et al. A systematic review of the effects of temperature on Anopheles mosquito development and survival: Implications for malaria control in a future warmer climate. Int. J. Environ. Res. Public Health 18, 7255 (2021).
Smith, M. W. et al. Incorporating hydrology into climate suitability models changes projections of malaria transmission in Africa. Nat. Commun. 11, 4353. https://doi.org/10.1038/s41467-020-18239-5 (2020).
Chemison, A. et al. Impact of an accelerated melting of Greenland on malaria distribution over Africa. Nat. Commun. 12, 3971. https://doi.org/10.1038/s41467-021-24134-4 (2021).
Thomas, C. J., Davies, G. & Dunn, C. E. Mixed picture for changes in stable malaria distribution with future climate in Africa. Trends Parasitol. 20, 216–220. https://doi.org/10.1016/j.pt.2004.03.001 (2004).
Carnevale, P. & Manguin, S. Review of issues on residual malaria transmission. J. Infect. Dis. 223, S61–S80. https://doi.org/10.1093/infdis/jiab084 (2021).
Killeen, G. F., Chaki, P. P., Reed, T. E., Moyes, C. L. & Govella, N. J. in Towards Malaria Elimination - A Leap Forward Ch. 17, (2018).
Killeen, G. F. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 13, 330. https://doi.org/10.1186/1475-2875-13-330 (2014).
Beebe, N. W. DNA barcoding mosquitoes: advice for potential prospectors. Parasitology 145, 622–633. https://doi.org/10.1017/S0031182018000343 (2018).
Lobo, N. F. et al. Unexpected diversity of Anopheles species in Eastern Zambia: implications for evaluating vector behavior and interventions using molecular tools. Sci. Rep. https://doi.org/10.1038/srep17952 (2015).
St Laurent, B. et al. Molecular characterization reveals diverse and unknown malaria vectors in the western Kenyan highlands. Am. J. Trop. Med. Hyg. 94, 327–335. https://doi.org/10.4269/ajtmh.15-0562 (2016).
Zhong, D. et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci. Rep. 10, 16139. https://doi.org/10.1038/s41598-020-73073-5 (2020).
Killeen, G. F. et al. Developing an expanded vector control toolbox for malaria elimination. BMJ Glob. Health 2, e000211. https://doi.org/10.1136/bmjgh-2016-000211 (2017).
Dambach, P. et al. Reduction of malaria vector mosquitoes in a large-scale intervention trial in rural Burkina Faso using Bti based larval source management. Malar J. 18, 311. https://doi.org/10.1186/s12936-019-2951-3 (2019).
Fillinger, U. & Lindsay, S. W. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop. Med. Int. Health 11, 1629–1642. https://doi.org/10.1111/j.1365-3156.2006.01733.x (2006).
Hardy, A., Makame, M., Cross, D., Majambere, S. & Msellem, M. Using low-cost drones to map malaria vector habitats. Parasit Vectors 10, 29. https://doi.org/10.1186/s13071-017-1973-3 (2017).
Lwetoijera, D. et al. Effective autodissemination of pyriproxyfen to breeding sites by the exophilic malaria vector Anopheles arabiensis in semi-field settings in Tanzania. Malar J. 13, 161. https://doi.org/10.1186/1475-2875-13-161 (2014).
Majambere, S., Lindsay, S. W., Green, C., Kandeh, B. & Fillinger, U. Microbial larvicides for malaria control in The Gambia. Malaria J. https://doi.org/10.1186/1475-2875-6-76 (2007).
Unlu, I., Faraji, A., Wang, Y., Rochlin, I. & Gaugler, R. Heterodissemination: precision insecticide delivery to mosquito larval habitats by cohabiting vertebrates. Sci. Rep. 11, 14119. https://doi.org/10.1038/s41598-021-93492-2 (2021).
Majambere, S. et al. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in The Gambia? A cross-over intervention trial. Am. J. Trop. Med. Hyg. 82, 176–184. https://doi.org/10.4269/ajtmh.2010.09-0373 (2010).
Dongus, S. et al. Participatory mapping of target areas to enable operational larval source management to suppress malaria vector mosquitoes in Dar es Salaam, Tanzania. Int. J. Health Geogr. 6, 37. https://doi.org/10.1186/1476-072X-6-37 (2007).
Ferguson, H. M. et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 7, e1000303. https://doi.org/10.1371/journal.pmed.1000303 (2010).
Gu, W., Utzinger, J. & Novak, R. J. Habitat-based larval interventions: A new perspective for malaria control. Am. J. Trop. Med. Hyg. 78, 2–6 (2008).
Cross, D. E. et al. Geographically extensive larval surveys reveal an unexpected scarcity of primary vector mosquitoes in a region of persistent malaria transmission in western Zambia. Parasit Vectors 14, 91. https://doi.org/10.1186/s13071-020-04540-1 (2021).
Orba, Y. et al. First isolation of West Nile virus in Zambia from mosquitoes. Transbound Emerg. Dis. 65, 933–938. https://doi.org/10.1111/tbed.12888 (2018).
Wastika, C. E. et al. Discoveries of exoribonuclease-resistant structures of insect-specific flaviviruses isolated in Zambia. Viruses https://doi.org/10.3390/v12091017 (2020).
Hulsman, P., Savenije, H. H. G. & Hrachowitz, M. Satellite-based drought analysis in the Zambezi River Basin: Was the 2019 drought the most extreme in several decades as locally perceived?. J. Hydrol. Reg. Stud. https://doi.org/10.1016/j.ejrh.2021.100789 (2021).
Hardy, A. et al. Automatic detection of open and vegetated water bodies using Sentinel 1 to map African malaria vector mosquito breeding habitats. Remote Sensing 11, 593. https://doi.org/10.3390/rs11050593 (2019).
Del Rio, T., Groot, J. C. J., DeClerck, F. & Estrada-Carmona, N. Integrating local knowledge and remote sensing for eco-type classification map in the Barotse Floodplain, Zambia. Data Brief 19, 2297–2304. https://doi.org/10.1016/j.dib.2018.07.009 (2018).
Timberlake, J. Biodiversity of the Zambezi Basin wetlands: Review and preliminary assessment of available information. IUCN - The World Conservation Union Regional Office for Southern Africa, Harare, Zimbabwe (1997).
Turpie, J., Smith, B., Emerton, L. & Barnes, J. Economic valuation of the Zambezi basin wetlands. IUCN - The World Conservation Union Regional Office for Southern Africa, Harare, Zimbabwe (1999).
Ciubotariu, I. I. et al. Genetic diversity of Anopheles coustani in high malaria transmission foci in southern and central Africa. J. Med. Entom. 57, 1–11. https://doi.org/10.1093/jme/tjaa132 (2020).
Jones, C. M. Vector biology and genomics of Anopheles in southern and central Africa PhD thesis, John Hopkins Bloomberg School of Public Health, (2019).
Stephen, A., Nicholas, K., Busula, A. O., Webale, M. K. & Omukunda, E. Detection of Plasmodium sporozoites in Anopheles coustani s.l; a hindrance to malaria control strategies in highlands of western Kenya. bioRxiv, https://doi.org/10.1101/2021.02.10.430589 (2021).
Tedrow, R. E. et al. Anopheles mosquito surveillance in Madagascar reveals multiple blood feeding behavior and Plasmodium infection. PLoS Negl. Trop. Dis. 13, e0007176. https://doi.org/10.1371/journal.pntd.0007176 (2019).
Taye, B., Lelisa, K., Emana, D., Asale, A. & Yewhalaw, D. Seasonal dynamics, longevity, and biting activity of anopheline mosquitoes in southwestern Ethiopia. J. Insect. Sci. https://doi.org/10.1093/jisesa/iev150 (2016).
Sikaala, C. H. et al. A cost-effective, community-based, mosquito-trapping scheme that captures spatial and temporal heterogeneities of malaria transmission in rural Zambia. Malar J. 13, 225. https://doi.org/10.1186/1475-2875-13-225 (2014).
De Meillon, B. The anophelini of the Ethiopian geographical region. Publ. South Afr. Inst. Med. Res. 49, 1–272 (1947).
Gillies, M. T. & De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Publ. South Afr. Inst. Med. Res. 54, 1–343 (1968).
Dida, G. O. et al. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect. Dis. Poverty 7, 2. https://doi.org/10.1186/s40249-017-0385-0 (2018).
Njoroge, M. M. et al. Exploring the potential of using cattle for malaria vector surveillance and control: a pilot study in western Kenya. Parasit Vectors 10, 18. https://doi.org/10.1186/s13071-016-1957-8 (2017).
Kibret, S. et al. The impact of a small-scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop. Med. Int. Health 15, 41–50. https://doi.org/10.1111/j.1365-3156.2009.02423.x (2010).
Coetzee, M. Anopheles crypticus, new species from South Africa is distinguished from Anopheles coustani (Diptera: Culicidae). Mosq. Syst. 26, 125–131 (1994).
Gillies, M. T. & Coetzee, M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Publ. South Afr. Inst. Med. Res. 55, 1–143 (1987).
Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 19, 70. https://doi.org/10.1186/s12936-020-3144-9 (2020).
Carter, T. E., Yared, S., Hansel, S., Lopez, K. & Janies, D. Sequence-based identification of Anopheles species in eastern Ethiopia. Malar J. 18, 135. https://doi.org/10.1186/s12936-019-2768-0 (2019).
Degefa, T. et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 16, 443. https://doi.org/10.1186/s12936-017-2098-z (2017).
Nepomichene, T. N. J. J., Tata, E. & Boyer, S. Malaria case in Madagascar, probable implication of a new vector, Anopheles coustani. Malaria J. 14, 475. https://doi.org/10.1186/s12936-015-1004-9 (2015).
Finney, M. et al. Widespread zoophagy and detection of Plasmodium spp. in Anopheles mosquitoes in southeastern Madagascar. Malar J. 20, 25. https://doi.org/10.1186/s12936-020-03539-4 (2021).
Mwangangi, J. M. et al. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors 6, 114. https://doi.org/10.1186/1756-3305-6-114 (2013).
Hoffman, J. E. et al. Phylogenetic complexity of morphologically identified Anopheles squamosus in southern Zambia. Insects 12, 146. https://doi.org/10.3390/insects12020146 (2021).
Fornadel, C. M., Norris, L. C., Franco, V. & Norris, D. E. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis. 11, 1173–1179. https://doi.org/10.1089/vbz.2010.0082 (2011).
Wilkes, T. J., Matola, Y. G. & Charlwood, J. D. Anopheles rivulorum, a vector of human malaria in Africa. Med. Vet. Entomol. 10, 108–110. https://doi.org/10.1111/j.1365-2915.1996.tb00092.x (1996).
Majambere, S., Fillinger, U., Sayer, D. R., Green, C. & Lindsay, S. W. Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. Am. J. Trop. Med. Hyg. 79, 19–27 (2008).
Thomas, C. J., Cross, D. E. & Bogh, C. Landscape movements of Anopheles gambiae malaria vector mosquitoes in rural Gambia. PLoS ONE https://doi.org/10.1371/journal.pone.0068679 (2013).
Hardy, A. J. et al. Habitat hydrology and geomorphology control the distribution of malaria vector larvae in rural Africa. PLoS ONE 8, e81931. https://doi.org/10.1371/journal.pone.0081931 (2013).
Kent, R. J., Thuma, P. E., Mharakurwa, S. & Norris, D. E. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am. J. Trop. Med. Hyg. 76, 267–274 (2007).
Imbahale, S. S. et al. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 10, 81. https://doi.org/10.1186/1475-2875-10-81 (2011).
Bayoh, M. N. et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 9, 62. https://doi.org/10.1186/1475-2875-9-62 (2010).
Mawejje, H. D. et al. Impact of seasonality and malaria control interventions on Anopheles density and species composition from three areas of Uganda with differing malaria endemicity. Malar J. 20, 138. https://doi.org/10.1186/s12936-021-03675-5 (2021).
Stevenson, J. C. et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: Implications for vector control. Parasit Vectors 9, 510. https://doi.org/10.1186/s13071-016-1786-9 (2016).
Dabire, K. R. et al. Year to year and seasonal variations in vector bionomics and malaria transmission in a humid savannah village in west Burkina Faso. J. Vector Ecol. 33, 70–75. https://doi.org/10.3376/1081-1710(2008)33[70:ytyasv]2.0.co;2 (2008).
Tuno, N., Githeko, A., Yan, G. & Takagi, M. Interspecific variation in diving activity among Anopheles gambiae Giles, An. arabiensis Patton, and An. funestus Giles (Diptera: Culicidae) larvae. J. Vector Ecol. 32, 112–117. https://doi.org/10.3376/1081-1710(2007)32[112:ividaa]2.0.co;2 (2007).
Nambunga, I. H. et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar J. 19, 219. https://doi.org/10.1186/s12936-020-03295-5 (2020).
Ageep, T. B. et al. Spatial and temporal distribution of the malaria mosquito Anopheles arabiensis in northern Sudan: influence of environmental factors and implications for vector control. Malar J. 8, 123. https://doi.org/10.1186/1475-2875-8-123 (2009).
Kweka, E. J. et al. Anopheline larval habitats seasonality and species distribution: a prerequisite for effective targeted larval habitats control programmes. PLoS ONE 7, e52084. https://doi.org/10.1371/journal.pone.0052084 (2012).
Libanda, B. & Ngonga, C. Projection of frequency and intensity of extreme precipitation in Zambia: a CMIP5 study. Climate Res. 76, 59–72. https://doi.org/10.3354/cr01528 (2018).
Zimba, H. et al. Assessment of trends in inundation extent in the Barotse Floodplain, upper Zambezi River Basin: A remote sensing-based approach. J. Hydrol. Reg. Stud. 15, 149–170. https://doi.org/10.1016/j.ejrh.2018.01.002 (2018).
Hamududu, B. H. & Killingtveit, A. Hydropower production in future climate scenarios; the case for the Zambezi River. Energies https://doi.org/10.3390/en9070502 (2016).
IUCN. Barotse Floodplain, Zambia: Local economic dependence on wetland resources. IUCN - The World Conservation Union, Harare, Zimbabwe (2003).
Moore, A. E., Cotterill, F.P.D., Main, M.P.L., Williams, H.B. in Large Rivers: Geomorphology and Management (ed Avijit Gupta) Ch. 15, (Wiley, 2007).
Heyden, C. J. V. D. The hydrology and hydrogeology of dambos: a review. Prog. Phys. Geog. 28, 544–564. https://doi.org/10.1191/0309133304pp424oa (2004).
Derua, Y. A. et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malaria J. https://doi.org/10.1186/1475-2875-11-188 (2012).
Kröckel, U., Rose, A., Eiras, Á. E. & Geier, M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Control Assoc. 22, 229–238. https://doi.org/10.2987/8756-971x(2006)22[229:Ntfsoa]2.0.Co;2 (2006).
Gama, R. A., Silva, I. M., Geier, M. & Eiras, A. E. Development of the BG-Malaria trap as an alternative to human-landing catches for the capture of Anopheles darlingi. Mem. Inst. Oswaldo Cruz 108, 763–771. https://doi.org/10.1590/0074-0276108062013013 (2013).
Ribeiro, J. M., Seulu, F., Abose, T., Kidane, G. & Teklehaimanot, A. Temporal and spatial distribution of anopheline mosquitos in an Ethiopian village: implications for malaria control strategies. Bull. World Health Organ. 74, 299–305 (1996).
Russell, T. L. et al. Geographic coincidence of increased malaria transmission hazard and vulnerability occurring at the periphery of two Tanzanian villages. Malar J. 12, 24. https://doi.org/10.1186/1475-2875-12-24 (2013).
Smith, D. L., Dushoff, J. & McKenzie, F. E. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2, e368. https://doi.org/10.1371/journal.pbio.0020368 (2004).
Midega, J. T. et al. Wind direction and proximity to larval sites determines malaria risk in Kilifi District in Kenya. Nat. Commun. 3, 674. https://doi.org/10.1038/ncomms1672 (2012).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Singh, B. et al. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 60, 687–692. https://doi.org/10.4269/ajtmh.1999.60.687 (1999).
QGIS Geographic Information System (Open Source Geospatial Foundation Project, 2021).
Postma, M. & Goedhart, J. PlotsOfData - A web app for visualizing data together with their summaries. PLoS Biol 17, e3000202. https://doi.org/10.1371/journal.pbio.3000202 (2019).
IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY, 2017).
Rita, H. & Komonen, A. Odds ratio: an ecologically sound tool to compare proportions. Ann. Zool. Fenn. 45, 66–72. https://doi.org/10.5735/086.045.0106 (2008).
Acknowledgements
The authors would like to thank collaborators in the University of Barotseland, particularly the field survey team: Chikenge Henry, Kundananji Silwenga, and Munukayumbwa Sitwala. We are grateful for the support of Tresford Kaniki of the National Malaria Elimination Centre at the beginning of fieldwork. We are indebted to Yasuko Orba and colleagues for agreeing to share unpublished sequences and count data from their contemporaneous dataset. Finally, we would like to thank the communities of Barotseland for their enthusiastic engagement with adult trapping.
Funding
This research was undertaken as part of the FLOODMAL project, funded by the UK Natural Environment Research Council (Grant Ref: NE/P013481/1), Principal Investigator CT. The funding body played no role in the design of the study, the collection, analysis and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
C.T., D.C., D.S., F.L., and A.S. conceptualized the study. C.T. and D.C. acquired funding. D.C., C.T., V.S., D.S., F.L. and J.S. contributed to the study design. D.C., V.S., A.H. and C.T. undertook collection of field data and samples; D.C., A.H., N.Mc.K. and N.M. performed laboratory analyses. D.C., A.H., N.Mc.K. and P.S. interpreted results of genetic analyses. D.C. and AH drafted the manuscript; N.Mc.K., C.T. and P.S. critically reviewed and edited subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cross, D.E., Healey, A.J.E., McKeown, N.J. et al. Temporally consistent predominance and distribution of secondary malaria vectors in the Anopheles community of the upper Zambezi floodplain. Sci Rep 12, 240 (2022). https://doi.org/10.1038/s41598-021-04314-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04314-4
- Springer Nature Limited