Abstract
Leaf functional traits support plant survival and growth in different stress and disturbed conditions and respond according to leaf habit. The present study examined 13 leaf traits (3 morphological, 3 chemical, 5 physiological, and 2 stoichiometry) of nine dominant forest tree species (3 coniferous, 3 deciduous broad-leaved, 3 evergreen broad-leafed) to understand the varied response of leaf habits. The hypothesis was to test if functional traits of the conifers, deciduous and evergreen differ significantly in the temperate forest and to determine the applicability of leaf economic theory i.e., conservative vs. acquisitive resource investment, in the temperate Himalayan region. The attributes of the functional traits i.e., leaf area (LA), specific leaf area (SLA), leaf dry matter content (LDMC), leaf water content (LWC), stomatal conductance (Gs), and transpiration (E) followed the order deciduous > evergreen > coniferous. Leaf carbon and leaf C/N ratio showed the opposite pattern, coniferous > evergreen > deciduous. Chlorophyll (Chl) and photosynthetic rate (A) were highest for evergreen species, followed by deciduous and coniferous species. Also, structural equation modelling determined that morphological factors were negatively related to physiological and positively with chemical factors. Nevertheless, physiological and chemical factors were positively related to each other. The physiological traits were mainly regulated by stomatal conductance (Gs) however the morphological traits were determined by LDMC. Stoichiometry traits, such as leaf C/N, were found to be positively related to leaf carbon, and leaf N/P was found to be positively related to leaf nitrogen. The result of the leaf functional traits relationship would lead to precise prediction for the functionality of the temperate forest ecosystem at the regional scale.
Similar content being viewed by others
Introduction
Leaf traits are morphological, physiological, chemical, phenological and stoichiometry characteristics influencing the growth, distribution, reproduction, and survival of the tree species1,2,3,4. Leaves regulate ecological processes, such as exchange of gases, carbon storage, photosynthesis assimilation, transpiration, respiration, nutrient cycling2. Leaf morphological traits, e.g., leaf area (LA) of a species determines canopy structure and surface availability for intercepting photosynthetically active radiation (PAR), and thus productivity in forest ecosystems5,6. Specific leaf area (SLA) determines the plant strategy of a species and provides information on photosynthetic capacity7,8. Leaf dry matter content (LDMC) explains plant growth rate and carbon assimilation9, while leaf nitrogen and phosphorus concentrations (LNC and LPC respectively) are good indicators of plant photosynthetic capacity and resource use strategy1,10. Similarly, photosynthetic rate (A), transpiration (E) and stomatal conductance (Gs) are indicators of CO2 and water exchanges. Therefore, knowledge of leaf morphological, chemical, physiological and stoichiometry traits can make a significant contribution to understanding the mechanisms of plant adaptation to future changes in environmental conditions11,12. Moreover, studying leaf functional traits at the regional scale is important for understanding trait-environment interaction at the global scale and develop predictive models. Therefore, unraveling the functional background of leaf traits can improve understanding of plant growth and its relationship with the environment8.
Changes in functional traits have significant impacts on the structure and composition of forests and the overall functioning of the ecosystem13. For example, functional trait changes provide support for trait-based habitat filtering in plant community14, species coexistence, and adaptive plant strategy15,16,17. However, the causes of high variability in leaf morphological and eco-physiological traits are due to different edaphic and climatic conditions, which assist in the coexistence and adaptation of the species in a forest ecosystem18. Moreover, biomass, carbon, and productivity of the forest are also dependent on the leaf traits5. Variation in the plant functional traits are regulated by the environmental constraints “i.e. climate, resource availability and disturbance level in the ecosystem19”. Moreover, trait-based modeling attempts to simulate ecological processes and requires detailed input parameters for ecosystem modelling20. The model-based information is useful for forest management and planning for ensuring the sustainable flow of ecosystem services.
Himalayan forests differ from other forests in terms of their climate, and are characterized by concentrated summer rainfall, mild winters, high sun angles, high elevation, and low annual variability in day length21. These conditions provide higher foliar biomass and nutrient sequestration, as well as higher net primary productivity to the forest ecosystem18. As a result, species found in the temperate ecosystem have particular and distinctive traits which allow them to function in these environmental conditions. Information regarding the temperate forests of the Himalayan region is limited, however, making it difficult for forest managers to understand the functioning of these forests. The lack of information prevents policymakers from formulating scientific-based forest management policies and thereby managing the degradation of the forests. With this in mind, we conducted trait variation evaluation in order to demonstrate the coexistence, adaptive strategy, and environmental constraints of the species in India’s temperate forests. Furthermore, the study was intended to facilitate better understanding of the functioning of the temperate forests, and to provide input for trait-based predictive modeling. The study attempted to evaluate variation among the traits (morphological, physiological, and chemical) of the conifers, deciduous broad-leaved, and evergreen broad-leaved tree species of the temperate forest, and to establish correlations between the functional traits of the tree species. We selected 13 leaf traits known to be indicators of plant morphological, physiological, and chemical status22,23, as well as important contributors to productivity, biomass production, decomposition, carbon storage, nitrogen utilization, and nutrient cycling24. We also analyzed the stoichiometry carbon/nitrogen ratio (C/N) and nitrogen/phosphorus ratio (N/P), as they can affect the environment25 and signal changes associated with the life history of plants. A complementary or alternative idea is that leaf economic theory may be applied to account for the functionality of temperate Himalayan forests, based on the trade-off strategy adopted by trees in exchanges such as conservative vs. acquisitive resource investment. Acquisitative species, which are characterized by high photosynthetic, respiration and growth rates, and short-lived and nitrogen-rich leaves, prefer nutrient-rich and/or growth-friendly environmental conditions. Conservative species, meanwhile, are those which display the opposite characteristics1. Leaf economic theory describes the relationships between a variety of leaf characteristics, such as carbon assimilation rate, leaf longevity, leaf mass-per-area, and nitrogen content1. The significance of leaf economic theory is that it can describe observed differences in plant strategies and indicate significant constraints on nutrient fluxes. Furthermore, leaf economic theory can be applied on a variety of scales, such as between species, within species, and even within individual plants26.
Our hypothesis is that functional traits of the conifers, deciduous broad-leaved and evergreen broad-leaved differ significantly in the temperate forest such as deciduous broad-leaved having highest values of SLA and lowest of LDMC, conifers having lowest values of SLA and highest of LDMC, and evergreen broad-leaved having intermediary values for both traits. These hypotheses were tested by evaluating the 13 selected key leaf functional traits in nine dominant tree species (3 coniferous, 3 deciduous broad-leaved, 3 evergreen broad-leafed) in a temperate forest in the Indian Himalayas. Trees were selected based on diameter at breast height (DBH 23.44 to 39.99 cm), to achieve uniformity amongst species in the same location. Morphological traits studied were: LA, SLA, LDMC; chemical traits: LCC, LNC, LPC; physiological traits: LWC, chlorophyll (Chl), A, Gs, E, and stoichiometry traits: leaf C/N and leaf N/P (Table 1). Productivity of the forest is dependent on these morphological, chemical, and physiological traits, and therefore variation in the traits can support in coexistence and adaptation of the species in a forest ecosystem. With this background, the main objectives of the study were to (a) quantify the variation in the 13 leaf traits among the different plant functional groups (conifer, deciduous broad-leafed, evergreen broad-leafed) and (b) applicability of leaf economic theory along with determination of the traits i.e. morphological, physiological, chemical and stoichiometry causal relationship for understanding the functionality of the temperate forest ecosystem. Structural Equation Modeling (SEM) was used to evaluate causal relationships among functional traits. Moreover, the information was also used to evaluate the applicability of leaf economic theory for the functionality of the temperate forests of the Himalayas.
Results
Variations in morphological traits
LA ranged from 34.00 to 173 cm2 for deciduous broad-leaved species, 6.00 to 71.50 cm2 for evergreens broad-leaved and 1.00 to 50 cm2 for conifers in the temperate forest studied (Fig. 1A). Mean SLA was 178.83, 112.00, and 43.31 cm2 g−1 in deciduous broad-leaved, evergreen broad-leaved, and coniferous tree species, respectively (Fig. 1B). However, LDMC was similar for conifers and evergreens broad-leaved (0.36%), but slightly higher for deciduous broad-leaved species (0.41%) (Fig. 1C). Therefore, LA and SLA were both maximum in deciduous broad-leaved species, followed by evergreen broad-leaved and coniferous species (Fig. 1A,B). LA and SLA differ significantly across the studied leaf habits, however, deciduous broad-leaved and evergreen broad-leaved were statistically similar and differ significantly with conifers for LDMC (Fig. 1A,B).
Distribution of values of various traits of tree species of different leaf habits in a temperate forest in Mussoorie Forest Division, Uttarakhand, Indian Himalayas (C = conifers, D = deciduous broad-leaved, E = evergreen broad-leaved): Morphological; LA = leaf area, cm2; SLA = specific leaf area, cm2 g−1; LDMC = leaf dry matter content, %; Chemical; LCC = leaf carbon content, %; LNC = leaf nitrogen content, %; LPC = leaf phosphorus content, %; Physiological; LWC = leaf water content, %; Chl = chlorophyll, mg g−1; A = photosynthetic rate, μmol CO2 m−2 s−1; Gs = stomatal conductance, mol H2O m−2 s−1; E = transpiration rate, mmol H2O m−2 s−1; Stoichiometry; Leaf C/N and Leaf N/P. The centerline indicates the median, upper and lower box heights indicate interquartile range. The alphabets within the figure represents results of post ANOVA multiple comparison by LSD. Different alphabets represents statistically different groups.
Variations in chemical and stoichiometry traits
LCC ranged from 28.00 to 48.36% (mean 42.21%) in deciduous broad-leaved species, from 20 to 60% (mean 45%) in evergreen broad-leaved species, and from 30.12 to 86.44% (mean 52.17%) in coniferous tree species (Fig. 1D). Mean LNC was 2.50%, 1.60%, and 2.00% in deciduous broad-leaved, evergreen broad-leaved, and coniferous tree species, respectively (Fig. 1E). Differences in LCC and LNC were significantly different with leaf habits (Fig. 1D,E) and LPC was more or less similar in all leaf habits i.e. non-significant at 5% level (Fig. 1F). Conifer and evergreen broad-leaved species were homogeneous group for LCC and LNC, and statistically differ with deciduous broad-leaved (Fig. 1D,E). Differences in stoichiometry traits i.e. leaf C/N and leaf N/P were statistically non-significant i.e. invariant across the three leaf habits (Fig. 1L,M).
Variations in physiological traits
LWC, Chl and A were insignificant among the three leaf habits (Fig. 1G–I). Chl was 21.70 mg g−1 in deciduous broad-leaved species, 18.12 mg g−1 in evergreens broad-leaved, and 13.16 mg g−1 in conifers (Fig. 1H). A ranged from 0.35 to 60 μmol CO2 m−2 s−1 (mean 15.42 μmol CO2 m−2 s−1) in deciduous broad-leaved species, from 0.23 to 81 μmol CO2 m−2 s−1 (mean 18.01 μmol CO2 m−2 s−1) in evergreens broad-leaved, and from 0.20 to 48.77 μmol CO2 m−2 s−1 (mean 14.93 μmol CO2 m−2 s−1) in conifers (Fig. 1I). Gs varied significantly between deciduous broad-leaved and conifers, but did not show significant variation between deciduous broad-leaved and evergreen broad-leaved, nor between evergreen broad-leaved and coniferous (Fig. 1J). E varied significantly among deciduous, evergreen broad-leaved, and conifers (Fig. 1K), while conifer and evergreen broad-leaved were homogeneous for E, as were deciduous broad-leaved and evergreen broad-leaved.
Causal relationship among leaf morphological, chemical, and physiological traits
Structural equation modeling (SEM) was applied to establish causal relationships among leaf morphological, chemical, physiological and stoichiometry traits (χ2 = 286.43, d.f. = 46, p = < 0.001, AIC = 10,613.33). SEM allowed identification of few significant causal relationships among leaf morphological, chemical, physiological, and stoichiometry traits (Fig. 2). Morphological traits were negatively (β = − 1.26) linked with physiological traits while positively with chemical traits. However physiological and chemical traits were positively related to each other (β = 1.96). Gs (β = 9.71) was having a strong direct effect followed by E (β = 0.26) for accounting the physiological features, with weak relationship for Chl (β = 0.04) and A (β = 0.03). The result indicated that with Gs increases, E decreases (r = − 0.21), however, Chl and A were positively (r = 0.29) related. LCC (β = 0.50), LNC (β = 0.57) and LPC (β = 0.41) were positively correlated with the functioning of the tree community. Morphological features were directly related to LDMC (β = 8.67) and LA, but indirectly related to SLA. Stoichiometry traits i.e., leaf C/N and leaf N/P were related with leaf chemical traits i.e. leaf C/N was positively related (r = 0. 26) with LCC, and leaf N/P were positively related (r = 0.12) with LNC.
Structural Equation Modeling (SEM) with JASP (Jeffreys’s Amazing Statistics Program) software (A free and Open Lience) “SEM package” (JASP 0.14.1) were used for evaluation of causal relationship among leaf morphological, physiological, chemical and stoichiometry traits in the temperate forest ecosystem. Square nodes indicate manifest variables, circular nodes indicate latent variables, and triangular nodes indicate constant variables (intercepts). Directed edges (single-headed) indicate one variable having an effect on another variable i.e. linear regression parameters and bidirectional edges indicate (co)variances (correlation “r”) between two variables, and the circular curved arrows represent the variance of a variable. The path coefficients represent standardized partial regression coefficients. Dashed line indicates weak relationship, thicker line strong relationship and numbers in brackets are regression coefficients. Morphological; LA = leaf area, cm2; SLA = specific leaf area, cm2 g−1; LDMC = leaf dry matter content, %; Chemical; LCC = leaf carbon content, %; LNC = leaf nitrogen content, %; LPC = leaf phosphorus content, %; Physiological; LWC = leaf water content, %; Chl = chlorophyll, mg g−1; A = photosynthetic rate, μmol CO2 m−2 s−1; Gs = stomatal conductance, mol H2O m−2 s−1; E = transpiration rate, mmol H2O m−2 s−1; Stoichiometry; Leaf C/N and Leaf N/P. The R2 value for LCC, LNC, LPC, E, and Chl was 0.25; 0.32; 0.17, 0.07, and 0.02 with respective latent factor. The latent factors are morphological traits (MPH) (LA + SLA + LDMC), chemical traits (CHE) (LCC + LNC + LPC) and physiological (PHY) (Chl + A + E + Gs); and regressions are CHE vs MPH and PHY vs MPH and CHE; and the residual covariances were LDMC vs SLA and LCC vs LPC.
Discussion
Evergreen leaves were characterized by higher leaf construction costs, slow nutrient returns, and tougher laminae. In contrast, deciduous leaves was associated with a higher photosynthetic rate per unit leaf mass, due to their higher LNC and SLA, higher intrinsic photosynthetic capacity, and less competition for light and carbon dioxide27. Evergreen broad-leaved favors infertile soils, longer leaf life span, and greater shade tolerance, that reduce seasonal variance in leaf exchange. In contrast, deciduous broad-leaved are favored by high seasonality, thermal, moisture, and light conditions, which are positively correlated across seasons28.
Morphological, chemical, physiological, and stoichiometry functional traits varied among different leaf habits in temperate forests and support for understanding the functioning of the forest ecosystem. In general, present evaluation observed variation in the traits and the order was: deciduous > evergreen > coniferous. However, LCC and leaf C/N ratio showed the reverse pattern (coniferous > evergreen > deciduous). There was little variation in LNC and LPC between the three leaf habits. Furthermore, present study found higher LA, SLA, and LDMC in deciduous broad-leaved species than in conifers and evergreen broad-leaved tree species. The higher LA and SLA in the deciduous broad-leaved species might be due to higher light interception1,29. Also, the higher SLA in deciduous broad-leaved species in tropical and subtropical forest indicates an acquisitive plant strategy8,30, while lower SLA in evergreens and conifers in subtropical forest indicates a conservative plant strategy1,31. LCC was significantly higher and LNC lower in conifers and evergreen broad-leaved plants in comparison to deciduous broad-leaved while LPC did not differ significantly among leaf-habits. A previous study on global data synthesis reported that leaves of conifers and evergreen broad-leaved plants have a higher carbon than deciduous broad-leaved species32. Higher carbon may be attributed to the presence of higher lignin content in conifers in comparison to the deciduous33. Leaf C/N was highest for conifers; however, leaf N/P was highest for deciduous species. The leaf C/N was high than subtropical forests8,34, possibly due to the high absorption capacity and utilize efficiency of nitrogen in the subtropical region8. High LWC is also reported for deciduous trees in temperate and boreal forests35,36. Chl and A were highest for evergreen broad-leaved tree species, possibly due to the higher availability of light and interaction with LNC. The availability of the leaf throughout the year enables the evergreen to use the nutrients to support the new growth and control photosynthesis18. Gs and E of leaves were lowest in deciduous species. The variation in the leave traits might be due to a robust leaf structure of evergreen species, which resists CO2 diffusion resulting in lower mesophyll conductance such as Gs37,38,39,40 and E41.
Our analysis for the evaluation of variations in leaf functional traits was across woody species in a temperate forest in the Indian Himalayan region. Among the different types of species studied, the three conifer species (Abies pindrow, Cedrus deodara, and Pinus wallichiana) had higher LCC and LDMC and are thus said to follow resource conservation strategy42. The three broad-leaved deciduous species (Aesculus indica, Pyrus pashia, and Toona ciliata) had higher SLA and LNC, indicating resource acquisition strategy. The three broad-leaved evergreen species (Euonymus pendulous, Quercus leucotrichophora, and Rhododendron arboretum) had high leaf Chl and A, and the traits leaf Chl and A did not exhibit significant differences among the three types. Although this study was limited to a specific region and climate, we do believe the results can be extended to other temperate forests dominated by the same representative species.
According to leaf economic theory coniferous have a resource conservation strategy with low SLA while the deciduous, have a strategy of fast acquisition with high SLA. Moreover, less productive plants tend to have low SLA, high LDMC, and leaves with long longevity (resource conservation strategy) however in the productive environment, the plants tend to have high growth rates, high SLA, low LDMC, low longevity leaves (fast acquisition strategy). The results of our study support the predictions of leaf economic theory, which is useful for understanding of functioning and a tool for predicting the responses of the forest vegetation to environmental changes, as plant strategies are dependent on the interactions among multiple traits1. This study provide information for further understanding the mechanism of species coexistence and predicting which kinds of species may assemble in a particular region in response to changes in environmental conditions.
We also observed that among all the morphological traits, LDMC was having strong relationship as observed by others7,8. LA and SLA were corelated as LA is directly affects the SLA43. Among physiological traits, Gs was having the strongest relationship, probablly due to the increase in CO2 concentration in the atmosphere as observed by others38,40,44. We found a negative relationship between Gs and E that might be due to the rise in CO2 level45,46. Our study also observed the positive relationship between Chl and A in the temperate region as reported by others36.
Conclusion
In the temperate forest studied, plant functional type classification explains forest ecosystem functioning. There have been few systematic studies on functional traits in temperate forest tree communities of the Himalayan region. This study investigated leaf functional traits concerning the morphology, physiology, chemical and stoichiometry component of the temperate forest tree community in this region. The results demonstrated that LA, SLA, LDMC, Gs, and E differed significantly between leaf habits, and their values followed the order deciduous > evergreen > conifers. The LCC showed the opposite pattern, conifers > evergreen > deciduous. Leaf Chl and A rates were highest for evergreen species, followed by deciduous and coniferous species. Overall, the variation in leaf functional traits affected leaf functions. Hence, species co-habiting in the same environment employ different plant adaptive strategies, i.e. conservative and acquisitive, for dealing with that environment. Moreover, variation in the functional traits among three-leaf habits largely supports the predictions of leaf economic theory.
Material and methods
Study site
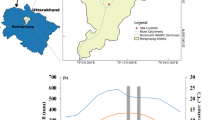
The temperate forest studied lies in the Indian Himalayan region of India, in Mussoorie Forest Division, Uttarakhand (30°28′02.6″N, 78°05′47.9″E; 2277 m asl). The mean annual rainfall in the region is around 2200 mm and the mean annual temperature is 20 °C. The region experiences three main seasons, winter (October to February), summer (March to June), and rainy (July to September)47. Soils of the region are Leptosols, Regosols, and Cambisols, developed mostly on dolomite48. The natural vegetation of the area is predominantly dense mixed forest (evergreen, deciduous, and coniferous tree species). Weather patterns differentiating this temperate forest and the region’s geographical features have enabled dominance of species such as oak, rhododendron, and conifers49.
Plant functional trait measurement
A vegetation survey was carried out using the quadrat method, where 20 quadrats, each measuring 10 m × 10 m, were laid out in the forest to study tree characteristics. The tree species were grouped into needle-leaved conifers, broad-leaved evergreens, and deciduous angiosperms (Table 1). LA, SLA, LDMC, LWC, and Chl were estimated based on measurements on five fresh, mature, fully expanded, and healthy leaves in five individuals per species, as described elsewhere23 (Table 2). LCC, LNC, and LPC were measured on fresh leaves collected from the forest, dried in the laboratory, crushed, and analyzed according to methods listed in Table 2. Leaf physiological traits, i.e., A, E, and Gs were measured by LICOR XT-6400 photosynthesis equipment. The youngest and fully expanded leaves were used preferentially for measuring physiological parameters and measurements were made between 9 am and 2 pm under clear-sky conditions. A total of 45 observations were made for each parameter, on nine trees (five replicates per tree) (Table 2). Permissions were granted from the Uttarakhand Forest Department for the collection of plant, wood, and soil samples and authors followed the guidelines with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. Dr Pravin Kumar Verma, Scientist, Division of Forest Botany, Forest Research Institute, Dehradun identified the plant species. The name of herbarium used for identification was DD Herbarium, Forest Research Institute, Dehradun. The specimens of the species were authenticated with the already available vouchers in the DD Herbarium of FRI Dehradun.
Data analysis
The mean values of all leaf traits was estimated by SPSS 23. The plant species are divided into three functional groups or leaf habits namely conifers, deciduous broad-leaved, and evergreen broad-leaved. ANOVA was applied to test the difference among the three functional groups for each functional trait and reported with Box-plot. Structural equation modeling was applied to examine the casual relationships among leaf morphological, physiological, chemical, and stoichiometric traits. SEM was conducted by the “lavaan package” and models were visualized with JASP software “SEM package”38.
Theoretical settings: a priori model
A theoretical framing of causal relationships between leaf morphological, physiological and chemical traits of temperate forest ecosystems, is explained in Fig. 3. According to an a priori model for the temperate forests, morphological and chemical features are understood to have a favorable relationship, while physiological traits and morphology are understood to have a negative relationship. This negative relationship may be attributed to the fact that many physiological processes are more plastic than structural processes50. Indeed, the physiology of trees can change dramatically without morphological changes in the short term50. Moreover, the difference in plasticity between morphological and physiological features is species-specific. Shade-intolerant species, for example, have greater physiological plasticity, while shade-tolerant species have greater morphological plasticity51. These complexities among the physiological, morphological and chemical features were used to assess species habitat affinities50, where leaf area was found to be positively associated with SLA1,8,28, as SLA influences canopy expansion and growth by changing total leaf area per plant and thereby affecting light interception and efficiency52. Chl, meanwhile, has a positive relationship with A14, as chloroplasts acclimatize to the environment and modulate the stoichiometry of components as per the requirements of plants53. A has a positive relationship with E51 for the management of leaf temperature54,55, and a negative relationship with Gs35,38. The stomata alter the aperture in response to external conditions in order to maximize the photosynthesis-water loss tradeoff56, and are therefore strategically linked to Chl and A. The responsiveness of leaf functional features to environmental variables, i.e. light and nutrient availability, is used by plant species to occupy environmental niches57. SLA is positively correlated with the relative growth rate of plants58, and reflects the potential rate of return on investment for a leaf intercepting light59. Leaf size accounts for water use efficiency and the amount of light intercepted for photosynthesis59, whereby SLA is positively correlated with the relative growth rate of plants58,60. Finally, the photosynthetic capacity of the leaves is positively associated with foliar N concentrations and specific leaf area61. Here we emphasize the significance of the ecological scale at which trait variation is considered, and suggests that common trait-by-trait scaling interactions should be handled with caution at regional to local scales. More specifically, PFT can be a useful predictor variable for inferring one feature from another. The results have significant implications for dynamic vegetation models at the local scale, and for using trait-based techniques to predict forest function at regional and local levels, depending on the availability of the data62,63.
(a) Leaf economic spectrum of different leaf habits (b) Theoretical framework of the casual relationships among morphological, physiological, chemical and stochiometry traits (Symbols; M: Morphology, P: Physiology, C: Chemical) of temperate forest ecosystem and (c) evidences for relationship along with direction between traits (Some other proposed relationship was hypothesized to exist in temperate forest).
References
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Lourens, P. & Frans, B. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87, 1733–1743 (2006).
Domínguez, M. T. et al. Relationships between leaf morphological traits, nutrient concentrations and isotopic signatures for Mediterranean woody plant species and communities. Plant Soil 357, 407–424 (2012).
Tian, M., Yu, G., He, N. & Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests Mechanisms and influencing factors. Sci. Rep. 6, 19703 (2016).
Paź-Dyderska, S. et al. Leaf traits and aboveground biomass variability of forest understory herbaceous plant species. Ecosystems 23, 555–569 (2020).
Lusk, C. H. Leaf functional trait variation in a humid temperate forest, and relationships with juvenile tree light requirements. PeerJ 7, e6855 (2019).
Liu, C., Li, Y., Xu, L., Chen, Z. & He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 9, 1–8 (2019).
Qin, J. & Shangguan, Z. Effects of forest types on leaf functional traits and their interrelationships of Pinus massoniana coniferous and broad-leaved mixed forests in the subtropical mountain, Southeastern China. Ecol. Evol. 9, 6922–6932 (2019).
Smart, S. M. et al. Leaf dry matter content is better at predicting above-ground net primary production than specific leaf area. Funct. Ecol. 31, 1336–1344 (2017).
Osnas, J. L. D., Lichstein, J. W., Reich, P. B. & Pacala, S. W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 340, 741–744 (2013).
Pierce, S. et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 31, 444–457 (2017).
Grime, J. P. Plant strategy theories: A comment on Craine (2005). J. Ecol. 95, 227–230 (2007).
Nam, K. J. & Lee, E. J. Variation in leaf functional traits of the Korean maple (Acer pseudosieboldianum) along an elevational gradient in a montane forest in Southern Korea. J. Ecol. Environ. 42, 33 (2018).
Li, Y. et al. Spatiotemporal variation in leaf size and shape in response to climate. J. Plant Ecol. 13, 87–96 (2020).
Liu, W., Zheng, L. & Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 10, 8166–8175 (2020).
Zhu, Z., Wang, X., Li, Y., Wang, G. & Guo, H. Predicting plant traits and functional types response to grazing in an alpine shrub meadow on the Qinghai-Tibet Plateau. Sci. China Earth Sci. 55, 837–851 (2012).
Wang, J. et al. Response of plant functional traits to grazing for three dominant species in alpine steppe habitat of the Qinghai-Tibet Plateau, China. Ecol. Res. 31, 515–524 (2016).
Negi, G. C. S. Leaf and bud demography and shoot growth in evergreen and deciduous trees of central Himalaya, India. Trees 20, 416–429 (2006).
Osnas, J. L. D. et al. Divergent drivers of leaf trait variation within species, among species, and among functional groups. PNAS 115, 5480–5485 (2018).
Liu, C., Li, Y., Xu, L., Chen, Z. & He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 9, 5803 (2019).
Zobel, D. B. & Singh, S. P. Himalayan forests and ecological generalizations. Bioscience 47, 735–745 (1997).
Kattge, J. et al. TRY—A global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011).
Pérez-Harguindeguy, N. et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167 (2013).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: Global convergence in plant functioning. PNAS 94, 13730–13734 (1997).
Güsewell, S. & Verhoeven, J. T. A. Litter N:P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 287, 131–143 (2006).
Niinemets, U. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol. 205, 79–96 (2015).
Devi, A. F. & Garkoti, S. C. Variation in evergreen and deciduous species leaf phenology in Assam, India. Trees 27, 985–997 (2013).
Givnish, T. Adaptive significance of evergreen vs. deciduous leaves: Solving the triple paradox. Silva Fenn. 36, 703–743 (2002).
Liu, Y. et al. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded?. Ann. Bot. 118, 1329–1336 (2016).
Derroire, G., Powers, J. S., Hulshof, C. M., Varela, L. E. C. & Healey, J. R. Contrasting patterns of leaf trait variation among and within species during tropical dry forest succession in Costa Rica. Sci. Rep. 8, 285 (2018).
Bai, K., He, C., Wan, X. & Jiang, D. Leaf economics of evergreen and deciduous tree species along an elevational gradient in a subtropical mountain. AoB Plants 7, plv064. https://doi.org/10.1093/aobpla/plv064 (2015).
Ma, S. et al. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 15, 693–702 (2018).
Singh, N. D. Leaf litter decomposition of evergreen and deciduous Dillenia species in humid tropics of north-east India. J. Trop. For. Sci. 14, 105–115 (2002).
Liang, X., Liu, S., Wang, H. & Wang, J. Variation of carbon and nitrogen stoichiometry along a chronosequence of natural temperate forest in northeastern China. J. Plant Ecol. 11, 339–350 (2018).
Lübbe, T., Schuldt, B. & Leuschner, C. Acclimation of leaf water status and stem hydraulics to drought and tree neighbourhood: Alternative strategies among the saplings of five temperate deciduous tree species. Tree Physiol. 37, 456–468 (2017).
Young-Robertson, J. M., Bolton, W. R., Bhatt, U. S., Cristóbal, J. & Thoman, R. Deciduous trees are a large and overlooked sink for snowmelt water in the boreal forest. Sci. Rep. 6, 1–10 (2016).
Hogan, K. P., Smith, A. P. & Samaniego, M. Gas exchange in six tropical semi-deciduous forest canopy tree species during the wet and dry seasons. Biotropica 27, 324–333 (1995).
Keel, S. G., Pepin, S., Leuzinger, S. & Körner, C. Stomatal conductance in mature deciduous forest trees exposed to elevated CO2. Trees 21, 151 (2006).
Kosugi, Y. & Matsuo, N. Seasonal fluctuations and temperature dependence of leaf gas exchange parameters of co-occurring evergreen and deciduous trees in a temperate broad-leaved forest. Tree Physiol. 26, 1173–1184 (2006).
Medlyn, B. E. et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: A synthesis. New Phytol. 149, 247–264 (2001).
Catovsky, S., Holbrook, N. M. & Bazzaz, F. A. Coupling whole-tree transpiration and canopy photosynthesis in coniferous and broad-leaved tree species. Can. J. For. Res. 32, 295–309 (2002).
Rawat, M., Arunachalam, K., Arunachalam, A., Alatalo, J. & Pandey, R. Associations of plant functional diversity with carbon accumulation in a temperate forest ecosystem in the Indian Himalayas. Ecol. Ind. 98, 861–868 (2019).
Weraduwage, S. M. et al. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 6, 167 (2015).
Sirisampan, S., Hiyama, T., Takahashi, A., Hashimoto, T. & Fukushima, Y. Diurnal and seasonal variations of stomatal conductance in a secondary temperate forest. J. Jpn. Soc. Hydrol. Water Resour. 16, 113–130 (2003).
Ghimire, C. P. et al. Transpiration and stomatal conductance in a young secondary tropical montane forest: Contrasts between native trees and invasive understorey shrubs. Tree Physiol. 38, 1053–1070 (2018).
Kirschbaum, M. U. F. & McMillan, A. M. S. Warming and elevated CO2 have opposing influences on transpiration. Which is more important?. Curr. For. Rep. 4, 51–71 (2018).
Saha, S., Rajwar, G. S. & Kumar, M. Soil properties along altitudinal gradient in Himalayan temperate forest of Garhwal region. Acta Ecol. Sin. 38, 1–8 (2018).
Raina, A. K. & Gupta, M. K. Soil characteristics in relation to vegetation and parent material under different forest covers in Kempty forest range, Uttarakhand. Indian Forester 135, 331–341 (2009).
Champion, S. H. G. & Seth, S. K. A Revised Survey of the Forest Types of India. (1968).
Belluau, M. & Shipley, B. Linking hard and soft traits: Physiology, morphology and anatomy interact to determine habitat affinities to soil water availability in herbaceous dicots. PLoS ONE 13, e0193130 (2018).
Rita, A. et al. Coordination of morphological and physiological traits in naturally recruited Abies alba Mill. saplings: Insights from a structural equation modeling approach. Ann. For. Sci. 74, 49 (2017).
Kumar, U., Singh, P. & Boote, K. J. Chapter two—effect of climate change factors on processes of crop growth and development and yield of groundnut (Arachis hypogaea L.). In Advances in Agronomy Vol. 116 (ed. Sparks, D. L.) 41–69 (Academic Press, 2012).
Gratani, L., Pesoli, P. & Crescente, M. F. Relationship between photosynthetic activity and chlorophyll content in an isolated Quercus ilex L. tree during the year. Photosynthetica 35, 445–451 (1998).
Lin, H., Chen, Y., Zhang, H., Fu, P. & Fan, Z. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 31, 2202–2211 (2017).
Damm, A., Haghighi, E., Paul-Limoges, E. & van der Tol, C. On the seasonal relation of sun-induced chlorophyll fluorescence and transpiration in a temperate mixed forest. Agric. For. Meteorol. 304–305, 108386 (2021).
Zhang, X. et al. Stomatal conductance bears no correlation with transpiration rate in wheat during their diurnal variation under high air humidity. PeerJ 8, e8927 (2020).
Wang, C., Zhou, J., Xiao, H., Liu, J. & Wang, L. Variations in leaf functional traits among plant species grouped by growth and leaf types in Zhenjiang, China. J. For. Res. https://doi.org/10.1007/s11676-016-0290-6 (2016).
Cornelissen, J. H. C., Castro Diez, P. & Hunt, R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 84, 755–765 (1996).
Zhang, S., Zhang, Y. & Ma, K. The association of leaf lifespan and background insect herbivory at the interspecific level. Ecology 98, 425–432 (2017).
Cunningham, S., Summerhayes, B. & Westoby, M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 69(4), 569–588. https://doi.org/10.1890/0012-9615(1999)069[0569:EDILSA]2.0.CO;2 (1999).
Reich, P. B. et al. Generality of leaf trait relationships: A test across six biomes. Ecology 80, 1955–1969 (1999).
Fyllas, N. M. et al. Functional trait variation among and within species and plant functional types in mountainous Mediterranean forests. Front. Plant Sci. 11, 212 (2020).
De Long, J. R. et al. Relationships between plant traits, soil properties and carbon fluxes differ between monocultures and mixed communities in temperate grassland. J. Ecol. 107, 1704–1719 (2019).
Acknowledgements
We gratefully acknowledge the Department of Science and Technology (DST), Government of India, for providing financial support through the INSPIRE Fellowship to MR. Laboratory facilities at Doon University were used for analytical work. JMA was supported by Qatar Petroleum.
Author information
Authors and Affiliations
Contributions
M.R. conceptualized, designed, and developed the data collection protocol; M.R. collected the data and analyzed the data with R.P.. M.R., K.A., A.A., J.A.M., and R.P. read and modified the draft manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rawat, M., Arunachalam, K., Arunachalam, A. et al. Assessment of leaf morphological, physiological, chemical and stoichiometry functional traits for understanding the functioning of Himalayan temperate forest ecosystem. Sci Rep 11, 23807 (2021). https://doi.org/10.1038/s41598-021-03235-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03235-6
- Springer Nature Limited
This article is cited by
-
Leaf and tree age-related changes in leaf ecophysiological traits, nutrient, and adaptive strategies of Alnus nepalensis in the central Himalaya
Journal of Biosciences (2024)
-
Exploring urban remnant forest soil bacterial diversity responses to woody plant leaf functional traits
Plant and Soil (2024)