Abstract
The concept that spinal manipulation therapy (SMT) outcomes are optimized when the treatment is aimed at a clinically relevant joint is commonly assumed and central to teaching and clinical use (candidate sites). This systematic review investigated whether clinical effects are superior when this is the case compared to SMT applied elsewhere (non-candidate sites). Eligible study designs were randomized controlled trials that investigated the effect of spinal manipulation applied to candidate versus non-candidate sites for spinal pain. We obtained data from four different databases. Risk of bias was assessed using an adjusted Cochrane risk of bias tool, adding four items for study quality. We extracted between-group differences for any reported outcome or, when not reported, calculated effect sizes from the within-group changes. We compared outcomes for SMT applied at a ‘relevant’ site to SMT applied elsewhere. We prioritized methodologically robust studies when interpreting results. Ten studies, all of acceptable quality, were included that reported 33 between-group differences—five compared treatments within the same spinal region and five at different spinal regions. None of the nine studies with low or moderate risk of bias reported statistically significant between-group differences for any outcome. The tenth study reported a small effect on pain (1.2/10, 95%CI − 1.9 to − 0.5) but had a high risk of bias. None of the nine articles of low or moderate risk of bias and acceptable quality reported that “clinically-relevant” SMT has a superior outcome on any outcome compared to “not clinically-relevant” SMT. This finding contrasts with ideas held in educational programs and clinical practice that emphasize the importance of joint-specific application of SMT.
Similar content being viewed by others
Introduction
Clinical guidelines recommend spinal manipulative therapy (SMT) as one possible intervention for spinal pain but do not provide specific details about how or where to deliver the intervention1,2. These generic recommendations do not consider the potential importance of applying manipulation at a specific application site, albeit such factors are considered important by many clinicians using manual therapy3. Much attention is invested in learning to determine the appropriate vertebral level, side, and thrust style (force–time profile), as this is believed to be important for clinical outcomes. SMT is, therefore, considered a highly skilled procedure that can only be mastered with extensive training. Consequently, the concept of treatment specificity of SMT is emphasized in clinical education3,4.
But what is, in fact, the evidence for this approach in relation to clinicians being able to identify a clinically relevant vertebra and that SMT produces a specific effect on or around this joint? A major hindrance to finding the exact site to treat is the poor diagnostic performance of many clinical tests used to locate aberrant spinal function5,6. On the other hand, once a segment has been selected, laboratory-based (animal) research has found biomechanical effects on the tissues and cell structures specific to the application site of SMT. For example, spinal stiffness at the treated vertebral level decreased at a higher rate at the site of SMT as compared to an adjacent vertebral level7. Similarly, higher muscle spindle discharge has been reported at the treated vertebral level than at the adjacent level8. However, it is unclear if such findings translate to humans and whether they have any clinical relevance. If indeed such effects are joint-specific in a clinical (human) context and of clinical relevance, the clinical outcomes would arguably differ depending on the SMT application site.
Therefore, the purpose of this review of clinical studies was to compare spine-related outcomes when SMT was applied at a candidate site presumed to be clinically relevant vs. when SMT was applied at any other spinal location.
Objectives
We explored whether SMT applied at a candidate site is superior to SMT applied at a non-candidate site in relation to the clinical outcome. Our primary outcome was between-group differences in patient-reported outcomes (e.g., pain intensity or disability). Secondary outcomes included objective measurements (e.g., pressure pain detection threshold (PPT) and range of motion).
Materials and methods
Design
This systematic review was submitted to The international prospective register of systematic reviews (PROSPERO) (ID = 202598). Minor additions were made to the protocol after registration. These included clarification of definitions for “candidate site” and “non-candidate site” and the addition of four items to rate study quality (in addition to the existing Cochrane risk of bias tool). The manuscript was prepared according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA2020) statement9. We have not published the protocol of the systematic review.
Eligibility criteria
SMT was defined as a high velocity, low amplitude force. This can be applied using two methods, either manually or via some instrument (e.g., an impulse device or a robotic arm). We included only randomized controlled study designs on humans with spinal pain in any region and of any duration, comparing SMT applied to any candidate site compared to SMT applied to any non-candidate site, where the between-group effect sizes were reported or estimable.
Non-thrust mobilization techniques (e.g., Maitland grades I through IV)10 were excluded. We also excluded studies that used different SMT applications (i.e., studies that compared manual SMT with any instrument-induced SMT) and studies in which some additional treatment was given to only one group. We also excluded studies that compared any SMT to sham SMT. Eligible studies had to be published in English or possible to be translated to English by a research team member. However, we did not find any relevant non-English articles.
The application site was determined as to where the treating clinician attempted to apply the force thrust of the SMT. We defined the candidate site as the SMT site determined to be relevant for clinical outcomes as i) prescribed by the treating clinician, regardless of the method used, or ii) if the clinician had to follow a procedure defined in a study protocol regardless of the method prescribed. As described above, the non-candidate site was SMT applied elsewhere in the spine but with no clinical indication.
We compared candidate SMT sites to the following three types of non-candidate SMT sites:
-
(i)
SMT at the candidate site compared to SMT to the opposite side of the indication (i.e., at the same spinal level but on the contralateral side—“same level”)
-
(ii)
SMT at the candidate site compared to SMT elsewhere in the same spinal region (i.e., cervical, thoracic, or lumbar—“same region”)
-
(iii)
SMT at the candidate site compared to SMT to a distant spinal region (“remote region”)
Search for literature
We systematically searched the literature in four electronic databases: PubMed, Embase, Index to Chiropractic Literature, and CINAHL from earliest to September 15th, 2020. The search strategy was initially developed for PubMed (S1) and afterward adopted to other databases in collaboration with a research librarian from the University of Southern Denmark. The search contained terms relating to (i) spinal pain, (ii) SMT applied at candidate sites, and iii) non-candidate SMT sites. MeSH terms and truncation (*) were elected as appropriate, allowing us to search multiple terms and portions of similar words.
Study selection
We used Covidence11 to handle the screening of potentially relevant studies. Titles and abstracts for all identified studies were screened for inclusion independently by two authors (CGN and AD), with differences discussed until consensus was reached. If consensus could not be reached, a third author would arbitrate the decision (CLY). After screening, the same two authors reviewed the relevant full texts until consensus was reached. If consensus could not be reached, the same third author would arbitrate the decision. However, no third opinions were necessary. Finally, CGN manually applied backward citation chaining by reviewing the references of each included study to identify potential additional studies.
Data extraction
One author (CGN) extracted data from included studies. A second author (SON) verified data extraction, resolving any discrepancy through consensus with a third author (AD). Data extraction included: study description, participant characteristics, description of intervention and control therapies, and outcome measurements at all time points. We extracted the between-group differences for all outcomes reported at all time points. If between-group differences were not reported, we calculated Cohen’s effect sizes based on the reported mean within-group changes in the SMT arms ([meancandidate − meannon-candidate]/SDpooled)12. We extracted only patient-reported outcomes if we had to calculate the effect sizes from the within-group changes due to statistical uncertainty about the assumptions12,13. Finally, if a study presented multiple different outcomes for the same domain (e.g., PPT at multiple regions), we extracted only the first reported result (e.g., PPT at the right arm).
We defined patient-reported outcomes as a subjective measurement if reported by the patient14 and objective measurements as assessments that are not subject to a large degree of subjective interpretation15. If > 20% of the data were missing, we did not extract that outcome. If it was apparent that outcome data necessary to compute between-group differences had been collected without being reported, we contacted the lead author to request the data.
Risk of bias and quality assessment
Each study was assessed for risk of bias by two authors independently (CGN (100%) and AD (50%), or CLY (50%)) using the Cochrane Risk of Bias tool (RoB) 116. We modified item (iii) “blinding of participants and personnel,” given that both study arms received SMT. Instead, we assessed whether the participants were naïve to SMT. Item (vii) “other sources of bias” assessed if the statistical analysis was performed in a blinded manner. The items are listed below with a description for “low risk of bias”:
-
(i)
Random sequence generation (i.e., reported that there was some independent sequence generation (including coin toss))
-
(ii)
Allocation concealment (i.e., reported that the allocation to study group was concealed to the assessor/clinician)
-
(iii)
Participants were naïve to SMT (i.e., the study subjects should be new to SMT or have no interest in the outcome. If they were likely to have been previous patients, the treatment must be such that they were unlikely to discern the difference between the candidate and the non-candidate site, thus considered to be effectively ‘blinded’ and unlikely to somehow ‘guide’ the outcomes)
-
(iv)
Blinding of outcome assessment (i.e., blinding of outcome assessors)
-
(v)
Incomplete outcome data (i.e., the drop-out rate must be clearly reported or discernible within the tables of results and not exceeding 20%)
-
(vi)
Selective reporting (i.e., all planned outcome variables reported in the Methods section must be reported in the Result section, and if available, also to be consistent with any trial registration or published protocols)
-
(vii)
Other sources of bias (this included blinded statistical analysis)
Authors (CGN, AD, CLY) undertook to pilot the risk of bias tool before independent assessment. Each item was reported as having “low” or “high” risk of bias and was considered to have “high risk” if the item was not reported. If we were unsure of an item, the item was reported as “unsure”. If consensus could not be reached, a third author (SON) would arbitrate the decision.
Risk of bias per study
The individual study’s overall RoB was considered to be “low risk” if there was a maximum of one “high risk” item and one “unsure” item. “Moderate risk” was defined if there were a maximum of two “high risk” items and one “unsure” item, and all other combinations were considered as “high risk”. This judgment was visualized using colors “low risk” (green), “unsure” (yellow), and “high risk” (red).
Risk of bias per item
We also collated the RoB for all included studies at the level of each item, using the same color labeling system. An item was considered to have a “low risk” of bias if it had a maximum of 2 red/yellow included studies, “moderate risk” if it had a maximum of 3 red/yellow included studies, and “high risk” for all the others.
The RoB is presented visually, and the figures were created in R vers. 4.117 for Ubuntu 20.04, using the add-on package dmetar18.
Quality assessment
In addition to the RoB tool, the following items were used to assess individual study quality19,20.
The quality assessment items were added, given that risk of bias assessment (alone) would not sufficiently capture study quality.
-
(i)
The SMT was sufficiently well described to be reproducible
-
(ii)
The experience of the investigator/therapist was sufficient to ensure competence in the delivery of SMT (e.g., not delivered by students)
-
(iii)
The primary outcome of the study was stated to have been validated. We considered pain and disability to be valid, regardless of whether this was stated in the article, as both are considered core outcomes in spine pain research21.
-
(iv)
The statistical analysis was reported to a sufficient level to facilitate re-analysis
Each item was marked as ‘yes’, ‘unsure’, or ‘no’. To be considered acceptable quality overall, studies had to satisfy ‘yes’ for at least items (iii) and (iv).
Study credibility
An individual study was considered credible if assessed as having either low or moderate RoB and acceptable quality.
Data synthesis
The synthesis is reported according to the Synthesis Without Meta-analysis (SWiM) in Systematic Reporting Guideline22. It was not possible to pool the results for meta-analysis due to heterogeneity in study design, the SMT application, and participant characteristics. We intended to report the differences in outcomes for the three control groups (“same level”, “same region”, or “remote region”) by counting the statistically significant between-group differences for all estimates. When interpreting results, we prioritized credible studies (low/moderate RoB and acceptable quality). All results are reported in tables.
Results
Description of studies
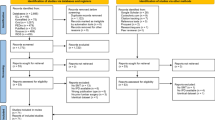
As shown in Fig. 1, we screened 3,288 articles, from which nine were included for analysis23,24,25,26,27,28,29,30,31. One additional article was found using backward citation tracking32, which resulted in ten included studies. All articles were in English and published between 2003 and 2020. All but three authors25,26,27 reported if there were any conflicts of interest, and four reported that they received funding23,24,31,32. We contacted the authors of three articles30,33,34 with insufficient data to estimate effect sizes. We received one response that allowed us to include that article30.
Table 1 lists descriptive information for each study. The study population ranged from 39 to 186, including patients with either cervical pain (n = 6) or lumbar pain (n = 4). Five studies included chronic pain patients, two included acute pain patients, and three did not specify this. The number of SMT sessions ranged from 1 to 10, and, most often, the outcomes were assessed immediately thereafter (n = 6). All but one study included patient-reported outcomes. Seven studies reported between-group differences for objective outcomes, most commonly PPT (n = 3). Four studies did not report between-group estimates. Therefore, we calculated effect sizes from the reported within-group differences25,26,30,32. No outcomes were excluded due to having more than 20% missing data.
Methodological quality and risk of bias
This area of research was considered to be credible based on RoB and quality. As shown in Table 2, the studies could be considered high quality, as nearly all achieved “yes” on the four domains (7/10). Specifically, all reported a valid outcome and included a reproducible statistical description.
Figure 2 shows that only one study was assessed as having high RoB28, four as moderate RoB27,29,31,32, and five as low RoB23,24,25,26,30. Items that commonly were deficient were “naïve study subjects” (to SMT) and “other sources of bias” (statistical analysis was performed blinded) (Fig. 3).
Summary of risk of bias for 10 studies in a systematic review comparing the outcome of applying spinal manipulative therapy at a candidate site versus a non-candidate site. The risk of bias was assessed using a modified version of the Cochrane Risk of Bias Tool for Randomized Controlled Trials. A ‘green + ’ indicates low risk of bias, a ‘red –’ indicates high risk of bias, and a ‘yellow ?’ indicates an unsure risk of bias.
Risk of bias for each item across 10 studies included in a systematic review comparing the outcome of applying spinal manipulative therapy at a candidate site versus a non-candidate site. The risk of bias was assessed using a modified version of the Cochrane Risk of Bias Tool for Randomized Controlled Trials. ‘Green’ indicates low risk of bias, ‘red’ indicates high risk of bias, and ‘yellow’ indicates an unsure risk of bias.
SMT applied at the same vertebral level
Only one study of moderate RoB examined whether SMT outcomes differed when applied at the same vertebral level32. The candidate site was determined by a clinician using palpation for movement restriction, and the control SMT was applied at the same vertebra but in counter-direction. Outcomes were measured immediately following two SMT sessions and at two weeks follow-up. The study reported no between-group differences in neck pain or disability when comparing these two approaches.
SMT applied in the same spinal region
Four studies applied SMT at a candidate site and SMT at a non-candidate site in the same spinal region. One study compared SMT at a candidate site to a random site23, and three to a non-specific or generalized regional SMT24,25,28 (i.e., the non-candidate SMT did not attempt to target a specific vertebral level).
In the first study23, assessed as low RoB, the clinician determined the candidate site by palpation assessing endplay and compared it to a non-candidate site determined as a matched random site in the cervical spine. Subjective neck pain, disability, and stiffness were measured immediately following one SMT session. No between-group differences were found.
Two studies assessed low back pain24,25, and the final study assessed neck pain28. The two low back pain studies were of low RoB, while the neck pain study was of high RoB. All outcomes were subjective and measured immediately after the first and only SMT session. However, one of the studies24 provided two sessions and repeated the measurements immediately following the second SMT session, at four weeks and 26 weeks. Only the neck pain study of high RoB28 reported a statistically significant but small between-group difference favoring the clinically relevant application, whereas the remaining two studies did not find any between-group differences.
SMT applied in a remote spinal region
The remaining five studies compared SMT applied at a candidate site in the symptomatic area to SMT applied at a non-candidate site at a remote region26,27,29,30,31. Two investigated low back pain and compared SMT at the symptomatic lumbar spine versus SMT in the asymptomatic thoracic spine27,31. Two compared symptomatic cervical SMT to asymptomatic thoracic SMT for neck pain26,29, and one study examined upper cervical SMT to a series of SMTs at non-candidate sites: lower cervical, cervicothoracic, and mid-thoracic30.
The two low back pain studies27,31 were both of moderate RoB. The first study examined immediate changes following a single SMT session at the symptomatic lower back compared to the asymptomatic thoracic spine27. They found no between-group difference for patient-reported low back pain or PPT at the lumbar spine. The same author group reproduced this trial in 2020, now including ten SMT sessions instead and measured changes in subjective low back pain, disability, and global perceived change, as well as objective PPT at four, 12, and 26 weeks31. Again, there were no statistically significant differences, with all between-group differences close to 0 and with narrow confidence intervals.
Three studies assessed neck pain26,29,30. Two studies reported immediate changes. The first was of low RoB26 and compared cervical SMT at both the right and left side to thoracic SMT. As no between-group differences were reported between the left and right sides, we extracted results only from the right side (candidate site) compared to thoracic SMT (non-candidate site). This study found no between-group difference in neck pain. The second study of low RoB30 compared SMT at the candidate site (upper cervical vertebrae) to multiple SMTs at non-candidate sites and reported no between-group difference in neck pain intensity. The final study, which was of moderate RoB29, chose C7 as the candidate site (the clinician determined whether it was to be treated on the left or right side) and compared it to SMT at a non-candidate site (T3 level) for neck pain participants. No subjective outcomes were reported, only multiple PPTs across both upper limbs and bilateral grip strength immediately following one SMT session. We extracted only the initial PPT assessment (right wrist) and grip strength for the right hand. The between-group differences were not statistically significant.
Summary of results
All results are reported in Table 3. We extracted a total of 33 between-group differences from ten studies. From these, nine studies23,24,25,26,27,29,30,31 (31 comparisons) reported no statistical between-group differences (low/moderate RoB, acceptable quality). Only one study28 (two comparisons) statistically favored SMT applied at the candidate site compared SMT at a non-candidate site for neck pain (mean difference of 1.2 out of 10 points (95% confidence interval = − 1.9 to − 0.5)) (high RoB, acceptable quality). Side effects were either not reported or were minimal and did not differ between groups receiving SMT at candidate and non-candidate sites.
Discussion
Statement of principal findings
This systematic review included ten randomized controlled clinical studies, of which nine were considered to have credible results. None of these nine studies detected any statistically significant differences in the 31 outcome measurements for the two treatment approaches. In other words, SMT given at a clinician-determined “correct” vertebral level did not have better outcomes than treatment given more haphazardly. These outcome measurements included pain, disability, and other objective outcomes. The only study to confirm the importance of treating the clinically relevant segment reported a small reduction in neck pain (1.2 points on an 11-point numerical rating scale)28. Although the magnitude of this effect is below the threshold for a minimally clinically important difference in this population35, the finding was statistically significant. However, that study was the only one assessed as having high RoB, which questions the validity of this result.
Methodological considerations
Strengths and weaknesses of this review
Our review had several strengths: We independently selected the studies and data extraction protocols. We cannot exclude the possibility that other relevant publications have been missed. However, as the manual perusal of reference lists resulted in only one additional study, our search was likely near exhaustive. In addition, one RoB assessment criterion (item iii) was amended to reflect actual participant blinding. Although the modification of the RoB and the addition of the quality items is an approach that has not undergone careful external validation, the modification is uncomplicated and meaningful. As it is a methodological adjustment that fits the current study types, it is probably more a strength than a potential weakness. Also, a different approach is unlikely to have resulted in a different overall assessment of the credibility.
Many of the included studies did not provide estimates for their between-group mean differences. Therefore, instead of omitting the data, we calculated effect sizes from the mean within-group changes. However, this approach may have introduced errors as we had no means of confirming the underlying statistical assumptions for such calculations, particularly relevant for small samples, where the data could be skewed, heteroscedastic, or include outliers12,13. For that reason, we opted to make this approach only for the primary outcome (i.e., the patient-reported outcomes).
The systematic search was intentionally sensitive, as we expected a broad range of study methods. When considering the heterogeneity in both study design and outcome measurements, a meta-analysis was not feasible to conduct. However, it could also be argued that this heterogeneity is a strength of the review, as all the outcomes, except one, nevertheless follow the same pattern. The lack of pooling, not possible with such a small number of studies in each subgroup, also precluded any statistical modeling (e.g., exploring other factors) that may explain the lack of effects such as technique, thrust direction, speed, and how the candidate site was selected or patient characteristics, such as pain duration. Also, we expected multiple different outcomes to be reported, which is why we did not limit ourselves to any specific outcomes but extracted what was reported in the included studies.
Strengths and weaknesses of the included studies
Nine of the ten included studies were assessed as credible. Considering the RoB assessment, it is important to notice that blinding of participants and personnel is impossible in trials comparing SMT at two different regions36, so we removed this domain and considered instead whether the participants were naïve to SMT or not. Thus, instead of being blind to the type of treatment, the subjects should not have had a pre-determined idea of where and how SMT should be best applied. The issue is that only a few studies that we reviewed reported clearly to have taken this into account, which is probably a weakness of the studies that did not report (or consider) this version of participant blinding. However, we argue that the presence of this potential bias should have increased the likelihood that SMT applied at a candidate site being more effective. On the contrary, the studies generally did not find any between-group differences, and we consider that this further confirms our conclusion. Additionally, no studies reported whether the participants could infer if they received SMT at the candidate or non-candidate site.
A strength of the studies was the methodologically and reproducible trials, however, this is also a weakness as most studies investigated a single intervention (often a single session of SMT) in patients with chronic pain. Thus, the lack of difference between groups could perhaps be explained by i) the short duration of the intervention and ii) the clinical presentation.
Clinical interpretation
To our knowledge, this is the first systematic review to explore the importance of the specificity of the application site of SMT in relation to clinical outcomes. As such, we are not able to compare these results against other studies. Our review advances evidence in this field and provides a more rigorous methodology to other narrative syntheses or evidence from individual studies on the subject.
The current systematic review failed to find any measurable difference in clinical outcome measurements based on whether the SMT was applied at a vertebral level based on clinical assessment (e.g., motion palpation) or not. This may run counter to the expectations and clinical experiences of those engaged in SMT. However, on reflection, this finding should not be surprising for several reasons.
The candidate site is a subjective concept
There are many lines of thinking regarding what tests to use to detect these presumed clinically relevant candidate sites to apply SMT5. Alas, there appear to be no studies that have succeeded in showing that such tests are reliable and reproducible. At the same time, it might be possible to locate a block vertebra using motion palpation37, and one chiropractor was able to recognize untreated patients by using this examination method, it was not possible to identify the treated patients38. Further, motion palpation cannot reliably distinguish between individuals from the general population with or without low back pain39. More recently, a systematic review recommended against the use of stand-alone tests for segmental motion assessment in patients with LBP6. Until demonstrated otherwise, reliable identification of a clinically relevant segment using manual assessment must be considered dubious.
Therefore, the detection method applied will depend on the profession, school of training, the fashion at the time of training, and own experience and preference. It is possible that, perhaps, clinically relevant candidate sites exist, but clinicians are unable to find them, which may explain the lack of difference in outcome between study groups. Therefore, the outcome in both groups may reflect not similarly promising results but similarly poor results. Thus, the results may simply capture the natural course of the condition in both groups at the time of assessment and indicate that the clinically relevant application site for SMT may, at present, be a nonsense concept. This is further supported by recent work concluding that the application site is not important for clinical outcomes despite attempting to target objectively determined clinically relevant sites, either in relation to stiffness or pain sensitivity40.
The manipulation is not specific
Another explanation relates not to the questionable validity of test procedures but in attempting to perform a specific SMT procedure. It has been shown that SMT has a wider effect on multiple vertebral joints, both in proximity and further away from the application site. Studies in which accelerometers or microphones have been used to record the location of the “crack”-sound associated with SMT have found that it does not necessarily stem from the SMT application site41,42,43. It is not obvious how to interpret such findings, but they certainly do not suggest that the mechanical effects of SMT are restricted to the application site.
A neuromuscular or biomechanical mechanism might explain the positive results of SMT
The positive changes observed after SMT may be unrelated to treatment specificity but an effect of a generalized (systemic) effect or biomechanical interactions, such as functional changes in a “biomechanical chain” and spinal regional interdependence44. This could explain why thoracic SMT seems to reduce cervical pain in clinical adult populations45,46,47. Examples of other potential biomechanical effects are increased disc diffusion and decreased posterior-anterior stiffness48. Other systemic effects could include changes in the functioning of descending anti-nociceptive system49, a widespread effect on muscle spindle response50, and central mechanisms of pain modulation51. These examples are not an exhaustive list of potential mechanisms, as this topic is outside the scope of this systematic review. Possibly, the benefits of SMT might come from mechanisms that have not yet been investigated thoroughly52 or complex interactions that cannot currently be understood.
Contextual contributions might explain the positive results of SMT
It is possible that at least some positive effects of SMT may be due to non-specific mechanisms such as contextual contributions (e.g., patient expectations and a response to the therapeutic alliance)53,54. These systemic and non-specific factors could contribute to an increased improvement following SMT. The same has been observed in acupuncture55 and exercise56, and it is a general finding across multiple interventions57. The same argument can be made for SMT in general, as it is non-superior to non-thrust mobilization or even sham SMT58. Thus, the application site (e.g., spinal level) and application type (high velocity, low amplitude or mobilization) would not be central to successful manual therapy. The results of this systematic review support this statement.
A more nuanced theory
Thus, while SMT appears to be an efficient intervention in some with spinal pain conditions58,59, the choice of the application site does not appear to modify this effect, and a more nuanced theory of treatment mechanism must account for this observation. Finally, these findings apply to all manual therapist professions as the efficacy of SMT does not appear to be therapist-dependent58.
Unanswered questions and future research
Future research
We acknowledge that further research is required to determine the underlying mechanisms of SMT. However, as clinicians cannot quantify or reliably locate spinal dysfunctions suitable for SMT application, clinicians must accept that the choice of SMT application site is based on an entirely subjective decision process. Therefore, there appears to be limited value in conducting further trials striving to optimize SMT by comparing specific applications as an intervention for spinal pain, at least until our knowledge of SMT mechanisms has improved.
Educational institutions
This review does not contradict the teaching and clinical use of SMT. However, it suggests that the best available evidence does not emphasize technical concepts of specificity related to improving clinical outcomes. We recommend that curricula should include how “non-specific SMT” can be used advantageously.
Conclusions
The current evidence does not support that SMT applied at a supposedly “clinically relevant” candidate site is superior to SMT applied at a supposedly “not clinically relevant” site for individuals with spinal pain. Whether this is true for objective outcomes is unknown. A more nuanced model related to the concept of specificity in spinal manipulation needs to be established and systematically tested for validity.
Data availability
All data are available in Supplementary information 2.
References
Oliveira, C. B. et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 27, 2791–2803 (2018).
Corp, N. et al. Evidence-based treatment recommendations for neck and low back pain across Europe: A systematic review of guidelines. Eur. J. Pain 25, 275–295 (2021).
Bergmann, T. F. & Peterson, D. H. Chiropractic Technique: Principles and Procedures, 3e (Mosby, 2010).
Stainsby, B. E., Clarke, M. C. S. & Egonia, J. R. Learning spinal manipulation: A best-evidence synthesis of teaching methods. J. Chiropr. Educ. 30, 138–151 (2016).
Triano, J. J. et al. Review of methods used by chiropractors to determine the site for applying manipulation. Chiropractic & Manual Therapies 21, 36 (2013).
Stolz, M., von Piekartz, H., Hall, T., Schindler, A. & Ballenberger, N. Evidence and recommendations for the use of segmental motion testing for patients with LBP—A systematic review. Musculoskelet. Sci. Pract. 45, 102076 (2020).
Edgecombe, T. L., Kawchuk, G. N., Long, C. R. & Pickar, J. G. The effect of application site of spinal manipulative therapy (SMT) on spinal stiffness. Spine J. 15, 1332–1338 (2015).
Reed, W. R., Long, C. R., Kawchuk, G. N. & Pickar, J. G. Neural responses to the mechanical characteristics of high velocity, low amplitude spinal manipulation: Effect of specific contact site. Man. Ther. 20, 797–804 (2015).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Maitland’s Peripheral Manipulation: Management of Neuromusculoskeletal Disorders—Volume 2. (Churchill Livingstone, 2013).
Veritas Health Innovation, A., Melbourne. Covidence—Better systematic review management.
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Academic Press, 2013).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 (2013).
Powers, J. H. et al. Clinician-Reported Outcome Assessments of Treatment Benefit: Report of the ISPOR Clinical Outcome Assessment Emerging Good Practices Task Force. Value Health 20, 2–14 (2017).
Velentgas, P., Dreyer, N. A., Nourjah, P., Smith, S. R. & Torchia, M. M. Outcome Definition and Measurement. (Agency for Healthcare Research; Quality (US), 2013).
Higgins, J. P. T. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
R Development Core Team. R: A Language and Environment for Statistical Computing. (2009).
Harrer, M., Cuijpers, P., Furukawa, T. & Ebert, D. D. Dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’ (2019).
Meyer, A.-L., Amorim, M.-A., Schubert, M., Schweinhardt, P. & Leboeuf-Yde, C. Unravelling functional neurology: Does spinal manipulation have an effect on the brain?—A systematic literature review. Chiropr. Man Therap. 27, 60 (2019).
Côté, P. et al. The global summit on the efficacy and effectiveness of spinal manipulative therapy for the prevention and treatment of non-musculoskeletal disorders: A systematic review of the literature. Chiropr. Man Therap. 29, 8 (2021).
Chiarotto, A. et al. Core outcome domains for clinical trials in non-specific low back pain. Eur. Spine J. 24, 1127–1142 (2015).
Campbell, M. et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 368, l6890 (2020).
Haas, M. et al. Efficacy of cervical endplay assessment as an indicator for spinal manipulation. Spine 28, 1091–1096 (2003).
Cleland, J. A. et al. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: A randomized clinical trial. Spine 34, 2720–2729 (2009).
Sutlive, T. G. et al. Comparison of short-term response to two spinal manipulation techniques for patients with low back pain in a military beneficiary population. Mil. Med. 174, 750–756 (2009).
Martínez-Segura, R., de-la-Llave-Rincón, A. I., Ortega-Santiago, R., Cleland, J. A. & Fernández-de-las-Peñas, C. Immediate changes in widespread pressure pain sensitivity, neck pain, and cervical range of motion after cervical or thoracic thrust manipulation in patients with bilateral chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports. Phys. Ther. 42, 806–814 (2012).
de Oliveira, R. F., Liebano, R. E., Costa, L. C. M., Rissato, L. L. & Costa, L. O. P. Immediate effects of region-specific and non-region-specific spinal manipulative therapy in patients with chronic low back pain: A randomized controlled trial. Phys. Ther. 93, 748–756 (2013).
Karas, S. & OlsonHunt, M. J. A randomized clinical trial to compare the immediate effects of seated thoracic manipulation and targeted supine thoracic manipulation on cervical spine flexion range of motion and pain. J. Man. Manip. Ther. 22, 108–114 (2014).
Bautista-Aguirre, F. et al. Effect of cervical vs. Thoracic spinal manipulation on peripheral neural features and grip strength in subjects with chronic mechanical neck pain: A randomized controlled trial. Eur. J. Phys. Rehabilitat. Med. 53, 333–341 (2017).
Romero del Rey, R., Saavedra Hernández, M., Rodríguez Blanco, C., PalomequedelCerro, L. & Alarcón Rodríguez, R. Short-term effects of spinal thrust joint manipulation on postural sway in patients with chronic mechanical neck pain: A randomized controlled trial. Disabil. Rehabil. https://doi.org/10.1080/09638288.2020.1798517 (2020).
de Oliveira, R. F., Costa, L. O. P., Nascimento, L. P. & Rissato, L. L. Directed vertebral manipulation is not better than generic vertebral manipulation in patients with chronic low back pain: A randomised trial. J. Physiother. 66, 174–179 (2020).
Karas, S. et al. The effect of direction specific thoracic spine manipulation on the cervical spine: A randomized controlled trial. Journal of Manual & Manipulative Therapy 26, 3–10 (2018).
Puentedura, E. J. et al. Thoracic spine thrust manipulation versus cervical spine thrust manipulation in patients with acute neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 41, 208–220 (2011).
McCarthy, C. J., Potter, L. & Oldham, J. A. Comparing targeted thrust manipulation with general thrust manipulation in patients with low back pain. A general approach is as effective as a specific one. A randomised controlled trial. BMJ Open Sport Exerc. Med. 5, e000514 (2019).
Bussières, A. E. et al. The treatment of neck pain-associated disorders and whiplash-associated disorders: A clinical practice guideline. J. Manipulative Physiol. Ther. 39, 523-564.e27 (2016).
Kamper, S. J. Blinding: Linking evidence to practice. J. Orthop. Sports Phys. Ther. 48, 825–826 (2018).
Humphreys, B. K., Delahaye, M. & Peterson, C. K. An investigation into the validity of cervical spine motion palpation using subjects with congenital block vertebrae as a ’gold standard’. BMC Musculoskelet. Disord. 5, 19 (2004).
Hansen, B. E., Simonsen, T. & Leboeuf-Yde, C. Motion palpation of the lumbar spine—A problem with the test or the tester?. J. Manipulative Physiol. Ther. 29, 208–212 (2006).
Leboeuf-Yde, C. et al. Motion palpation findings and self-reported low back pain in a population-based study sample. J. Manipulative Physiol. Ther. 25, 80–87 (2002).
Nim, C. G., Kawchuk, G. N., Schiøttz-Christensen, B. & O’Neill, S. The effect on clinical outcomes when targeting spinal manipulation at stiffness or pain sensitivity: A randomized trial. Sci. Rep. 10, 14615 (2020).
Ross, J. K., Bereznick, D. E. & McGill, S. M. Determining cavitation location during lumbar and thoracic spinal manipulation: Is spinal manipulation accurate and specific?. Spine 29, 1452–1457 (2004).
Beffa, R. & Mathews, R. Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds. J. Manipulative Physiol. Ther. 27, 118–122 (2004).
Dunning, J. et al. Bilateral and multiple cavitation sounds during upper cervical thrust manipulation. BMC Musculoskelet. Disord. 14, 24 (2013).
McDevitt, A., Young, J., Mintken, P. & Cleland, J. Regional interdependence and manual therapy directed at the thoracic spine. J. Man. Manip. Ther. 23, 139–146 (2015).
Cross, K. M., Kuenze, C., Grindstaff, T. & Hertel, J. Thoracic spine thrust manipulation improves pain, range of motion, and self-reported function in patients with mechanical neck pain: A systematic review. J. Orthop. Sports Phys. Ther. 41, 633–642 (2011).
Huisman, P. A., Speksnijder, C. M. & de Wijer, A. The effect of thoracic spine manipulation on pain and disability in patients with non-specific neck pain: A systematic review. Disabil. Rehabil. 35, 1677–1685 (2013).
Masaracchio, M. et al. Thoracic spine manipulation for the management of mechanical neck pain: A systematic review and meta-analysis. PLoS ONE 14, e0211877 (2019).
Wong, A. Y. L., Parent, E. C., Dhillon, S. S., Prasad, N. & Kawchuk, G. N. Do participants with low back pain who respond to spinal manipulative therapy differ biomechanically from nonresponders, untreated controls or asymptomatic controls?. Spine 40, 1329–1337 (2015).
Skyba, D. A., Radhakrishnan, R., Rohlwing, J. J., Wright, A. & Sluka, K. A. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain 106, 159–168 (2003).
Reed, W. R. & Pickar, J. G. Paraspinal muscle spindle response to intervertebral fixation and segmental thrust level during spinal manipulation in an animal model. Spine 40, E752–E759 (2015).
Randoll, C. et al. The mechanism of back pain relief by spinal manipulation relies on decreased temporal summation of pain. Neuroscience 349, 220–228 (2017).
Matyas, J. R., Klein, C., Ponjevic, D., Duncan, N. A. & Kawchuk, G. N. Repetitive in vivo manual loading of the spine elicits cellular responses in porcine annuli fibrosi. PLoS ONE 16, e0248104 (2021).
Newell, D., Lothe, L. R. & Raven, T. J. L. Contextually Aided Recovery (CARe): A scientific theory for innate healing. Chiropractic & Manual Therapies 25, 6 (2017).
Lennep, J.H.P.A. et al. Placebo effects in low back pain: A systematic review and meta‐analysis of the literature. European Journal of Pain 25, 1876–1897 (2021).
Linde, K., Niemann, K., Schneider, A. & Meissner, K. How large are the nonspecific effects of acupuncture? A meta-analysis of randomized controlled trials. BMC Med. 8, 75 (2010).
Miller, C. T. et al. Attempting to separate placebo effects from exercise in chronic pain: A systematic review and meta-analysis. Sports Med. https://doi.org/10.1007/s40279-021-01526-6 (2021).
Hafliðadóttir, S. H. et al. Placebo response and effect in randomized clinical trials: Meta-research with focus on contextual effects. Trials 22, 493 (2021).
Rubinstein, S. M. et al. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: Systematic review and meta-analysis of randomised controlled trials. The BMJ 364, l689 (2019).
Hidalgo, B. et al. The efficacy of manual therapy and exercise for treating non-specific neck pain: A systematic review. J. Back Musculoskelet. Rehabil. 30, 1149–1169 (2018).
Acknowledgements
The authors would like to acknowledge research librarian Anne Faber Hansen, PhD at the University of Southern Denmark, to help with the literature search and for having ‘translated’ the search method from PubMed to the other databases. The project was supported by The Danish Chiropractic Fund for Research and Post Graduate Research (R32-A977-B845).
Author information
Authors and Affiliations
Contributions
C.G.N.: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Writing—Original Draft Preparation. A.D.: Data Curation, Formal Analysis, Investigation, Writing—Review & Editing. S.O.N.: Data Curation, Supervision, Writing–Review & Editing. G.N.K.: Conceptualization, Supervision, Writing—Review & Editing. S.M.P.: Supervision, Writing—Review & Editing. C.L.Y.: Conceptualization, Data Curation, Formal Analysis, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nim, C.G., Downie, A., O’Neill, S. et al. The importance of selecting the correct site to apply spinal manipulation when treating spinal pain: Myth or reality? A systematic review. Sci Rep 11, 23415 (2021). https://doi.org/10.1038/s41598-021-02882-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02882-z
- Springer Nature Limited
This article is cited by
-
A modern way to teach and practice manual therapy
Chiropractic & Manual Therapies (2024)
-
A new role for spinal manual therapy and for chiropractic? Part I: weaknesses and threats
Chiropractic & Manual Therapies (2024)
-
A randomized controlled trial comparing different sites of high-velocity low amplitude thrust on sensorimotor integration parameters
Scientific Reports (2024)
-
Clinician approaches to spinal manipulation for persistent spinal pain after lumbar surgery: systematic review and meta-analysis of individual patient data
Chiropractic & Manual Therapies (2023)
-
The effectiveness of spinal manipulative therapy procedures for spine pain: protocol for a systematic review and network meta-analysis
Chiropractic & Manual Therapies (2023)