Abstract
Daratumumab has shown clinical benefit in multiple myeloma. We aimed to evaluate the safety and efficacy of adding daratumumab to backbone anti-myeloma treatments. Systematic search was performed up to August 2021 to identify randomised controlled trials comparing the outcomes of backbone therapy with and without daratumumab in relapsed/refractory and newly diagnosed myeloma (RRMM and NDMM, respectively). Odds ratios (ORs) and hazard ratios (HRs) were calculated with 95% confidence intervals (CIs). Primary outcomes were death or disease progression, minimal residual disease (MRD) negativity, and stringent complete response (sCR). Secondary outcomes were complete response or better and safety endpoints prespecified in the study protocol: PROSPERO (CRD42020222904). In NDMM, MRD negativity [OR = 3.61 (CI 2.33–5.61)] and sCR [OR = 2.29 (CI 1.49–3.51)] were more likely and death or disease progression [HR = 0.47 (CI 0.39–0.57)] was less likely to occur with daratumumab compared to control. Regarding RRMM, MRD negativity [OR = 5.43 (CI 2.76–10.66)] and sCR [OR = 3.08 (CI 2.00–4.76)] were more likely and death or disease progression was less likely [HR = 0.50 (CI 0.37–0.67)] with daratumumab compared to control. The addition of daratumumab has shown high clinical efficacy and acceptable toxicity profile for the treatment of NDMM and RRMM regarding the endpoints examined.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is the second most common haematologic malignancy, accounting for 1% of all cancers1. In the early 2000s, the approval of modern therapeutic options, such as immunomodulatory drugs (IMiD) and proteasome inhibitors (PI), greatly improved the relative survival rate in MM2. Although new innovative agents have brought a considerable breakthrough, this disease’s the treatment is still a challenging pursuit because of the frequent relapses. The 5-year survival rate is only 52.2%, despite the tremendous advances and continuous evolving therapeutic strategies3 Over the past decade, monoclonal antibodies were also proved to be an essential part of the therapeutic arsenal, especially in combination with the aforementioned novel agents4.
CD38 is overexpressed on myeloma cells’ surface, making this transmembrane glycoprotein a good target for immunotherapy in MM. Daratumumab is a CD38-targeted human IgG monoclonal antibody that exerts its antineoplastic effect through complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity and phagocytosis, programmed cell death after crosslinking, and inhibition of ectoenzyme function of CD385.
This agent was approved as a monotherapy for relapsed MM by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2015 and 2016, respectively. Additionally, daratumumab is also indicated for combination therapy (e.g. with bortezomib, melphalan, and prednisone in newly diagnosed MM (NDMM) or with lenalidomide and dexamethasone in patients who have received at least one prior therapy6). Furthermore, new regimens including daratumumab are evaluated by ongoing clinical trials (NCT04288765, NCT03180736, NCT04649060, NCT03710603).
Since the FDA and EMA approved daratumumab, its incorporation into myeloma treatment regimens has significantly improved the outcomes of myeloma treatment including stringent complete response (sCR) and minimal residual disease (MRD) negativity, translating to prolonged progression-free survival (PFS) and overall survival (OS) with a relatively safe toxicity profile. Recent meta-analyses have assessed the safety and efficacy of daratumumab as an addition to backbone treatments in MM. Xu et al. found that addition of daratumumab to first-line regimens (bortezomib, melphalan, and prednisone or lenalidomide and dexamethasone) significantly improves PFS, compared to the same regimens alone, in NDMM. Furthermore, in their study, patients receiving daratumumab, compared to patients on the control arms, had a higher chance to achieve complete response (CR) rate or better7. Similar benefit of daratumumab was found by Wang et al. regarding CR or better in relapsed/refractory MM (RRMM)8. In another meta-analysis, Giri et al. revealed longer PFS in patients receiving daratumumab both in NDMM and RRMM regardless of cytogenetic risk9. The publication by Cao et al. focused on RRMM. Their results also suggested that daratumumab- based therapies enhance PFS in RRMM regardless of patient's baseline characteristics or previous therapeutic agents10. Another meta-analysis also investigated the impact of cytogenetic risk on the PFS benefit provided by the addition of daratumumab. They observed an increased PFS in RRMM patients with daratumumab; however, they did not reveal benefit of that agent in high genetic risk NDMM11. Similarly to that publication, the findings of two other studies could not support the survival benefit of daratumumab in high cytogenetic risk NDMM12,13.
Since these studies' publication, more evidence has become available, allowing us to re-evaluate these results performing meta-analytic calculations and to assess new endpoints such as sCR or MRD negativity. Furthermore, our objective was to resolve the discrepancies between the findings in previous reports. Besides these goals, our meta-analysis aims to summarise evidence on daratumumab containing backbone regimens' safety profile compared to the same combinations without daratumumab.
Materials and methods
We report this study in accordance with the PRISMA 2020 statement: an updated guideline for reporting systematic reviews14. We fully adhered to our pre-study protocol registered in PROSPERO (CRD42020222904).
Search strategy
We ran a systematic search in five electronic databases [MEDLINE (via PubMed), Embase, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and Scopus], dated from inception to August 3rd, 2021, with the query ‘(daratumumab) OR (humax-CD38) OR (humax-CD 38) OR (Darzalex) OR (anti-CD38) OR (antiCD38) OR (L01XC24) OR (945721–28-8) OR (DB09331) OR (4Z63YK6E0E) OR (D10777)’. No filter was applied. Reference lists of the eligible studies were also screened to identify relevant publications.
Selection and eligibility criteria
Two independent review authors assessed all records at title, abstract, and full-text level. At each level of selection, inter-rater reliability was evaluated by calculating Cohen's kappa coefficient (κ)15. κ values ≤ 0 were interpreted as no agreement, 0.01–0.20 as none to slight agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement, 0.81–1.00 as almost perfect agreement, and 1.00 as a perfect agreement15. Disagreements have been resolved by third-party arbitration.
Only randomised controlled trials (RCTs) that compared the outcomes of a daratumumab containing regimen with the same treatment without daratumumab in patients with MM were eligible for inclusion. Furthermore, eligible studies had to provide data on least one of the following outcomes in both treatment arms: death or disease progression, sCR, CR or better, MRD negativity, thrombocytopenia, neutropenia, lymphopenia, anaemia, second primary malignancy, peripheral neuropathy, hypertension, cardiac failure, ischemic heart disease, acute renal failure. If more publication reported on the same trial (e.g. in case of CASTOR trial), the one that provided the most recent data was included in each outcome. If a study was not published in full-text article, the record was deemed ineligible for inclusion.
Data extraction
Two independent review authors extracted the following data from the full text and corresponding supplementary information of eligible articles: study name, first author, year of publication, Digital Object Identifier (DOI), title, number of patients in the study and each treatment group, age and gender distribution, number of patients with the above-listed outcomes. For death or disease progression, hazard ratios (HRs) with the corresponding 95% confidence intervals (CIs) were extracted. If there were available data, we collected information on high- and standard cytogenetic risk subtypes. High cytogenetic risk subtype was defined as t(4;14) translocation, t(14;16) translocation, or 17p deletion and standard cytogenetic risk subtype as patients lacking all of these. Data on NDMM and RRMM were collected and analysed separately.
Risk of bias assessment and certainty of the evidence
Two independent review authors evaluated the quality of the included studies using the RoB 2: a revised tool for assessing risk of bias in randomised trials16. The risk of bias assessment comprises five main domains: randomisation process, deviation from the intended intervention, missing outcome data, and selection of the reported results. These were rated as low risk, some concerns, or high risk of bias. Disagreements have been resolved by an independent third investigator.
Based on the approach proposed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group, the certainty of the evidence was assessed by two review authors independently with the help of GRADE profiler software (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). Available from gradepro.org.). Disagreements have been resolved by third-party arbitration.
Statistical analysis
Pooled odds ratios (ORs) and hazard ratios (HRs) were calculated for dichotomous outcomes. A random-effect model was applied in all analyses with the DerSimonian–Laird estimation17. If there was an overlap between the two study populations, the study with the higher patient number was included in the analysis. Statistical heterogeneity was analysed using the I2 and χ2 tests to gain probability values; p < 0.10 was defined to indicate significant heterogeneity. The I2 test represents the percentage of total variability across studies because of heterogeneity. I2 values of 30–60%, 50–90%, and 75–100% corresponded to moderate, substantial, and considerable heterogeneity, respectively, based on Cochrane's handbook18. Forest plots displayed the results of the meta-analysis. Trial sequential boundaries for cumulative meta-analyses and the meta-analyses were performed with Stata 16 SE (Stata Corp).
Ethics approval and consent to participate
Not required as data is not individualized and primary data was not collected. Not required as data is not individualized and primary data was not collected.
Consent for publication
The corresponding author accepts responsibility for releasing this material on behalf of any and all co-authors.
Results
Systematic search and selection
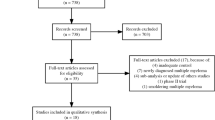
A total of 13,521 records were identified, 12 of which proved to be eligible for inclusion in qualitative synthesis (Fig. 1)19,20,21,22,23,24,25,26,27,28,29,30. Reasons for exclusion regarding full-text assessment are provided in Appendix A. The inter-rater reliability was rated as almost perfect or perfect at all steps of selection.
Characteristics of the studies included
Five and seven publications reported on patients with newly diagnosed and relapsed/refractory MM, respectively. The eligible papers reported on seven daratumumab containing regimens. The main characteristics of the studies included are summarised in Table 1.
Synthesis
Newly diagnosed multiple myeloma
Efficacy
Figure 2 summarises the results of the meta-analyses. Death or disease progression were less likely to occur with daratumumab-containing regimens in overall (HR: 0.47, CI 0.39–0.57) and standard-risk MM (HR: 0.43, CI 0.35–0.53) compared to control treatment (all with statistical power ≥ 80%); however, we failed to reach the level of significance in high cytogenetic risk MM (with statistical power < 80%) (Fig. 3). MM patients receiving daratumumab-containing regimens were more likely to achieve CR or better (OR 2.14, CI 1.66–2.75), sCR (OR 2.29, CI 1.49–3.51), and MRD negativity (OR 3.61, CI 2.33–5.61) compared to control treatment, all with statistical power ≥ 80%.
Effect of adding daratumumab to backbone anti-myeloma regimens on the risk of death of disease progression in (A) newly-diagnosed multiple myeloma with high cytogenetic risk, (B) newly-diagnosed multiple myeloma with standard cytogenetic risk, (C) relapsed/refractory multiple myeloma with high cytogenetic risk, and (D) relapsed/refractory multiple myeloma with standard cytogenetic risk.
Safety
Figure 4 summarises the results of meta-analyses on haematological toxicity. Incidence of anaemia and thrombocytopenia was not higher with daratumumab compared to the control group. On the other hand, lymphopenia and neutropenia occurred more frequently in the daratumumab group (all with statistical power ≥ 80% except for anaemia). Findings were consistent for grade 3–4 haematological toxicities. MM patients receiving daratumumab-containing treatment were less likely to develop peripheral neuropathy of any grade compared to control treatment (OR 0.76, CI 0.63–0.92, with statistical power ≥ 80%), whereas the frequency of grade 3–4 neuropathy was not significantly different between the groups (OR 0.80, CI 0.40–1.60, with statistical power < 80%). The second primary malignancy frequency was similar between the groups (OR 0.88, CI 0.54–1.45, with statistical power < 80%). No studies reported the other pre-specified outcomes, the risk of hypertension, acute cardiac and renal failure, and ischemic heart disease.
Relapsed/refractory multiple myeloma multiple myeloma
Efficacy
Death or disease progression were less likely to occur with daratumumab-containing regimens in overall (HR: 0.50, CI 0.37–0.67), standard-risk (HR: 0.38, CI 0.29–0.50), and high cytogenetic risk MM (HR: 0.52, CI 0.35–0.76) compared to the control treatment (all with statistical power ≥ 80%) (Fig. 3). MM patients receiving daratumumab-containing regimens were more likely to achieve CR or better (OR 3.50, CI 2.33–5.25), sCR (OR 3.08, CI 2.00–4.76), and MRD negativity (OR 5.43, CI 2.76–10.66) compared to control treatment, all with statistical power ≥ 80%.
Safety
Figure 3 summarises the results of the meta-analyses on haematological toxicity. Grade 3–4 lymphopenia, all grade neutropenia, and all grade and grade 3–4 thrombocytopenia were more common with daratumumab-containing vs control treatment (with statistical power ≥ 80%). We failed to detect a significant difference between the groups regarding other haematological toxicities. However, the comparison of all grade lymphopenia and grade 3–4 anaemia had less than 80% statistical power. We have not found difference in all grade (OR 2.21, CI 0.92–5.29, with statistical power ≥ 80%) and grade 3–4 hypertension (OR 3.21, CI 0.97–10.61, with statistical power ≥ 80%).
Data were insufficient for meta-analysis in the case of peripheral neuropathy and second primary malignancies (two non-overlapping study populations each); and in the case of acute cardiac failure, acute renal failure, ischemic heart disease (one non-overlapping population each) (shown in Table 2), and haematological toxicity in cytogenetic subgroups (two non-overlapping study populations each) (shown in Table 3).
Risk of bias assessment and certainty of the evidence
The overall risk of bias was assessed as 'low risk' or 'some concern' for all studies. The most common reasons for 'some concern' assessments were the insufficient description of randomisation and allocation concealment processes or the lack of a statistical analysis plan. Detailed assessments for each endpoint are provided in Appendix A.
Certainty of evidence ranged between 'very low' and 'moderate'. A detailed assessment is shown in the GRADE evidence profile tables in Appendix B.
Discussion
One of the main objectives of treatment in myeloma patients is to improve survival, both in NDMM and RRMM. In NDMM, the addition of daratumumab was associated with increased chance for PFS in each individual RCT and in our meta-analysis as well (moderate certainty). Regardless of these promising results, genetic risk stratification is a particularly important aspect of MM. About 15% of these patients carry myeloma with a high cytogenetic risk31. They are prone to worse therapeutic response, earlier relapse, and shorter PFS. While the survival benefit is still present in the standard cytogenetic risk population (moderate certainty), we have not found survival benefit in patients with high cytogenetic risk MM (low certainty).
The results about PFS benefit of daratumumab in high cytogenetic risk NDMM are controversial in the previous meta-analyses. We identified some possible explanations of this. Firstly, the paper of Giri et al.9, which had significant results in this analysis, incorporated their results from a conference abstract of the MAIA trial32 with a higher PFS benefit in the daratumumab arm compared to the one in other two publications11,12 and our paper. These three meta-analyses were more conservative and included only peer-reviewed full-text publications. Secondly, although all meta-analyses used the random effect model, the weights of the included studies slightly differ among the meta-analyses regarding this analysis. It has to be noted that the meta-analysis of Mohyuddin et al. did not reveal PFS benefit of adding daratumumab in high cytogenetic risk NDMM13; however that study did not use the HRs provided by the original publication and recalculated them from raw data. This resulted HRs that differed much from the ones calculated by the studies included. Thirdly, the four papers with neutral results used PFS values from more similar median follow up times (ALCYONE: 16.5 months; CASSIOPEIA: 18.8 months; MAIA: 28 months) and the one with significant PFS benefit in this population used HR from the MAIA trial with a median follow up of 36.4 months. This heterogeneity could also contribute to the difference between previous publications and reflect on the long term PFS benefit of daratumumab. However, regardless of the discrepancy in these findings, our TSA analysis confirmed that the statistical power was insufficient in this subgroup analysis, it seems to be early to preclude the benefit on this outcome.
In RRMM, PFS benefit was also observed in the daratumumab group (low certainty). This benefit was also found in the standard cytogenetic risk (low certainty) and in the high cytogenetic population (moderate certainty) as well. Therefore, our finding supports the incorporation of daratumumab regardless of the results of the cytogenetic assessment.
Several studies support that a better therapeutic response translates into longer PFS and OS in MM33. Based on this meta-analysis, patients on daratumumab have a better chance of achieving CR or better compared to control in NDMM (low certainty) and RRMM (moderate certainty). As modern therapeutic strategies led to deeper therapeutic response, sCR became an essential surrogate for survival endpoints34. Kapoor et al. found that patients achieving sCR had longer time to progression and longer OS after transplantation than those who only achieved CR, which underlines the prognostic significance of sCR34. Concerning sCR, we found that it is more likely to be achieved both in NDMM (low certainty) and RRMM patients (moderate certainty) receiving additional daratumumab compared to controls.
Detection of MRD is emerging as an important tool to assess the efficacy of treatments in MM35. Several studies have confirmed that MRD negativity is associated with improved survival both in patients with NDMM (ALCYONE, CASSIOPEIA, GRIFFIN, and MAIA trial) and RRMM (APOLLO, CANDOR, and LEPUS trial)19,21,22,24,26,27,28,29. In RRMM, MRD-negativity rates favoured daratumumab arm (moderate certainty). Regarding NDMM patients, the same associations were observed, and our pooled analyses support that the addition of daratumumab increases the chance of achieving MRD negativity (low certainty).
Despite the enhanced cytotoxicity on myeloma cells, better survival results, and therapeutic response, safety concerns arise36 as the target of this antibody is expressed on haematopoietic cells37. In NDMM, both all grade and grade 3–4 lymphopenia (moderate certainty) and neutropenia (low certainty) were more likely to appear in patients on daratumumab. These were consistent with our RRMM population results; however, the chance for all grade thrombocytopenia and grade 3–4 thrombocytopenia was also found to be increased (low certainty). Regarding the likelihood of anaemia, no difference was demonstrated.
Besides the bone marrow, other tissues, like peripheral and central neurons, also express CD38 which could raise clinical concern37. A previous meta-analysis demonstrated that the risk for peripheral neuropathy does not increase with the addition of daratumumab36. This study covered the literature until June 2019. Now all of their eligible studies have updated results; therefore, we could re-evaluate their findings and perform analyses on all grade and grade 3–4 neuropathy separately. Our results also support their finding.
With the increasingly longer survival of MM, second primary malignancies gained more significance38. In a population-based study of Sweden, the incidence of second primary malignancy was 5.5% after a median follow-up of 2.5 years39. These disorders mostly consist of mostly acute leukaemia or myelodysplasia and their incidence is about 2.19 times higher in MM compared to the general population39. Htut et al. found no increase in the incidence of second primary malignancies after the addition of daratumumab to backbone therapies36. We support their finding (very low certainty). However, as they pointed out, long-term follow-up results are required to confirm this observation.
This study has multiple strengths and limitations. First of all, the research was conducted with rigorous methodology adhering to the latest methodological recommendations and we reported our analyses transparently. Furthermore, our results are consistent with previous meta-analyses on the topic. Nevertheless, many of new publications on the subject emerged since these reviews were published, counting the longer follow-up data for the studies included in the precedent meta-analyses and the results of the GRIFFIN trial, the LEPUS trial, and the APOLLO trial21,24,29. This enabled us to re-evaluate their results and to assess new endpoints such as MRD negativity and sCR and to evaluate RRMM with subgroup analyses, which have not been included in a meta-analysis yet. There are limitations in this meta-analysis, including that different backbone regimens are evaluated in the individual RCTs. This issue was addressed by lowering the level of evidence due to indirectness in each evaluation. As the Trial Sequential Analyses have pointed out, some assessments are exposed to the possibility of imprecision. The overall risk of bias was generally ‘low’; however, certain domains had ‘some concern’ evaluation. Publication bias could not be assessed because the number of studies included in each analysis was insufficient for statistical analysis.
Implication for practice: our results support incorporating daratumumab in backbone therapies in MM which was associated with better therapeutic response and survival and favourable safety profile both in NDMM and RRMM.
Implication for science: additional studies are needed to specify further the population that gains the most benefits from this treatment, especially in high cytogenetic risk NDMM, where the OIS was not reached.
Conclusion
Daratumumab has shown high clinical efficacy and acceptable toxicity profile for the treatment of both NDMM and RRMM for the endpoints examined.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CENTRAL:
-
Cochrane central register of controlled trials
- CI:
-
Confidence interval
- CR:
-
Complete response
- DPd:
-
Daratumumab, pomalidomide, and dexamethasone
- DRd:
-
Daratumumab, lenalidomide and dexamethasone
- DRVd:
-
Daratumumab, bortezomib, lenalidomide and dexamethasone
- DVd:
-
Daratumumab, bortezomib and dexamethasone
- DVMP:
-
Daratumumab, bortezomib, melphalan, and prednisone
- DVTd:
-
Daratumumab, bortezomib, thalidomide, and dexamethasone
- GRADE:
-
Grading of recommendations, assessment, development and evaluation
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- IRR:
-
Infusion-related reaction
- Kd:
-
Carfilzomib and dexamethasone
- KdD:
-
Carfilzomib, dexamethasone, and daratumumab
- MM:
-
Multiple myeloma
- MRD:
-
Minimal residual disease negativity
- NDMM:
-
Newly diagnosed multiple myeloma
- n.r.:
-
Not reported
- OIS:
-
Optimal information size
- OR:
-
Odds ratio
- OS:
-
Overall survival
- Pd:
-
Pomalidomide and dexamethasone
- PFS:
-
Progression-free survival
- PRISMA:
-
Preferred reporting items for systematic reviews and meta‐analyses
- RCT:
-
Randomised controlled trial
- Rd:
-
Lenalidomide and dexamethasone
- RRMM:
-
Relapsed/refractory multiple myeloma
- RVd:
-
Bortezomib, lenalidomide and dexamethasone
- sCR:
-
Stringent complete response
- TSA:
-
Trial sequential analysis
- Vd:
-
Bortezomib and dexamethasone
- VMP:
-
Bortezomib, melphalan, and prednisone
- VTd:
-
Bortezomib, thalidomide, and dexamethasone
References
Rajkumar, S. V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 95, 548–567. https://doi.org/10.1002/ajh.25791 (2020).
Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 43, 676–681. https://doi.org/10.1053/j.seminoncol.2016.11.004 (2016).
Merrill, R. M. & Dearden, K. A. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States?. Cancer Causes Control 15, 1027–1034. https://doi.org/10.1007/s10552-004-1324-5 (2004).
Thanendrarajan, S. et al. Monoclonal antibody therapy in multiple myeloma: Where do we stand and where are we going?. Immunotherapy 8, 367–384. https://doi.org/10.2217/imt.15.118 (2016).
Plesner, T. & Krejcik, J. Daratumumab for the treatment of multiple myeloma. Front. Immunol. 9, 1228–1228. https://doi.org/10.3389/fimmu.2018.01228 (2018).
Nastoupil, L. J. When to use targeted therapy for the treatment of follicular lymphoma. Curr. Hematol. Malig. Rep. 16, 45–51. https://doi.org/10.1007/s11899-021-00617-5 (2021).
Xu, W. et al. Daratumumab added to standard of care in patients with newly diagnosed multiple myeloma: A network meta-analysis. Eur. J. Haematol. 103, 542–551. https://doi.org/10.1111/ejh.13317 (2019).
Wang, Y., Li, Y. & Chai, Y. Efficacy and safety of daratumumab in the treatment of multiple myeloma: A systematic review and meta-analysis. J. Int. Med. Res. 49, 03000605211038135. https://doi.org/10.1177/03000605211038135 (2021).
Giri, S. et al. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: A systematic review and meta-analysis. JAMA Oncol. 6, 1–8. https://doi.org/10.1001/jamaoncol.2020.4338 (2020).
Cao, C., Zhou, X. & Ma, Q. Daratumumab provides a survival benefit in relapsed and refractory multiple myeloma, independent of baseline clinical characteristics: A meta-analysis. Pharmacol. Res. Perspect. 9, e00797. https://doi.org/10.1002/prp2.797 (2021).
Premkumar, V., Pan, S., Lentzsch, S. & Bhutani, D. Use of daratumumab in high risk multiple myeloma: A meta-analysis. eJHeam 1, 267–271. https://doi.org/10.1002/jha2.47 (2020).
Chong, L. L. et al. Daratumumab-based induction therapy for multiple myeloma: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 159, 103211. https://doi.org/10.1016/j.critrevonc.2020.103211 (2021).
Mohyuddin, G. R. et al. Impact of anti-CD38 therapy in multiple myeloma with high-risk cytogenetics: Systematic review and meta-analysis. Leuk. Lymphoma 61, 2519–2522. https://doi.org/10.1080/10428194.2020.1772475 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
McHugh, M. L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb) 22, 276–282 (2012).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 366, l4898. https://doi.org/10.1136/bmj.l4898 (2019).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188. https://doi.org/10.1016/0197-2456(86)90046-2 (1986).
Jonathan J Deeks, Julian PT Higgins, Douglas G Altman & Group, o. b. o. t. C. S. M. (2021).
Dimopoulos, M. et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 396, 186–197 (2020).
Dimopoulos, M. A. et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375, 1319–1331. https://doi.org/10.1056/NEJMoa1607751 (2016).
Dimopoulos, M. A. et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol. 22, 801–812. https://doi.org/10.1016/s1470-2045(21)00128-5 (2021).
Facon, T. et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 380, 2104–2115. https://doi.org/10.1056/NEJMoa1817249 (2019).
Kaufman, J. L. et al. Daratumumab, lenalidomide, and dexamethasone in relapsed/refractory myeloma: A cytogenetic subgroup analysis of POLLUX. Blood Cancer J. 10, 111. https://doi.org/10.1038/s41408-020-00375-2 (2020).
Lu, J. et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in chinese patients with relapsed or refractory multiple myeloma: Phase 3 LEPUS (MMY3009) study. Clin. Lymphoma Myeloma Leuk. 21, e699–e709. https://doi.org/10.1016/j.clml.2021.04.012 (2021).
Mateos, M. V. et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 395, 132–141. https://doi.org/10.1016/s0140-6736(19)32956-3 (2020).
Mateos, M. V. et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N. Engl. J. Med. 378, 518–528. https://doi.org/10.1056/NEJMoa1714678 (2018).
Moreau, P. et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394, 29–38. https://doi.org/10.1016/s0140-6736(19)31240-1 (2019).
Palumbo, A. et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375, 754–766. https://doi.org/10.1056/NEJMoa1606038 (2016).
Voorhees, P. M. et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 136, 936–945. https://doi.org/10.1182/blood.2020005288 (2020).
Weisel, K. et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: Subgroup analysis of CASTOR based on cytogenetic risk. J. Hematol. Oncol. 13, 115. https://doi.org/10.1186/s13045-020-00948-5 (2020).
Joseph, N. S., Gentili, S., Kaufman, J. L., Lonial, S. & Nooka, A. K. High-risk multiple myeloma: Definition and management. Clin. Lymphoma Myeloma Leukemia 17S, S80–S87. https://doi.org/10.1016/j.clml.2017.02.018 (2017).
Bahlis, N. et al. Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant: Updated analysis of maia. Blood 134, 1875–1875. https://doi.org/10.1182/blood-2019-123426%JBlood (2019).
Chanan-Khan, A. A. & Giralt, S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 28, 2612–2624. https://doi.org/10.1200/jco.2009.25.4250 (2010).
Kapoor, P. et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 31, 4529–4535. https://doi.org/10.1200/JCO.2013.49.0086 (2013).
Perrot, A. et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 132, 2456–2464. https://doi.org/10.1182/blood-2018-06-858613 (2018).
Htut, T. W. et al. Incidence of second primary malignancies and peripheral sensory neuropathy in patients with multiple myeloma receiving daratumumab containing regimen. Blood 134, 5550–5550. https://doi.org/10.1182/blood-2019-123156%JBlood (2019).
Jain, A. & Ramasamy, K. Evolving role of daratumumab: From backbencher to frontline agent. Clin. Lymphoma Myeloma Leuk. 20, 572–587. https://doi.org/10.1016/j.clml.2020.03.010 (2020).
Poh, C., Keegan, T. & Rosenberg, A. S. Second primary malignancies in multiple myeloma: A review. Blood Rev. 46, 100757. https://doi.org/10.1016/j.blre.2020.100757 (2021).
Dong, C. & Hemminki, K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: A search for common mechanisms. Br. J. Cancer 85, 997–1005. https://doi.org/10.1038/sj.bjc.6691998 (2001).
Acknowledgements
This work was supported by the Economic Development and Innovation Operative Programme Grant [GINOP-2.3.4-15-2020-00010]; by the Human Resources Development Operational Programme Grant [EFOP-3.6.1-16-2016-00004], and the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund [ÚNKP-20-3]. This research was not a company-initiated study. Sponsors were not involved in the design, data collection, analysis, interpretation, or preparation of the manuscript. We thank Mária Földi and Andrea Szentesi for their comments that greatly improved the manuscript.
Funding
This work was supported by the Economic Development and Innovation Operative Programme Grant [GINOP-2.3.4-15-2020-00010]; by the Human Resources Development Operational Programme Grant [EFOP-3.6.1-16-2016-00004], and the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund [ÚNKP-20-3]. This research was not a company-initiated study. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Author information
Authors and Affiliations
Contributions
SK: conceptualisation, project administration, formal analysis, writing-original draft; NG: conceptualisation, formal analysis, visualization, writing-original draft; PH: conceptualisation, funding acquisition, writing-review & editing; BN: conceptualisation, data curation, writing-review & editing; RD: conceptualisation, data curation, writing-review & editing; FD: conceptualisation, methodology, writing-review & editing, SB: conceptualisation, writing-review & editing; BE: conceptualisation, methodology, writing-review & editing; TL: conceptualisation, writing-review & editing; ZS: conceptualisation, methodology, visualization, writing-original draft; HA: conceptualisation; supervision; writing-original draft. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiss, S., Gede, N., Hegyi, P. et al. Addition of daratumumab to multiple myeloma backbone regimens significantly improves clinical outcomes: a systematic review and meta-analysis of randomised controlled trials. Sci Rep 11, 21916 (2021). https://doi.org/10.1038/s41598-021-01440-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01440-x
- Springer Nature Limited